The Effect of Resistance Training on Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
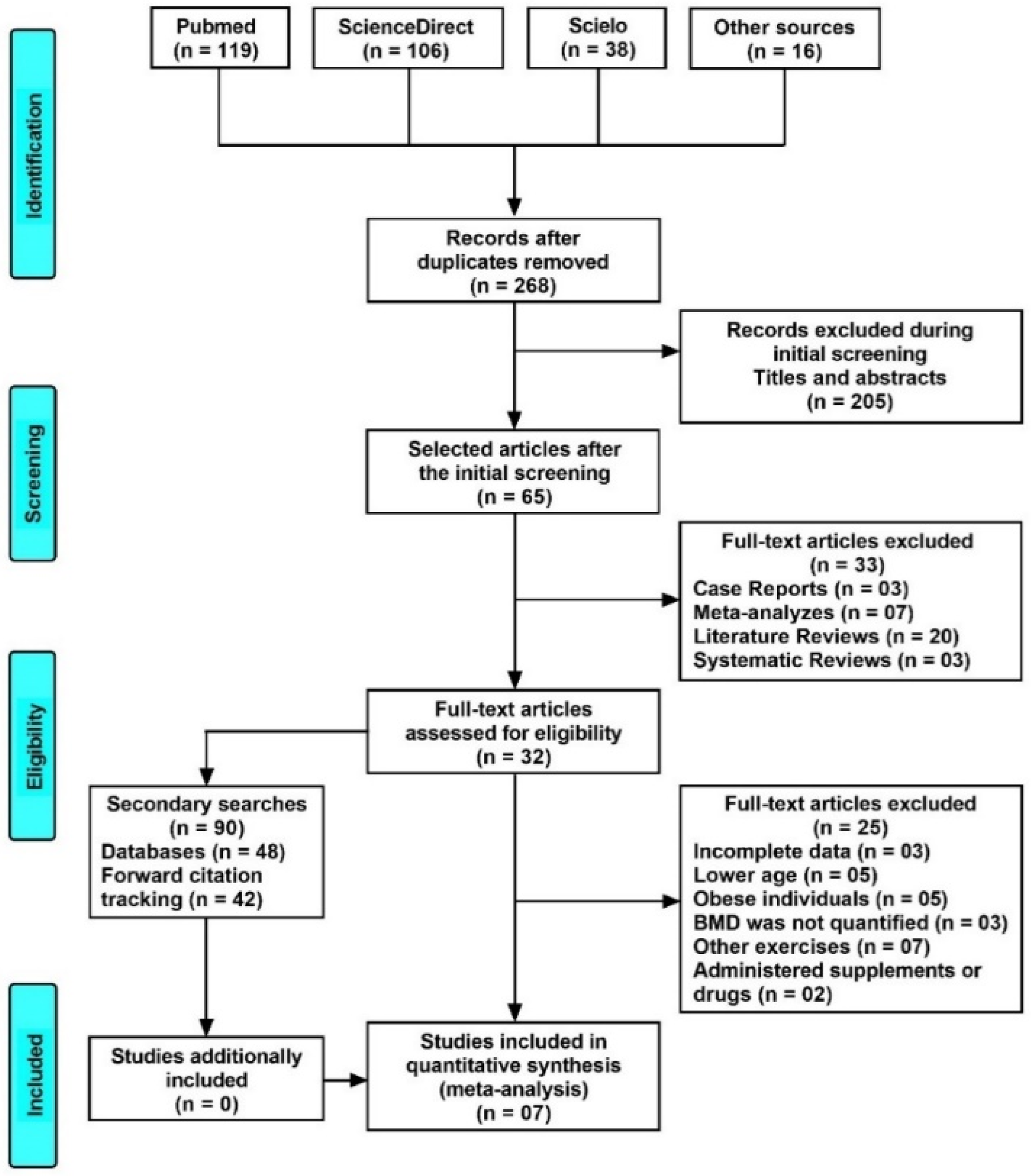
2. Materials and Methods
2.1. Study Selection Criteria
2.2. Data Extraction
2.3. Assessment of Methodological Quality and Risk of Bias
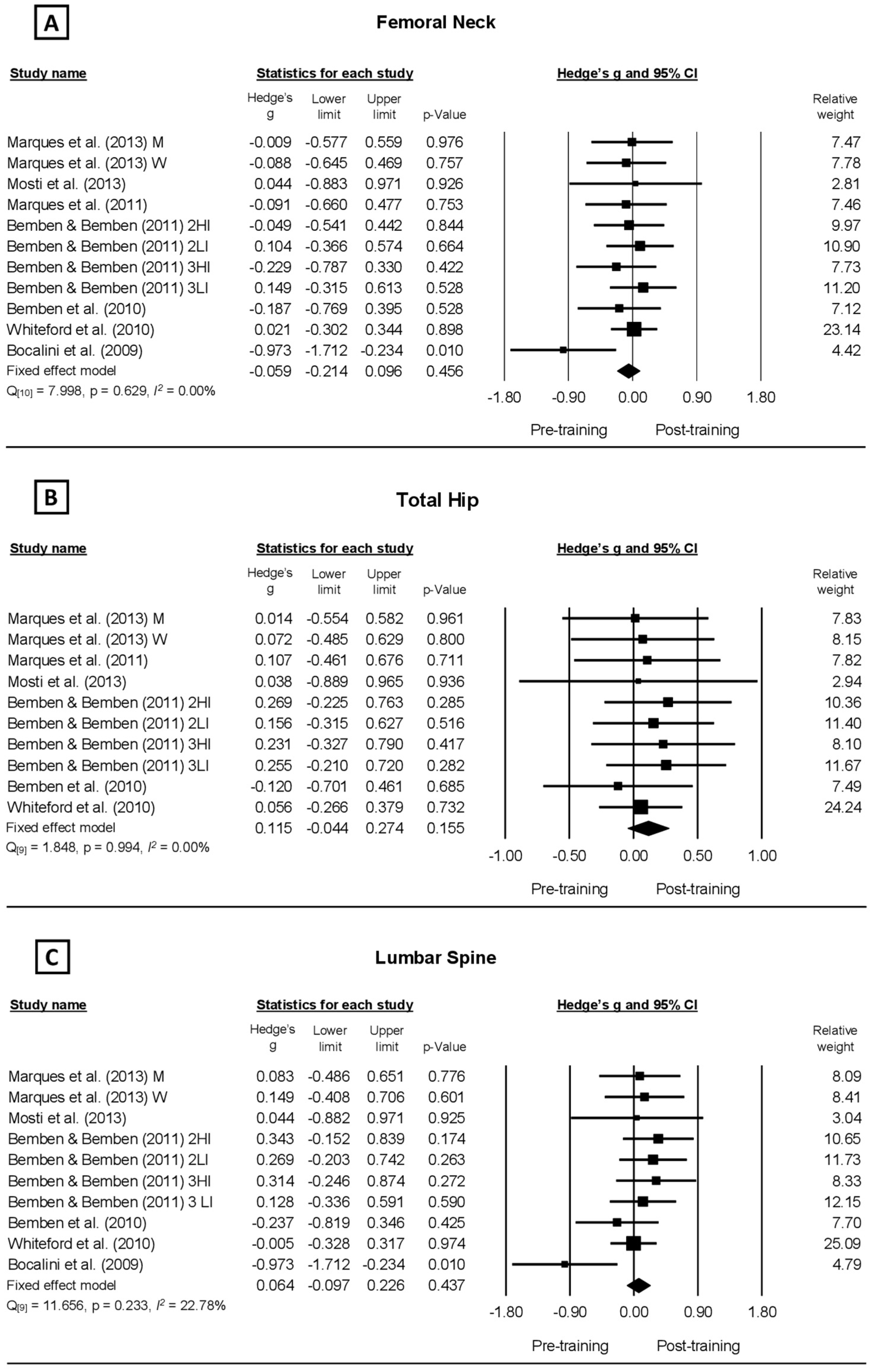
2.4. Statistical Analysis
3. Results
| Study | Participants | Resistance Training Protocols | Bone Mineral Density (g/cm2) | PEDro | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age | Height | Body Mass | BMI | Exercise | Sets | Rep | Intensity | Rest | Weekly Frequency | Duration | Bone | Interventions | ||||
| (years) | (cm) | (kg) | (kg/m2) | (Body Region) | (%1 RM) | (s) | (Weeks) | (Region) | Pre | Post | ∆ (%) | Scores | |||||
| Mosti et al., 2013 [4] | 8 W | 61.9 ± 5.0 | 169.3 ± 6.5 | 72.3 ± 7.7 | 25.3 ± 2.9 | 1 ex. LL (SM) | 24 | 8–12 3–5 | 50 90 | 120 180 | 3 | 12 | FN LS TH | 0.651 ± 0.084 0.759 ± 0.061 0.751 ± 0.125 | 0.655 ± 0.088 0.762 ± 0.067 0.756 ± 0.123 | 0.61 0.40 0.67 | 6 Excellent |
| Marques et al., 2011 [12] | 23 W | 67.3 ± 5.2 | 28.8 ± 4.6 | 4 ex. LL (LP, KE, LC, HAb) 4 ex. T and UL (CP, LR, SP, AC) | 3 | 6–8 | 75–80 | 120 | 3 | 32 | FN TH | 0.684 ± 0.082 0.859 ± 0.124 | 0.676 ± 0.090 0.873 ± 0.132 | −1.17 1.63 | 5 Good | ||
| Whiterford et al., 2010 [13] | 73 M | 64.6 ± 6.0 | 176.6 ± 6.8 | 82.4 ± 10.7 | 26.4 ± 3.1 | 4 ex. LL (HF, HE, Hab, CR) 5 ex. T and UL (WC, WE, BC, TP, FPS) | 3 | 10 | 85 | 60 | 3 | 52 | FN LS TH | 0.966 ± 0.142 1.236 ± 0.189 1.045 ± 0.161 | 0.969 ± 0.140 1.235 ± 0.185 1.054 ± 0.157 | 0.31 −0.08 0.86 | 6 Excellent |
| Bemben et al., 2010 [17] | 22 W | 64 ± 0.9 | 160.6 ± 1.7 | 76.6 ± 3.1 | 5 ex. LL (LP, HF, HE, HAb, HAd) 3 ex. T and UL (SP, LPD, SR) | 3 | 10 | 80 | 60 | 3 | 32 | FN LS TH | 0.902 ± 0.021 1.163 ± 0.028 0.955 ± 0.025 | 0.898 ± 0.021 1.156 ± 0.030 0.952 ± 0.024 | −0.44 −0.60 −0.44 | 5 Good | |
| Bocalini et al., 2009 [20] | 40 W | 69 ± 9.0 | *≅155 | 68 ± 6.0 | 28 ± 4.0 | 5 ex. LL (LP, LC, KE, HAb, HAd) 7 ex. UL (CP, LPD, BC, TP, SR, SP, AC) | 3 | 10 | 85 | 60 | 3 | 24 | FN LS | 0.705 ± 0.001 0.881 ± 0.001 | 0.704 ± 0.001 0.880 ± 0.001 | −0.14 −0.14 | 6 Excellent |
| Marques et al., 2013 [36] | 23 M 24 W | 68.2 ± 5.2 68.2 ± 5.7 | *≅169 *≅150 | 83.0 ± 11.7 64.2 ± 10.2 | 29.2 ± 3.4 28.6 ± 4.1 | 4 ex. LL (LP, KE, LC, HAb) 4 ex. T and UL (CP, LR, SP, AC) | 3 | 6–8 | 75–80 | 150 | 3 | 32 | FN LS TH FN LS TH | 0.822 ± 0.113 1.051 ± 0.161 1.004 ± 0.140 0.715 ± 0.119 0.877 ± 0.122 0.864 ± 0.108 | 0.821 ± 0.115 1.065 ± 0.172 1.006 ± 0.138 0.705 ± 0.104 0.896 ± 0.129 0.872 ± 0.111 | 0.12 1.33 0.20 −1.40 2.17 0.93 | 6 Excellent |
| Bemben & Bemben 2011 [37] | 45 M 79 W | 65.2 ± 0.5 63.8 ± 0.4 | 176.8 ± 1.0 163.2 ± 0.6 | 83.5 ± 1.6 69.7 ± 1.5 | 26.7 ± 0.5 26.1 ± 0.5 | 7 ex. LL (KF, KE, LP, HF, HE, HAb, HAd) 5 ex. T and UL (FF, FE, SP, LPD, SR) | 3 3 3 3 | 8 16 8 16 | 80 40 80 40 | 2 2 3 3 | 40 | FN LS TH FN LS TH FN LS TH FN LS TH | 0.903 ± 0.020 1.143 ± 0.035 0.943 ± 0.022 0.902 ± 0.019 1.186 ± 0.032 0.940 ± 0.019 0.894 ± 0.022 0.889 ± 0.021 0.950 ± 0.026 0.928 ± 0.026 1.186 ± 0.031 0.976 ± 0.031 | 0.902 ± 0.020 1.155 ± 0.034 0.949 ± 0.022 0.904 ± 0.019 1.195 ± 0.034 0.943 ± 0.019 0.889 ± 0.021 1.190 ± 0.034 0.956 ± 0.025 0.932 ± 0.026 1.190 ± 0.031 0.984 ± 0.031 | −0.11 1.05 0.64 0.22 0.76 0.32 −0.56 0.96 0.63 0.43 0.34 0.82 | 5 Good | |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamoscinska, M.; Faber, I.R.; Busch, D. Do older adults with reduced bone mineral density benefit from strength training? A critically appraised topic. J. Sport Rehabil. 2020, 29, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Brentano, M.A.; Kruel, L.F.M. Efeitos da atividade física na densidade mineral óssea e na remodelação do tecido ósseo. Rev. Bras. Med. Esporte. 2005, 11, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes, B.R.; Pimenta, L.D.; Massini, D.A.; Dos Santos, D.; Siqueira, L.; Simionato, A.R.; Dos Santos, L.G.A.; Neiva, C.M.; Pessôa Filho, D.M. Muscle strength and regional lean body mass influence on mineral bone health in young male adults. PLoS ONE 2018, 13, e0191769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosti, M.P.; Kaehler, N.; Stunes, A.K.; Hoff, J.; Syversen, U. Maximal strength training in postmenopausal women with osteoporosis or osteopenia. J. Strength Cond. Res. 2013, 27, 2879–2886. [Google Scholar] [CrossRef]
- Pimenta, L.D.; Massini, D.A.; Dos Santos, D.; Siqueira, L.O.C.; Sancassani, A.; Dos Santos, L.G.A.; Guimarães, B.R.; Neiva, C.M.; Pessôa Filho, D.M. Women’s femoral mass content correlates to muscle strength independently of lean body mass. Rev. Bras. Med. Esporte. 2019, 25, 485–489. [Google Scholar] [CrossRef]
- Daly, R.M.; Dalla Via, J.; Duckham, R.L.; Fraser, S.F.; Helge, E.W. Exercise for the prevention of osteoporosis in postmenopausal women: An evidence-based guide to the optimal prescription. Braz. J. Phys. Ther. 2019, 23, 170–180. [Google Scholar] [CrossRef]
- Choe, Y.R.; Jeong, J.R.; Kim, Y.P. Grip strength mediates the relationship between muscle mass and frailty. J. Cachexia Sarcopenia Muscle 2020, 11, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, H.R.; Kennedy, J.A.; Mellon, S.J.; Judge, A.; Dodd, C.A.; Murray, D.W. The clinical outcomes of cementless unicompartmental knee replacement in patients with reduced bone mineral density. J. Orthop. Surg. Res. 2020, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabello, A.; Ara, I.; Gonzalez-Aguero, A.; Casajus, J.A.; Vicente-Rodriguez, G. Effects of training on bone mass in older adults: A systematic review. Sports Med. 2012, 42, 301–325. [Google Scholar] [CrossRef] [PubMed]
- Westcott, W.L. Resistance training is medicine: Effects of strength training on health. Curr. Sports Med. Rep. 2012, 11, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.A.; Wanderley, F.; Machado, L.; Sousa, F.; Viana, J.L.; Moreira-Goncalves, D.; Moreira, P.; Mota, J.; Carvalho, J. Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp. Gerontol. 2011, 46, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, J.; Ackland, T.R.; Dhaliwal, S.S.; James, A.P.; Woodhouse, J.J.; Price, R.; Prince, R.L.; Kerr, D.A. Effects of a 1-year randomized controlled trial of resistance training on lower limb bone and muscle structure and function in older men. Osteoporos. Int. 2010, 21, 1529–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, S.L.; Weeks, B.K.; Weis, L.J.; Harding, A.T.; Horan, S.A.; Beck, B.R. High-Intensity Resistance and Impact Training Improves Bone Mineral Density and Physical Function in Postmenopausal Women with Osteopenia and Osteoporosis: The LIFTMOR Randomized Controlled Trial. J. Bone Miner. Res. 2018, 33, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, M.; Xu, Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: A meta-analysis. Osteoporos Int. 2015, 26, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, L.M.; Papaioannou, A.; Macintyre, N.J.; Ashe, M.C.; Heinonen, A.; Shipp, K.; Wark, J.; McGill, S.; Keller, H.; Jain, R.; et al. Too Fit to Fracture: Exercise recommendations for individuals with osteoporosis or osteoporotic vertebral fracture. Osteoporos. Int. 2014, 25, 821–835. [Google Scholar] [CrossRef] [Green Version]
- Bemben, D.A.; Palmer, I.J.; Bemben, M.G.; Knehans, A.W. Effects of combined whole-body vibration and resistance training on muscular strength and bone metabolism in postmenopausal women. Bone 2010, 47, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, E.C.; Martin, A.D.; Taunton, J.E.; Donnelly, M.; Warren, J.; Elliot, J. Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br. J. Sports Med. 2000, 34, 18–22. [Google Scholar] [CrossRef]
- Bocalini, D.S.; Serra, A.J.; Dos Santos, L. Moderate resistive training maintains bone mineral density and improves functional fitness in postmenopausal women. J. Aging Res. 2010, 2010, 760818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocalini, D.S.; Serra, A.J.; dos Santos, L.; Murad, N.; Levy, R.F. Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J. Aging Health 2009, 21, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolan, E.; Dumas, A.; Keane, K.M.; Bestetti, G.; Freitas, L.H.M.; Gualano, B.; Kohrt, W.; Kelley, G.A.; Pereira, R.M.R.; Sale, C.; et al. The influence of acute exercise on bone biomarkers: Protocol for a systematic review with meta-analysis. Syst. Rev. 2020, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, W.M.; Bloomfield, S.A.; Little, K.D.; Nelson, M.E.; Yingling, V.R. American College of Sports Medicine Position Stand: Physical activity and bone health. Med. Sci. Sports Exerc. 2004, 36, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Rourke, D.; Beck, B.R.; Harding, A.T.; Watson, S.L.; Pivonka, P.; Martelli, S. Assessment of femoral neck strength and bone mineral density changes following exercise using 3D-DXA images. J. Biomech. 2021, 119, 110315. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Kummel, J.; Kramer, A.; Giboin, L.S.; Gruber, M. Specificity of balance training in healthy individuals: A systematic review and meta-analysis. Sports Med. 2016, 46, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Lazinica, B.; Mikulic, P.; Krieger, J.W.; Schoenfeld, B.J. The effects of short versus long inter-set rest intervals in resistance training on measures of muscle hypertrophy: A systematic review. Eur. J. Sport Sci. 2017, 17, 983–993. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Su, L.; Fu, J.; Sun, S.; Zhao, G.; Cheng, W.; Dou, C.; Quan, M. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: A meta-analysis. PLoS ONE 2019, 14, e0210644. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grgic, J.; Rodriguez, R.F.; Garofolini, A.; Saunders, B.; Bishop, D.J.; Schoenfeld, B.J.; Pedisic, Z. Effects of sodium bicarbonate supplementation on muscular strength and endurance: A systematic review and meta-analysis. Sports Med. 2020, 50, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.; Wewege, M.A.; Hackett, D.A.; Keogh, J.W.L.; Hagstrom, A.D. Sex differences in adaptations in muscle strength and size following resistance training in older adults: A systematic review and meta-analysis. Sports Med. 2021, 51, 503–517. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Marques, E.A.; Mota, J.; Viana, J.L.; Tuna, D.; Figueiredo, P.; Guimaraes, J.T.; Carvalho, J. Response of bone mineral density, inflammatory cytokines, and biochemical bone markers to a 32-week combined loading exercise programme in older men and women. Arch. Gerontol. Geriatr. 2013, 57, 226–233. [Google Scholar] [CrossRef]
- Bemben, D.A.; Bemben, M.G. Dose-response effect of 40 weeks of resistance training on bone mineral density in older adults. Osteoporos. Int. 2011, 22, 179–186. [Google Scholar] [CrossRef]
- Arija-Blazquez, A.; Ceruelo-Abajo, S.; Diaz-Merino, M.S.; Godino-Duran, J.A.; Martinez-Dhier, L.; Martin, J.L.; Florensa-Vila, J. Effects of electromyostimulation on muscle and bone in men with acute traumatic spinal cord injury: A randomized clinical trial. J. Spinal Cord Med. 2014, 37, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Vainionpaa, A.; Korpelainen, R.; Sievanen, H.; Vihriala, E.; Leppaluoto, J.; Jamsa, T. Effect of impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone 2007, 40, 604–611. [Google Scholar] [CrossRef]
- Going, S.B.; Laudermilk, M. Osteoporosis and strength training. Am. J. Lifestyle Med. 2009, 3, 310–319. [Google Scholar] [CrossRef]
- Min, S.K.; Oh, T.; Kim, S.H.; Cho, J.; Chung, H.Y.; Park, D.H.; Kim, C.S. Position statement: Exercise guidelines to increase peak bone mass in adolescents. J. Bone Metab. 2019, 26, 225–239. [Google Scholar] [CrossRef]
- Wolff, I.; van Croonenborg, J.J.; Kemper, H.C.; Kostense, P.J.; Twisk, J.W. The effect of exercise training programs on bone mass: A meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos. Int. 1999, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Samadpour, M.; Eghbali, E. The effects of concurrent training (aerobic-resistance) and milk consumption on some markers of bone mineral density in women with osteoporosis. BMC Womens Health 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Maddalozzo, G.F.; Snow, C.M. High intensity resistance training: Effects on bone in older men and women. Calcif. Tissue Int. 2000, 66, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 275, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- De Matos, O.; Lopes da Silva, D.J.; Martinez de Oliveira, J.; Castelo-Branco, C. Effect of specific exercise training on bone mineral density in women with postmenopausal osteopenia or osteoporosis. Gynecol. Endocrinol. 2009, 25, 616–620. [Google Scholar] [CrossRef]
- Turner, C.H.; Robling, A.G. Designing exercise regimens to increase bone strength. Exerc. Sport Sci. Rev. 2003, 31, 45–50. [Google Scholar] [CrossRef]
- Warren, M.; Petit, M.A.; Hannan, P.J.; Schmitz, K.H. Strength training effects on bone mineral content and density in premenopausal women. Med. Sci. Sports Exerc. 2008, 40, 1282–1288. [Google Scholar] [CrossRef]
- Laurent, M.R.; Dubois, V.; Claessens, F.; Verschueren, S.M.; Vanderschueren, D.; Gielen, E.; Jardí, F. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol. Cell Endocrinol. 2016, 432, 14–36. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massini, D.A.; Nedog, F.H.; de Oliveira, T.P.; Almeida, T.A.F.; Santana, C.A.A.; Neiva, C.M.; Macedo, A.G.; Castro, E.A.; Espada, M.C.; Santos, F.J.; et al. The Effect of Resistance Training on Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1129. https://doi.org/10.3390/healthcare10061129
Massini DA, Nedog FH, de Oliveira TP, Almeida TAF, Santana CAA, Neiva CM, Macedo AG, Castro EA, Espada MC, Santos FJ, et al. The Effect of Resistance Training on Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis. Healthcare. 2022; 10(6):1129. https://doi.org/10.3390/healthcare10061129
Chicago/Turabian StyleMassini, Danilo A., Flávio H. Nedog, Thiago P. de Oliveira, Tiago A. F. Almeida, Caroline A. A. Santana, Cassiano M. Neiva, Anderson G. Macedo, Eliane A. Castro, Mário C. Espada, Fernando J. Santos, and et al. 2022. "The Effect of Resistance Training on Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis" Healthcare 10, no. 6: 1129. https://doi.org/10.3390/healthcare10061129
APA StyleMassini, D. A., Nedog, F. H., de Oliveira, T. P., Almeida, T. A. F., Santana, C. A. A., Neiva, C. M., Macedo, A. G., Castro, E. A., Espada, M. C., Santos, F. J., & Pessôa Filho, D. M. (2022). The Effect of Resistance Training on Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis. Healthcare, 10(6), 1129. https://doi.org/10.3390/healthcare10061129










