Implementing a New Electronic Health Record System in a University Hospital: The Effect on Reported Medication Errors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Data Collection and Analysis
2.3. Study Ethics
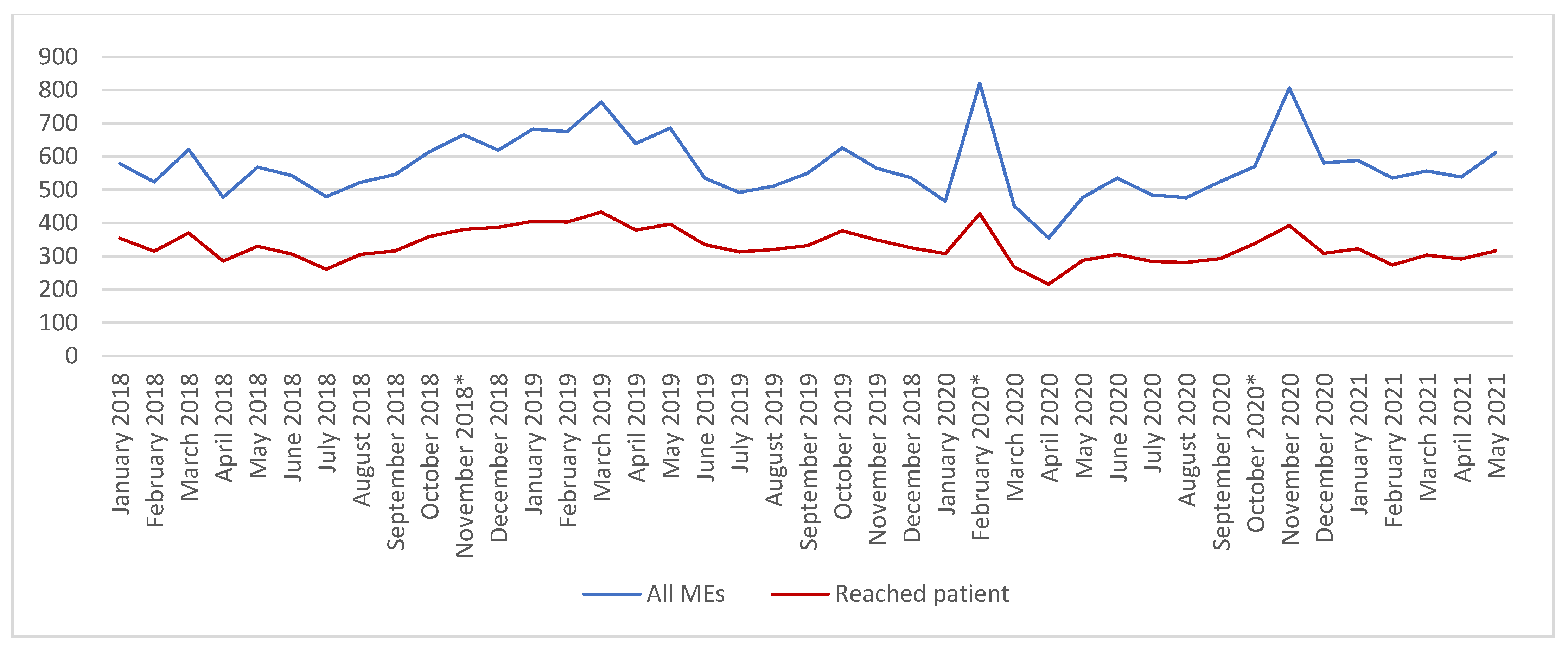
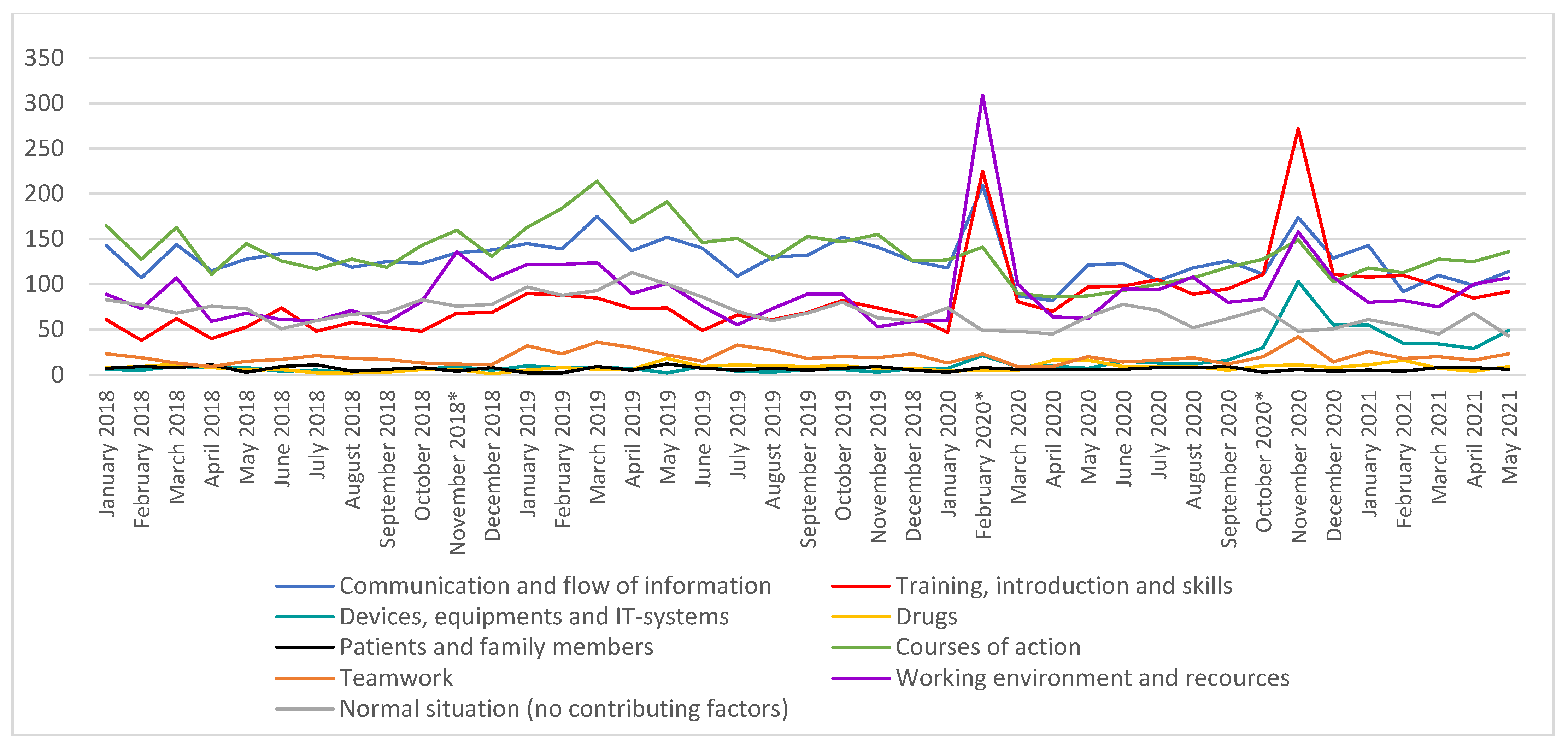
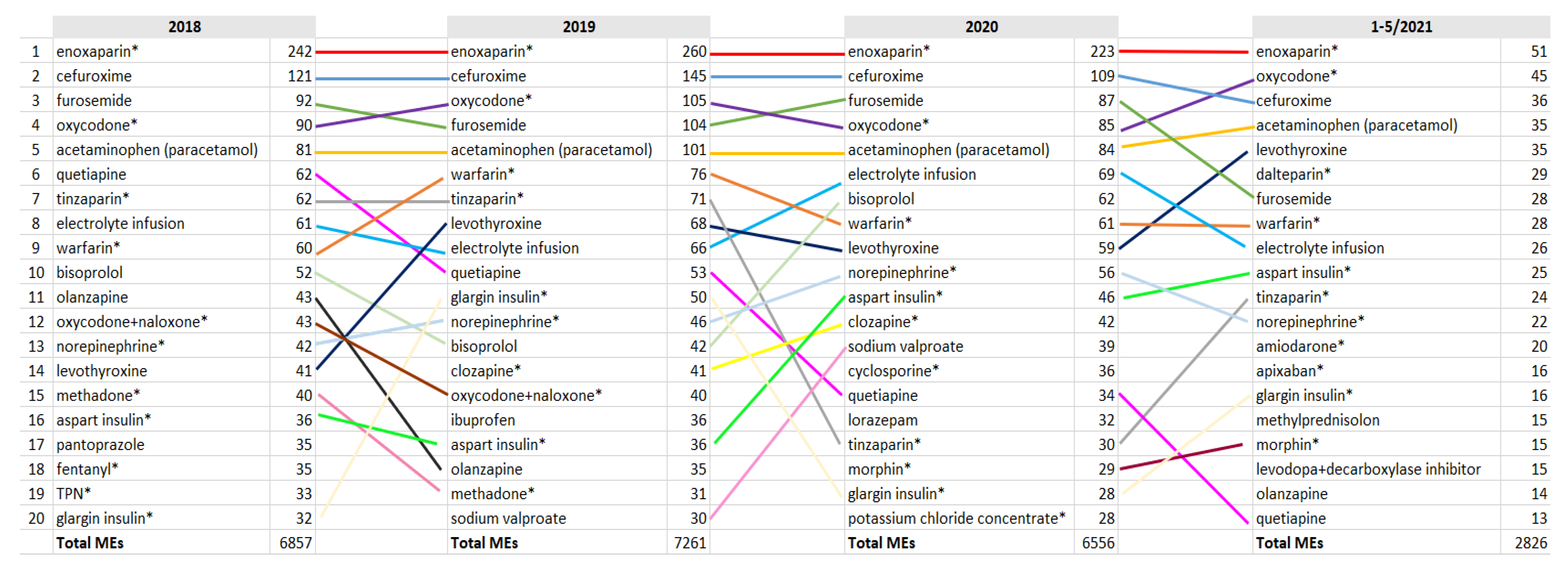
3. Results
3.1. TOP Medications Related to MEs
3.2. Severe MEs
4. Discussion
4.1. Changes in the ME Report Profile
4.2. Creating a Structured Home Medication List Is Challenging
4.3. Structured Ordering Process Still Needs Special Attention
4.4. Implementing Barcode Scanning Is Beneficial
4.5. Other Lessons Learned in Implementing a New EHR System from a Medication Safety Perspective
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohn, L.T.; Corrigan, J.M.; Donaldson, M.S. To err is Human—Building a Safer Health System; Institute of Medicine, National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- The World Health Organization (WHO). Medication Without Harm—Global Patient Safety Challenge on Medication Safety. Geneva, 2017. License: CC BY-NC-SA 3.0 IGO. Available online: http://apps.who.int/iris/bitstream/10665/255263/1/WHO-HIS-SDS2017.6-eng.pdf?ua=1&ua=1 (accessed on 15 March 2022).
- National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP): What Is a Medication Error? Available online: https://www.nccmerp.org/about-medication-errors (accessed on 27 March 2022).
- Rauhala, A.; Kinnunen, M.; Kuosmanen, A.; Liukka, M.; Olin, K.; Sahlström, M.; Roine, R.P. What does voluntary reporting system of patient safety incidents tell? (english summary). Lääkärilehti 2018, 46, 2716–2720. [Google Scholar]
- Mulac, A.; Taxis, K.; Hagesaether, E.; Granas, A.G. Severe and fatal medication errors in hospitals: Findings from the Norwegian Incident Reporting System. Eur. J. Hosp. Pharm. 2020, 28, e56–e61. [Google Scholar] [CrossRef] [PubMed]
- Linden-Lahti, C.; Takala, A.; Holmström, A.-R.; Airaksinen, M. What Severe Medication Errors Reported to Health Care Supervisory Authority Tell About Medication Safety? J. Patient Saf. 2021, 17, e1179–e1185. [Google Scholar] [CrossRef] [PubMed]
- Institute for Safe Medication Practices. ISMP’s List of High-alert Medications in Acute Care Settings. 2018. Available online: www.ismp.org/tools/institutionalhighAlert.asp (accessed on 15 March 2022).
- Schepel, L.; Lehtonen, L.; Airaksinen, M.; Lapatto-Reiniluoto, O. How to Identify Organizational High-Alert Medications. J. Patient Saf. 2018, 17, e1358–e1363. [Google Scholar] [CrossRef]
- Zheng, W.Y.; Lichtner, V.; Van Dort, B.A.; Baysari, M.T. The impact of introducing automated dispensing cabinets, barcode medication administration, and closed-loop electronic medication management systems on work processes and safety of controlled medications in hospitals: A systematic review. Res. Soc. Adm. Pharm. 2020, 17, 832–841. [Google Scholar] [CrossRef]
- Austin, J.A.; Smith, I.R.; Tariq, A. The impact of closed-loop electronic medication management on time to first dose: A comparative study between paper and digital hospital environments. Int. J. Pharm. Pr. 2018, 26, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.D.; O’Grady, K.; Donyai, P.; Jacklin, A.; Barber, N. The impact of a closed-loop electronic prescribing and administration system on prescribing errors, administration errors and staff time: A before-and-after study. Qual. Saf. Health Care 2007, 16, 279–284. [Google Scholar] [CrossRef]
- Pearce, R.; Whyte, I. Electronic medication management: Is it a silver bullet? Aust. Prescr. 2018, 41, 32–33. [Google Scholar] [CrossRef] [Green Version]
- Whalen, K.; Lynch, E.; Moawad, I.; John, T.; Lozowski, D.; Cummings, B. Transition to a new electronic health record and pediatric medication safety: Lessons learned in pediatrics within a large academic health system. J. Am. Med Inform. Assoc. 2018, 25, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Goudra, B.; Singh, P.; Borle, A.; Gouda, G. Effect of introduction of a new electronic anesthesia record (Epic) system on the safety and efficiency of patient care in a gastrointestinal endoscopy suite-comparison with historical cohort. Saudi J. Anaesth. 2016, 10, 127–131. [Google Scholar] [CrossRef]
- Hensley, N.B.; Koch, C.G.; Pronovost, P.J.; Mershon, B.H.; Boyd, J.; Franklin, S.; Moore, D.; Sheridan, K.; Steele, A.; Stierer, T.L. Wrong-Patient Blood Transfusion Error: Leveraging Technology to Overcome Human Error in Intraoperative Blood Component Administration. Jt. Comm. J. Qual. Patient Saf. 2018, 45, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.; Bondarenka, C.; Edwards, K.; Hartwell, R.; Letton, C.; Perez, A. Evaluation of electronic health record implementation on pharmacist interventions related to oral chemotherapy management. J. Oncol. Pharm. Pr. 2016, 23, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Awanic Oy 2016: Reporting System for Safety Incidents in Health Care Organizations. Available online: https://awanic.fi/haipro/eng/ (accessed on 15 March 2022).
- World Health Organization Collaborating Centre for Drug Statistics Methodology (WHOCC). Anatomical Therapeutic Chemical Classification System. Last Updated 2018. Available online: http://www.whocc.no/atc/structure_and_principles/ (accessed on 15 March 2022).
- Hegarty, J.; Flaherty, S.J.; Saab, M.M.; Goodwin, J.; Walshe, N.; Wills, T.; McCarthy, V.J.; Murphy, S.; Cutliffe, A.; Meehan, E.; et al. An International Perspective on Definitions and Terminology Used to Describe Serious Reportable Patient Safety Incidents: A Systematic Review. J. Patient Saf. 2020, 17, e1247–e1254. [Google Scholar] [CrossRef]
- Kanta 2021: What Are Kanta Services? Available online: https://www.kanta.fi/en/professionals/what-are-kanta-services (accessed on 15 March 2022).
- Hsieh, H.-F.; Shannon, S.E. Three Approaches to Qualitative Content Analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Berry, J.; Smith, B.; Esterman, A.; Selim, P.; O’Shaughnessy, J.; DeWit, M. Attitudes and barriers to incident reporting: A collaborative hospital study. Qual. Saf. Health Care 2006, 15, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Sarvadikar, A.; Prescott, G.; Williams, D. Attitudes to reporting medication error among differing healthcare professionals. Eur. J. Clin. Pharmacol. 2010, 66, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Schepel, L.; Lehtonen, L.; Airaksinen, M.; Ojala, R.; Ahonen, J.; Lapatto-Reiniluoto, O. Medication reconciliation and review for older emergency patients requires improvement in Finland. Int. J. Risk Saf. Med. 2018, 30, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Samaranayake, N.; Cheung, S.; Chui, W.; Cheung, B. Technology-related medication errors in a tertiary hospital: A 5-year analysis of reported medication incidents. Int. J. Med Inform. 2012, 81, 828–833. [Google Scholar] [CrossRef]
- Lichtner, V.; Baysari, M.; Gates, P.; Dalla--Pozza, L.; Westbrook, J.I. Medication safety incidents in paediatric oncology after electronic medication management system implementation. Eur. J. Cancer Care 2019, 28, e13152. [Google Scholar] [CrossRef]
- Kinlay, M.; Ho, L.M.R.; Zheng, W.Y.; Burke, R.; Juraskova, I.; Moles, R.; Baysari, M. Electronic Medication Management Systems: Analysis of Enhancements to Reduce Errors and Improve Workflow. Appl. Clin. Inform. 2021, 12, 1049–1060. [Google Scholar] [CrossRef]
- De Winter, S.; Spriet, I.; Indevuyst, C.; Vanbrabant, P.; Desruelles, D.; Sabbe, M.; Gillet, J.B.; Wilmer, A.; Willems, L. Pharmacist- versus physician-acquired medication history: A prospective study at the emergency department. BMJ Qual. Saf. 2010, 19, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.K.; Sponsler, K.C.; Kripalani, S.; Schnipper, J.L. Hospital-based Medication Reconciliation Practices: A Systematic Review. Arch. Intern. Med. 2012, 172, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.; Baysari, M.T.; Lake, R.; Richardson, L.; McCullagh, C.; Westbrook, J.I. User Perceptions of the Implementation of an Electronic Medication Management System in a Paediatric Setting. Stud Health Technol Inf. 2017, 239, 41–47. [Google Scholar]
- Vaghasiya, M.R.; Penm, J.; Kuan, K.K.Y.; Gunja, N.; Liu, Y.; Kim, E.D.; Petrina, N.; Poon, S. Implementation of an Electronic Medication Management System in a large tertiary hospital: A case of qualitative inquiry. BMC Med Inform. Decis. Mak. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- van der Sijs, H.; Aarts, J.; Vulto, A.; Berg, M. Overriding of drug safety alerts in computerized physician order entry. J. Am. Med. Inform. Assoc. 2006, 13, 138–147. [Google Scholar] [CrossRef]
- Cerqueira, O.; Gill, M.; Swar, B.; Prentice, K.A.; Gwin, S.; Beasley, B.W. The effectiveness of interruptive prescribing alerts in ambulatory CPOE to change prescriber behaviour & improve safety. BMJ Qual. Saf. 2021, 30, 1038–1046. [Google Scholar] [CrossRef]
- Hassink, J.J.M.; Jansen, M.M.P.M.; Helmons, P.J. Effects of bar code-assisted medication administration (BCMA) on frequency, type and severity of medication administration errors: A review of the literature. Eur. J. Hosp. Pharm. 2012, 19, 489–494. [Google Scholar] [CrossRef]
- What is the Minimum Viable Product? Venture Hacks. 2009. Available online: http://venturehacks.com/articles/minimum-viable-product (accessed on 15 March 2022).
- Chan, J.; Shojania, K.G.; Easty, A.C.; E Etchells, E. Usability evaluation of order sets in a computerised provider order entry system. BMJ Qual. Saf. 2011, 20, 932–940. [Google Scholar] [CrossRef]
- Meehan, R.A.; Mon, D.T.; Kelly, K.M.; Rocca, M.; Dickinson, G.; Ritter, J.; Johnson, C.M. Increasing EHR system usability through standards: Conformance criteria in the HL7 EHR-system functional model. J. Biomed. Inform. 2016, 63, 169–173. [Google Scholar] [CrossRef]
- Landex, N. The Epic healthcare system in Denmark. Ugeskr Laeger 2017, 179, V69572. [Google Scholar]

| Medication Process before APOTTI | Medication Process with APOTTI |
|---|---|
| Multiple EHRs in the hospital and medications are ordered in multiple systems. | There is one EHR and ordering system in the entire hospital. |
| Medication reconciliation in the EHR system is based only on hospital policy and documented in free text. Pharmacists are not widely involved in the process. | Medication reconciliation and a structured home-medication list are mandatory for in-patient medication. A home-medication list is integrated into the Kanta system, which holds electronic prescriptions [20]. Pharmacists are involved in medication reconciliation in many units. |
| Prescribing with free text orders and prescriptions in variable places in EHR systems. | Prescribing with structured order and prescription forms in specific medication applications in one EHR system. |
| Prescribing and ordering with the brand name. | Prescribing and ordering with the generic name. |
| Clinical decision support system (CDSS) for interactions and allergy warnings | More sophisticated CDSS, e.g., with dose warnings (including dosing with older patients and renal impairment), duplicate medications, and electronic best practice advice (BPA) |
| Primarily nurses transcribe orders to patients’ medication list. Verbal orders are common. | Primarily physicians document orders directly to patients’ medication list. Verbal orders are allowed only in limited situations. |
| Orders are not verified. | Pharmacists verify orders in some units. |
| Automated dispensing cabinets not integrated into the EHR system | Automated dispensing cabinets integrated with APOTTI enable the dispensing of medicines according to electronic orders. |
| Dispensing and preparing the medicines in units for the next shift or day (24 hours), some of the units use paper-based medication lists. | Dispensing and preparing the medicines in a timely manner (max. 2 hours before administration) by using the EHR system’s medication application and barcode scanning. |
| Medicines dispensed in the unit are double-checked by another nurse (manual double-check process). | Dispensing the right medicine is assured by scanning the barcodes of the medicine packages (no unit doses). A manual double-check process is used only when the barcode is not available or in use and for high alert medications (in addition to scanning). |
| Medicines prepared (e.g., dissolved and diluted) are double-checked in a few units, paper-based instructions for preparing medicines. | Preparing is documented by scanning the barcodes of the medicines, and EHR’s medication application gives the instructions for preparing. The manual double-check process is used only when the barcode is not available or in use and for the high alert medications (in addition to scanning). |
| The right patient and right medicine are assured manually when administering the medicine. | When administering the medicine, the right patient and medicines are assured by using the barcodes. |
| Medication administration is recorded with delay and only some of the medicines are recorded (e.g., high alert medications). | Medication administration is recorded in a timely manner at the bedside, and all medicines are recorded. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindén-Lahti, C.; Kivivuori, S.-M.; Lehtonen, L.; Schepel, L. Implementing a New Electronic Health Record System in a University Hospital: The Effect on Reported Medication Errors. Healthcare 2022, 10, 1020. https://doi.org/10.3390/healthcare10061020
Lindén-Lahti C, Kivivuori S-M, Lehtonen L, Schepel L. Implementing a New Electronic Health Record System in a University Hospital: The Effect on Reported Medication Errors. Healthcare. 2022; 10(6):1020. https://doi.org/10.3390/healthcare10061020
Chicago/Turabian StyleLindén-Lahti, Carita, Sanna-Maria Kivivuori, Lasse Lehtonen, and Lotta Schepel. 2022. "Implementing a New Electronic Health Record System in a University Hospital: The Effect on Reported Medication Errors" Healthcare 10, no. 6: 1020. https://doi.org/10.3390/healthcare10061020
APA StyleLindén-Lahti, C., Kivivuori, S.-M., Lehtonen, L., & Schepel, L. (2022). Implementing a New Electronic Health Record System in a University Hospital: The Effect on Reported Medication Errors. Healthcare, 10(6), 1020. https://doi.org/10.3390/healthcare10061020





