Prevalence and Correlates of Vitamin D Deficiency in Children Aged Less than Two Years: A Cross-Sectional Study from Aseer Region, Southwestern Saudi Arabia
Abstract
:1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Strengths and Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.M.; Del Giudice, M.M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thacher, T.D.; Pludowski, P.; Shaw, N.J.; Mughal, M.Z.; Munns, C.F.; Högler, W. Nutritional rickets in immigrant and refugee children. Public Health Rev. 2016, 37, 3. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Leonardi, A.; Lanciotti, L.; Cofini, M.; Muzi, G.; Penta, L. Vitamin D and growth hormone in children: A review of the current scientific knowledge. J. Transl. Med. 2019, 17, 87. [Google Scholar] [CrossRef] [Green Version]
- Sizar, O.; Khare, S.; Goyal, A.; Givler, A. Vitamin D Deficiency. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532266/ (accessed on 1 May 2022).
- van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef]
- Wahl, D.A.; Cooper, C.; Ebeling, P.R.; Eggersdorfer, M.; Hilger, J.; Hoffmann, K.H.; Josse, R.G.; Kanis, J.A.; Mithal, A.; Pierroz, D.D.; et al. A global representation of vitamin D status in healthy populations. Arch. Osteoporos. 2012, 7, 155–172. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Schleicher, R.L.; Sternberg, M.R.; Looker, A.C.; Yetley, E.A.; Lacher, D.A.; Sempos, C.T.; Taylor, C.L.; Durazo-Arvizu, R.A.; Maw, K.L.; Chaudhary-Webb, M.; et al. National Estimates of Serum Total 25-Hydroxyvitamin D and Metabolite Concentrations Measured by Liquid Chromatography–Tandem Mass Spectrometry in the US Population during 2007–2010. J. Nutr. 2016, 146, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Sarafin, K.; Durazo-Arvizu, R.; Tian, L.; Phinney, K.W.; Tai, S.; Camara, J.E.; Merkel, J.; Green, E.; Sempos, C.T.; Brooks, S.P.J. Standardizing 25-hydroxyvitamin D values from the Canadian Health Measures Survey. Am. J. Clin. Nutr. 2015, 102, 1044–1050. [Google Scholar] [CrossRef] [Green Version]
- Mogire, R.M.; Mutua, A.; Kimita, W.; Kamau, A.; Bejon, P.; Pettifor, J.; Adeyemo, A.; Williams, T.N.; Atkinson, S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e134–e142. [Google Scholar] [CrossRef] [Green Version]
- Bassil, D.; Rahme, M.; Hoteit, M.; Fuleihan, G.E.-H. Hypovitaminosis D in the Middle East and North Africa: Prevalence, risk factors and impact on outcomes. Dermato-Endocrinol. 2013, 5, 274–298. [Google Scholar] [CrossRef] [Green Version]
- Al-Mahroos, F.T.; Al-Sahlawi, H.S.; Al-Amer, E.; Mahmood, N.A.; Sandhu, A.K.; Sharida, H.; Nagalla, D.S.; Jaradat, A.A.; Jibrel, S.O.; Bin Jamal, S.A.S.; et al. Prevalence and Risk Factors for Vitamin D Deficiency among Mothers in Labor and their Newborns. Bahrain Med. Bull. 2013, 35, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Khuri-Bulos, N.; Lang, R.D.; Blevins, M.; Kudyba, K.; Lawrence, L.; Davidson, M.; Faouri, S.; Halasa, N.B. Vitamin D Deficiency among Newborns in Amman, Jordan. Glob. J. Health Sci. 2013, 6, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Fouda, M.A.; Turkestani, I.Z.; Almusharraf, S.; Al-Ajlan, A.; Angkaya-Bagayawa, F.F.; Sabico, S.; Mohammed, A.G.; Hassanato, R.; Al-Serehi, A.; Alshingetti, N.M.; et al. Extremely High Prevalence of Maternal and Neonatal Vitamin D Deficiency in the Arab Population. Neonatology 2017, 112, 225–230. [Google Scholar] [CrossRef]
- Mohamed, W.A.W.; Al-Shehri, M.A. Cord Blood 25-Hydroxyvitamin D Levels and the Risk of Acute Lower Respiratory Tract Infection in Early Childhood. J. Trop. Pediatr. 2013, 59, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Farhat, K.H.; Arafa, M.A.; Rabah, D.M.; Amin, H.S.; Ibrahim, N.K. Vitamin D status and its correlates in Saudi male population. BMC Public Health 2019, 19, 211. [Google Scholar] [CrossRef] [Green Version]
- Nichols, E.K.; Khatib, I.M.D.; Aburto, N.J.; Serdula, M.K.; Scanlon, K.S.; Wirth, J.P.; Sullivan, K.M. Vitamin D status and associated factors of deficiency among Jordanian children of preschool age. Eur. J. Clin. Nutr. 2015, 69, 90–95. [Google Scholar] [CrossRef]
- Graham, L. IOM Releases Report on Dietary Reference Intakes for Calcium and Vitamin D. Available online: http://www.iom.edu/Reports/2010/DietaryReference-Intakes-for-Calcium-and-Vitamin-D.aspx (accessed on 1 January 2022).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Atapattu, N.; Shaw, N.; Högler, W. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of vitamin D deficiency in children. Pediatr. Res. 2013, 74, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, S.; Noor, S.S.; Barakzai, Q. Serum alkaline phosphatase screening for vitamin D deficiency states. J. Coll. Physicians Surg. Pak. 2012, 22, 424–427. [Google Scholar]
- Abdul-Razzak, K.K.; Ajlony, M.-J.A.; Khoursheed, A.M.; Obeidat, B.A. Vitamin D deficiency among healthy infants and toddlers: A prospective study from Irbid, Jordan. Pediatr. Int. 2011, 53, 839–845. [Google Scholar] [CrossRef]
- Chaudhry, A.B.; Hajat, S.; Rizkallah, N.; Abu-Rub, A. Risk factors for vitamin A and D deficiencies among children under-five in the state of Palestine. Confl. Health 2018, 12, 13. [Google Scholar] [CrossRef]
- Jayashri, R.; Venkatesan, U.; Shanthirani, C.S.; Deepa, M.; Anjana, R.M.; Mohan, V.; Pradeepa, R. Prevalence of vitamin D deficiency in urban south Indians with different grades of glucose tolerance. Br. J. Nutr. 2020, 124, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Khor, G.L.; Chee, W.S.S.; Shariff, Z.M.; Poh, B.K.; Arumugam, M.; Ab Rahman, J.; Theobald, H.E. High prevalence of vitamin D insufficiency and its association with BMI-for-age among primary school children in Kuala Lumpur, Malaysia. BMC Public Health 2011, 11, 95. [Google Scholar] [CrossRef] [Green Version]
- Griffin, T.P.; Wall, D.; Blake, L.; Griffin, D.G.; Robinson, S.; Bell, M.; Mulkerrin, E.C.; O’Shea, P.M. Higher risk of vitamin D insufficiency/deficiency for rural than urban dwellers. J. Steroid Biochem. Mol. Biol. 2019, 197, 105547. [Google Scholar] [CrossRef]
- Surve, S.; Chauhan, S.; Amdekar, Y.; Joshi, B. Vitamin D deficiency in Children: An update on its Prevalence, Therapeutics and Knowledge gaps. Indian J. Nutr. 2017, 4, 167. [Google Scholar]
- Chacham, S.; Rajput, S.; Gurnurkar, S.; Mirza, A.; Saxena, V.; Dakshinamurthy, S.; Chaturvedi, J.; Goyal, J.P.; Chegondi, M. Prevalence of Vitamin D Deficiency Among Infants in Northern India: A Hospital Based Prospective Study. Cureus 2020, 12, e11353. [Google Scholar] [CrossRef]
- He, H.; Zeng, Y.; Wang, X.; Yang, L.; Zhang, M.; An, Z. Meteorological Condition and Air Pollution Exposure Associated with Vitamin D Deficiency: A Cross-Sectional Population-Based Study in China. Risk Manag. Healthc. Policy 2020, 13, 2317–2324. [Google Scholar] [CrossRef]
- Lionel, B.P.; Gnanaraj, R.; Paranjape, M.; Moses, P.; John, J.; Geethanjali, F.; Rose, W. Vitamin-D deficiency and its association with breast feeding among children at 1 year of age in an urban community in South India. J. Fam. Med. Prim. Care 2020, 9, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Dhanalakshmi, K.; Amperayani, S. Vitamin D deficiency in childhood—A review of current guidelines on diagnosis and management. Indian Pediatr. 2013, 50, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Ncayiyana, J.; Martinez, L.; Goddard, E.; Myer, L.; Zar, H. Prevalence and Correlates of Vitamin D Deficiency among Young South African Infants: A Birth Cohort Study. Nutrients 2021, 13, 1500. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, M.K.; Jeong, S.J. Vitamin D deficiency in infants aged 1 to 6 months. Korean J. Pediatr. 2013, 56, 205–210. [Google Scholar] [CrossRef]
- Shati, A.A.; Khalil, S.N.; Asiri, K.A.; Alshehri, A.A.; Deajim, Y.A.; Al-Amer, M.S.; Alshehri, H.J.; Alqahtani, F.S. Occurrence of Diarrhea and Feeding Practices among Children below Two Years of Age in Southwestern Saudi Arabia. Int. J. Environ. Res. Public Health 2020, 17, 722. [Google Scholar] [CrossRef] [Green Version]
| Background Characteristics | n% | Mean & SD ng/mL | p-Value |
|---|---|---|---|
| Gender | |||
| Male | 295 61.0% | 24.64 | 0.002 |
| Female | 189 39.0% | 27.26 | |
| Age group | |||
| 2–6 months | 160 33.1% | 24.3 | 0.001 |
| 7–12 months | 144 29.8% | 24.7 | |
| >12 months | 180 37.1% | 27.7 | |
| Residence | |||
| Rural | 274 56.6% | 26.57 | 0.012 |
| Urban | 210 43.4% | 24.48 | |
| Housing status | |||
| Villa | 136 28.1% | 26.62 | 0.149 |
| Apartment | 384 71.9% | 25.29 | |
| Birth weight | |||
| <3 kg | 118 24.4% | 24.45 | 0.097 |
| ≥3 kg | 366 75.6% | 26.05 | |
| Feeding history | |||
| Breast milk only | 78 16.1% | 20.92 | <0.001 |
| Breast milk & formula milk | 194 40.1% | 25.54 | |
| Formula milk only | 221 43.8% | 27.52 | |
| Weekly exposure to sunlight | |||
| <3 times/week | 454 93.8% | 25.22 | <0.001 |
| ≥3 times/week | 30 6.2% | 32.43 | |
| Family history of Rickets | |||
| Yes | 98 20.2% | 25.52 | 0.858 |
| No | 386 79.8% | 25.70 | |
| Mother uses vitamin D supplements | |||
| Yes | 62 12.8% | 26.56 | 0.566 |
| No | 422 87.2% | 25.60 |
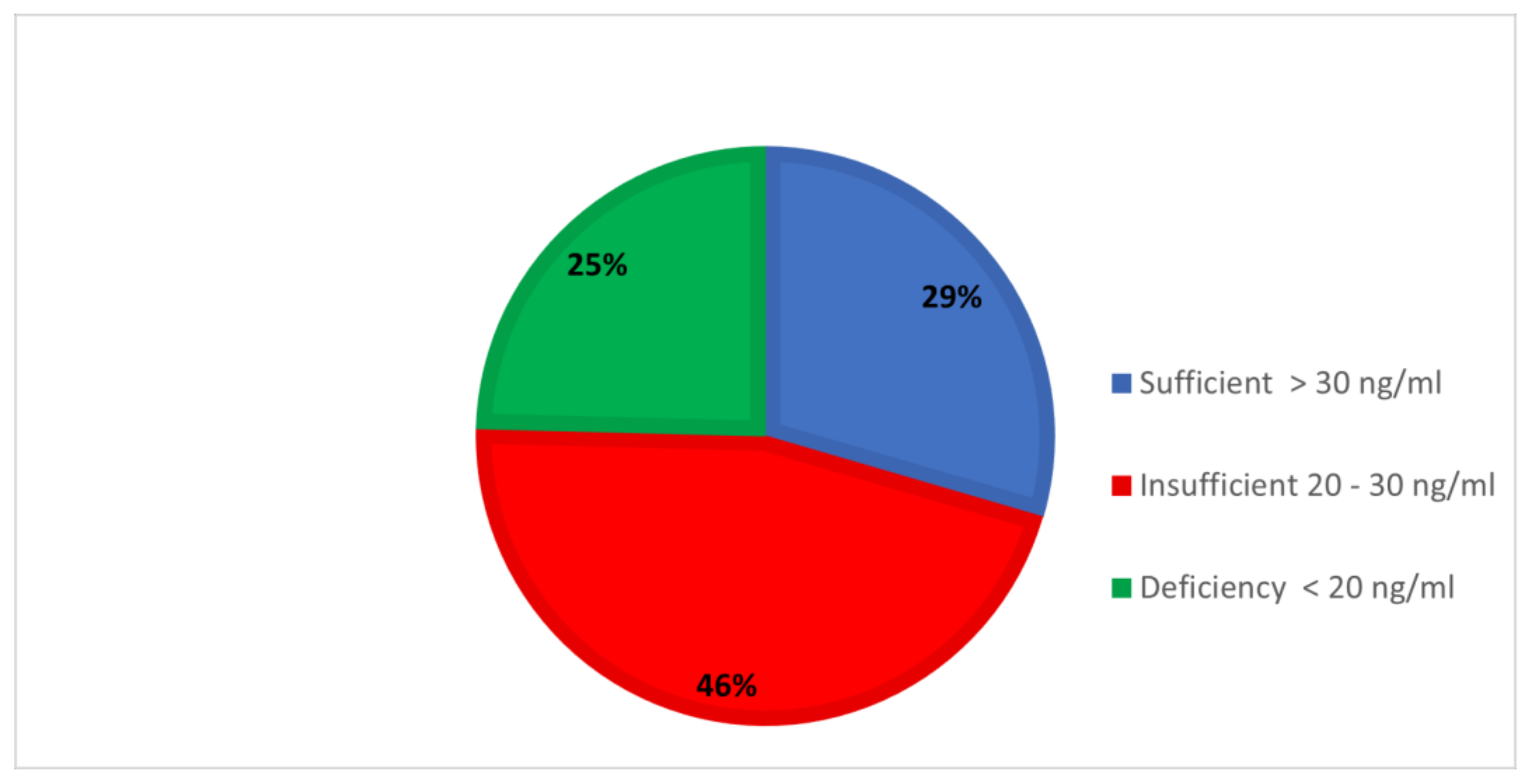
| Biochemical Markers | Reference | Overall n = 484 | Sufficient n = 143 | Insufficiency n = 222 | Deficiency n= 119 | F Value & df | p Value |
|---|---|---|---|---|---|---|---|
| Vitamin D (ng/mL) | 20–50 | 25.66 ± 9.11 | 35.89 ± 4.59 | 25.63 ± 3.0 | 13.43 ± 4.46 | 1071.7 (2) | <0.001 |
| Calcium (mg/dL) | 8.80–10.80 | 8.82 ± 0.58 | 9.05 ± 0.48 | 8.85 ± 0.47 | 8.50 ± 0.71 | 33.17 (2) | <0.001 |
| ALP (U/L) | 100–550 | 262 ± 90.36 | 240.45 ± 87.81 | 260.48 ± 79.66 | 291.16 ± 104.1 | 10.7 (2) | <0.001 |
| Phosphorous (mg/dL) | 4.0–7.0 | 5.06 ± 0.661 | 4.99 ± 0.64 | 5.07 ± 0.59 | 5.14 ± 0.77 | 1.68 (2) | NS (0.187) |
| Variables | Status of Vitamin D | |||
|---|---|---|---|---|
| Deficiency n% | Insufficiency n% | Sufficiency n% | p Value | |
| Gender | ||||
| Male | 83 (28.1) | 135 (45.8) | 77 (26.1) | 0.032 |
| Female | 36 (19.0) | 87 (46.0) | 66 (34.9) | |
| Age | ||||
| 2–12 months (infant) | 85 (28.0) | 148 (48.7) | 71 (23.4) | <0.001 |
| More than 12 months | 34 (18.9) | 74 (41.1) | 72 (40.0) | |
| Residence | ||||
| Rural | 66 (24.1) | 108 (39.4) | 100 (36.5) | <0.001 |
| Urban | 53 (25.2) | 114 (54.3) | 43 (20.5) | |
| Housing status | ||||
| Villa | 21 (15.4) | 79 (58.1) | 36 (20.5) | 0.001 |
| Apartment | 98 (28.2) | 143 (41.1) | 107 (30.7) | |
| Birth weight | ||||
| <3 kg | 39 (33.1) | 43 (36.4) | 36 (30.5) | 0.02 |
| ≥3 kg | 80 (21.9) | 179 (48.9) | 107 (29.2) | |
| Feeding history | ||||
| Breast milk only | 32 (41.0) | 35 (44.9) | 11 (14.1) | <0.001 |
| Breast milk & formula milk | 52 (26.8) | 80 (41.2) | 62 (32.0) | |
| Formula milk only | 35 (16.5) | 107 (50.5) | 70 (33.0) | |
| Exposure to sunlight | ||||
| <3 times/week | 118 (26.0) | 211 (46.5) | 125 (27.5) | <0.001 |
| ≥3 times/week | 01 (3.3) | 11 (36.7) | 18 (60.0) | |
| Family history of Rickets | ||||
| Yes | 22 (22.4) | 51 (52.0) | 25 (25.5) | 0.382 |
| No | 97 (25.1) | 171 (44.3) | 118 (30.6) | |
| Mother uses vitamin D supplements | ||||
| Yes | 12 (37.5) | 14 (43.8) | 06 (18.8) | 0.537 |
| No | 113 (25.0) | 208 (46.0) | 131 (29.0) | |
| Variables | β | aOR * | p-Value | 95% Confidence Interval (CI) |
|---|---|---|---|---|
| Age | ||||
| >12 months | Reference | |||
| 2–12 months | 0.540 | 1.71 | 0.017 | 1.10–2.67 |
| Residence | ||||
| Rural | Reference | |||
| Urban | 1.101 | 3.0 | <0.001 | 1.78–5.08 |
| Exposure to sunlight | ||||
| ≥3 days/week | Reference | |||
| <3 days/week | 1.551 | 4.71 | <0.001 | 2.04–10.88 |
| Feeding | ||||
| Formula feeding | Reference | |||
| Breast milk & formula feeding | 1.09 | 0.703 | 0.684–1.75 | |
| Only breast milk | 0.887 | 2.42 | 0.024 | 1.12–5.23 |
| Constant | 1.554 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qahtani, S.M.; Shati, A.A.; Alqahtani, Y.A.; Dawood, S.A.; Siddiqui, A.F.; Zaki, M.S.A.; Khalil, S.N. Prevalence and Correlates of Vitamin D Deficiency in Children Aged Less than Two Years: A Cross-Sectional Study from Aseer Region, Southwestern Saudi Arabia. Healthcare 2022, 10, 1064. https://doi.org/10.3390/healthcare10061064
Al-Qahtani SM, Shati AA, Alqahtani YA, Dawood SA, Siddiqui AF, Zaki MSA, Khalil SN. Prevalence and Correlates of Vitamin D Deficiency in Children Aged Less than Two Years: A Cross-Sectional Study from Aseer Region, Southwestern Saudi Arabia. Healthcare. 2022; 10(6):1064. https://doi.org/10.3390/healthcare10061064
Chicago/Turabian StyleAl-Qahtani, Saleh M., Ayed A. Shati, Youssef A. Alqahtani, Samy A. Dawood, Aesha F. Siddiqui, Mohamed Samir A. Zaki, and Shamsun N. Khalil. 2022. "Prevalence and Correlates of Vitamin D Deficiency in Children Aged Less than Two Years: A Cross-Sectional Study from Aseer Region, Southwestern Saudi Arabia" Healthcare 10, no. 6: 1064. https://doi.org/10.3390/healthcare10061064
APA StyleAl-Qahtani, S. M., Shati, A. A., Alqahtani, Y. A., Dawood, S. A., Siddiqui, A. F., Zaki, M. S. A., & Khalil, S. N. (2022). Prevalence and Correlates of Vitamin D Deficiency in Children Aged Less than Two Years: A Cross-Sectional Study from Aseer Region, Southwestern Saudi Arabia. Healthcare, 10(6), 1064. https://doi.org/10.3390/healthcare10061064






