Osgood-Schlatter Disease: Appearance, Diagnosis and Treatment: A Narrative Review
Abstract
1. Introduction
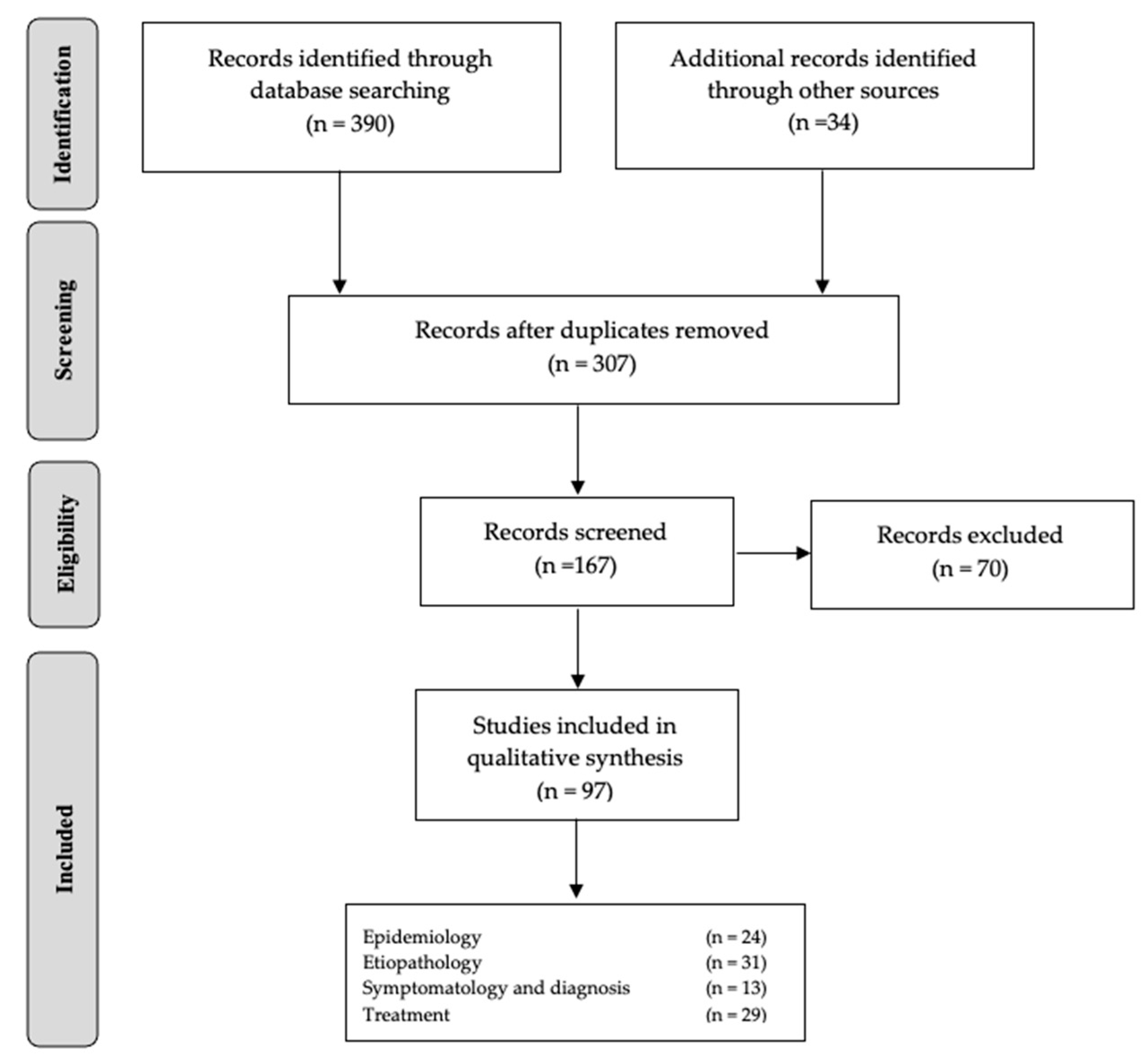
2. Material and Methods
2.1. Information Sources
2.2. Study Inclusion Criteria
2.3. Study Exclusion Criteria
3. Results
4. Epidemiology
4.1. Gender
4.2. Practice Level
4.3. Sports
4.4. Bilaterality of the Disease
5. Etiopathology
5.1. Mechanical Factors
5.2. Functional Factors
5.3. Morphological Factors
5.4. Environmental Factors
5.5. Psychological Factors
6. Symptomatology and Diagnosis
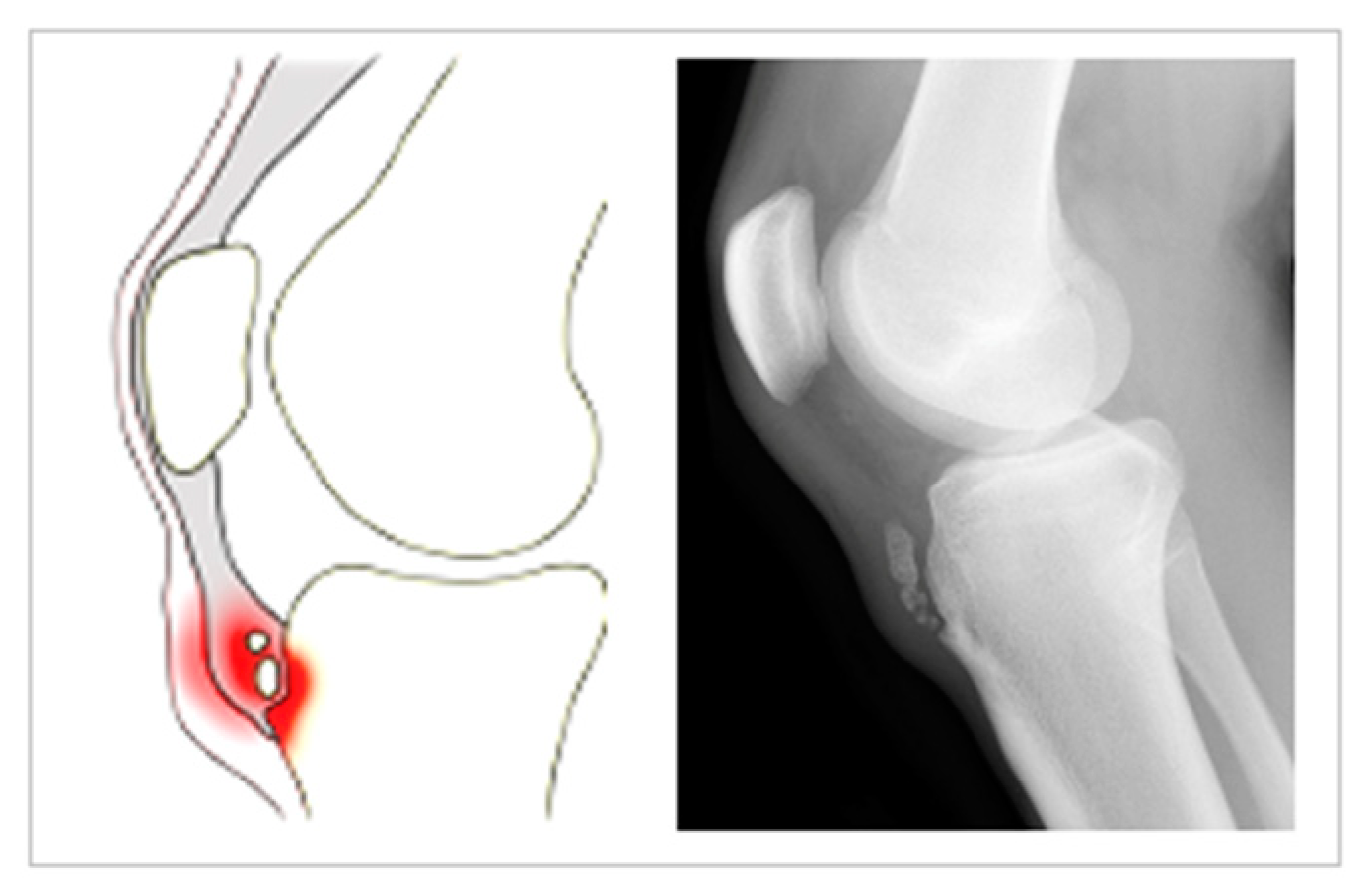
6.1. Conventional Radiology
6.2. Ultrasound
6.3. Nuclear Magnetic Resonance (NMR)
7. Treatment
8. Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaishya, R.; Azizi, A.T.; Agarwal, A.K.; Vijay, V. Apophysitis of the Tibial Tuberosity (Osgood-Schlatter Disease): A Review. Cureus 2016, 13, e780. [Google Scholar] [CrossRef] [PubMed]
- Osgood, R.B. Lesions of the tibial tubercle occurring during adolescence. Clin. Orthop. Relat. Res. 1993, 286, 4–9. [Google Scholar] [CrossRef]
- Dwek, J.R.; Chung, C.B. The patellar extensor apparatus of the knee. Pediatr. Radiol. 2008, 38, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, G.; Tunay, V.B. Rehabilitation of avulsion fracture of the tibial tuberosity following Osgood-Schlatter disease. Knee Surg. Sports Traumatol. Arthrosc. 2004, 12, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Holden, S.; Olesen, J.L.; Winiarski, L.M.; Krommes, K.; Thorborg, K.; Hölmich, P.; Rathleff, M.S. Is the Prognosis of Osgood-Schlatter Poorer Than Anticipated? A Prospective Cohort Study With 24-Month Follow-up. Orthop. J. Sports Med. 2021, 19, 23259671211022239. [Google Scholar] [CrossRef]
- Bezuglov, E.N.; Tikhonova, A.A.; Chubarovskiy, P.V.; Repetyuk, D.; Khaitin, V.Y.; Lazarev, A.M.; Usmanova, E.M. Conservative treatment of Osgood-Schlatter disease among young professional soccer players. Int. Orthop. 2020, 44, 1737–1743. [Google Scholar] [CrossRef]
- Zonfrillo, M.R.; Spicer, R.S.; Lawrence, B.A.; Miller, T.R. Incidence and costs of injuries to children and adults in the United States. Inj. Epidemiol. 2018, 5, 4–9. [Google Scholar] [CrossRef]
- De Lucena, G.L.; Gomes, C.D.S.; Guerra, R.O. Prevalence and Associated Factors of Osgood-Schlatter Syndrome in a Population-Based Sample of Brazilian Adolescents. Am. J. Sports Med. 2011, 39, 415–420. [Google Scholar] [CrossRef]
- Kujala, U.; Kvist, M.; Heinonen, O. Osgood-Schlatter’s disease in athletes retrospective study of incidence and duration. Am. J. Sports Med. 1985, 13, 236–241. [Google Scholar] [CrossRef]
- Pascarella, F.; Ziranu, A.; Maccauro, G. Tibial Tubercle Fracture in a 14-Year-Old Athlete with Bilateral Lower Pole Bipartite Patella and Osgood-Schlatter Disease. Case Rep. Orthop. 2015, 2015, 815061. [Google Scholar] [CrossRef][Green Version]
- Balmat, P.; Vichard, P.; Pem, R. The Treatment of Avulsion Fractures of the Tibial Tuberosity in Adolescent Athletes. Sports Med. 1990, 9, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data: The PRISMA-IPD statement. JAMA 2015, 28, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Nkaoui, M.; El Alouani, E.M. Osgood-schlatter disease: Risk of a disease deemed banal. Pan Afr. Med. J. 2017, 28, 56. [Google Scholar] [CrossRef]
- Kellersmann, R.; Blattert, T.R.; Weckbach, A. Bilateral patellar tendon rupture without predisposing systemic disease or steroid use: A case report and review of the literature. Arch. Orthop. Trauma. Surg. 2005, 125, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka, M.; Hiramoto, I.; Minagawa, H.; Matsuzaki, M.; Kodama, H. Screening of the Maturity Status of the Tibial Tuberosity by Ultrasonography in Higher Elementary School Grade Schoolchildren. Int. J. Environ. Res. Public Health 2019, 16, 2138. [Google Scholar] [CrossRef] [PubMed]
- Kaneuchi, Y.; Otoshi, K.; Hakozaki, M.; Sekiguchi, M.; Watanabe, K.; Igari, T.; Konno, S. Bony Maturity of the Tibial Tuberosity with Regard to Age and Sex and Its Relationship to Pathogenesis of Osgood-Schlatter Disease: An Ultrasonographic Study. Orthop. J. Sports Med. 2018, 6, 2325967117749184. [Google Scholar] [CrossRef] [PubMed]
- Gholve, P.A.; Scher, D.; Khakharia, S.; Widmann, R.; Green, D.W. Osgood Schlatter syndrome. Curr. Opin. Pediatr. 2007, 19, 44–50. [Google Scholar] [CrossRef]
- Hart, E.; Meehan, W.P.; Bae, D.S.; D’Hemecourt, P.; Stracciolini, A. The Young Injured Gymnast: A literature review and discussion. Curr. Sports Med. Rep. 2018, 17, 366–375. [Google Scholar] [CrossRef]
- Foss, K.D.B.; Myer, G.D.; Hewett, T.E. Epidemiology of Basketball, Soccer, and Volleyball Injuries in Middle-School Female Athletes. Physician Sportsmed. 2014, 42, 146–153. [Google Scholar] [CrossRef]
- Perhamre, S.; Lundin, F.; Norlin, R.; Klässbo, M. Sever’s injury; treat it with a heel cup: A randomized, crossover study with two insole alternatives. Scand. J. Med. Sci. Sports 2011, 21, e42–e47. [Google Scholar] [CrossRef]
- O’Kane, J.W.; Neradilek, M.; Polissar, N.; Sabado, L.; Tencer, A.; Schiff, M.A. Risk Factors for Lower Extremity Overuse Injuries in Female Youth Soccer Players. Orthop. J. Sports Med. 2017, 5, 2325967117733963. [Google Scholar] [CrossRef] [PubMed]
- Orava, S.; Malinen, L.; Karpakka, J.; Kvist, M.; Leppilathi, J.; Rantonen, J.; Kujala, U.M. Results of surgical treatment of unresolved Osgood-Schlatter lesion. Ann. Chir. Gynaecol. 2000, 89, 298–302. [Google Scholar] [PubMed]
- Ehrenborg, G. The Osgood-Schlatter lesion. A clinical and experimental study. Acta Chir. Scand. 1962, (Suppl. 288), 1–36. Available online: https://pubmed.ncbi.nlm.nih.gov/13889498/ (accessed on 21 January 2022).
- Vreju, F.; Ciurea, P.; Rosu, A. Osgood-Schlatter disease—Ultrasonographic diagnostic. Med. Ultrason. 2010, 12, 336–339. [Google Scholar]
- Omodaka, T.; Ohsawa, T.; Tajika, T.; Shiozawa, H.; Hashimoto, S.; Ohmae, H.; Shitara, H.; Ichinose, T.; Sasaki, T.; Hamano, N.; et al. Relationship Between Lower Limb Tightness and Practice Time Among Adolescent Baseball Players with Symptomatic Osgood-Schlatter Disease. Orthop. J. Sports Med. 2019, 7, 2325967119847978. [Google Scholar] [CrossRef]
- Halilbašić, A.; Kreso, A.; Klepić, M.; Jaganjac, A.; Avdic, D. The Algorithm for overload syndrome prevention: Osgood-Schlatter’s syndrome (OSD) as an overload syndrome caused by early inclusion of children in sports and excessive physical activity (sports and recreation). J. Health Sci. 2019, 9, 151–158. [Google Scholar] [CrossRef]
- Wu, A.C.; Rauh, M.J.; DeLuca, S.; Lewis, M.; Ackerman, K.E.; Barrack, M.T.; Heiderscheit, B.; Krabak, B.J.; Roberts, W.O.; Tenforde, A.S. Running-related injuries in middle school cross-country runners: Prevalence and characteristics of common injuries. PM R 2022, 30. online ahead of print. [Google Scholar] [CrossRef]
- Tzalach, A.; Lifshitz, L.; Yaniv, M.; Kurz, I.; Kalichman, L. The Correlation between Knee Flexion Lower Range of Motion and Osgood-Schlatter’s Syndrome among Adolescent Soccer Players. Br. J. Med. Med. Res. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Moy, A.; Song, E.; Wallace, S.J.; Teixeira, R.; Torres, D. Simultaneous Bilateral Patellar Tendon Rupture in a Young Adult Male: A Case Report and Review of the Literature. Cureus 2020, 25, e10649. [Google Scholar] [CrossRef]
- Demirag, B.; Ozturk, C.; Yazici, Z.; Sarisozen, B. The pathophysiology of Osgood-Schlatter disease: A magnetic resonance investigation. J. Pediatr. Orthop. B 2004, 13, 379–382. [Google Scholar] [CrossRef]
- Lyng, K.D.; Rathleff, M.S.; Dean, B.J.F.; Kluzek, S.; Holden, S. Current management strategies in Osgood Schlatter: A cross-sectional mixed-method study. Scand. J. Med. Sci. Sports 2020, 30, 1985–1991. [Google Scholar] [CrossRef]
- Krause, J.B.; Williams, A.; Catterall, A. Natural history of Osgood-Schlatter disease. J. Pediatr. Orthop. 1990, 10, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Kartini, C.; Wayan-Suryanto, D.I. Osgood-Schlatter disease: A review of current diagnosis and management. Curr. Orthop. Pract. 2022, 33, 294–298. [Google Scholar] [CrossRef]
- Enomoto, S.; Tsushima, A.; Oda, T.; Kaga, M. The Passive Mechanical Properties of Muscles and Tendons in Children Affected by Osgood-Schlatter Disease. J. Pediatr. Orthop. 2020, 40, e243–e247. [Google Scholar] [CrossRef]
- Tachdjian, O. Peditric Orthopaedics; Saunders: Philadelphia, PA, USA, 1990. [Google Scholar]
- Woolfrey, B.; Chandler, E. Manifestations of Osgood-Chlatter’s disease in late teen age and early adulthood. J. Bone Joint Surg. Am. 1960, 42, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Gaulrapp, H.; Nührenbörg, C. The Osgood-Schlatter disease: A large clinical series with evaluation of risk factors, natural course, and outcomes. Int. Orthop. 2022, 46, 197–204. [Google Scholar] [CrossRef]
- Nakase, J.; Aiba, T.; Goshima, K.; Takahashi, R.; Toratani, T.; Kosaka, M.; Ohashi, Y.; Tsuchiya, H. Relationship between the skeletal maturation of the distal attachment of the patellar tendon and physical features in preadolescent male football players. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Osawa, T.; Saito, K.; Kobayashi, T.; Tajika, T.; Yamamoto, A.; Iizuka, H.; Takagishi, K. Assessment of Osgood-Schlatter Disease and the Skeletal Maturation of the Distal Attachment of the Patellar Tendon in Preadolescent Males. Orthop. J. Sports Med. 2014, 18, 3–6. [Google Scholar] [CrossRef]
- Šarčević, Z. Limited ankle dorsiflexion: A predisposing factor to Morbus Osgood Schlatter? Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 726–728. [Google Scholar] [CrossRef]
- Katoh, K. An analysis of quadriceps muscle force in boys with Osgood-Schlatter disease. Nihon Seikeigeka Gakkai Zasshi 1988, 62, 523–533. [Google Scholar]
- Itoh, G.; Ishii, H.; Kato, H.; Nagano, Y.; Hayashi, H.; Funasaki, H. Risk assessment of the onset of Osgood-Schlatter disease using kinetic analysis of various motions in sports. PLoS ONE 2018, 8, e0190503. [Google Scholar] [CrossRef]
- Enomoto, S.; Oda, T.; Sugisaki, N.; Toeda, M.; Kurokawa, S.; Kaga, M. Muscle stiffness of the rectus femoris and vastus lateralis in children with Osgood-Schlatter disease. Knee 2021, 32, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Fujii, M.; Yoshimoto, M.; Abe, H.; Toda, N.; Higashiyama, R.; Takahira, N. Pathogenic Factors Associated with Osgood-Schlatter Disease in Adolescent Male Soccer Players: A Prospective Cohort Study. Orthop. J. Sports Med. 2018, 6, 2325967118792192. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Bevilacqua, C.; Bonetti, M.; Greco, F. Increased external tibial torsion in Osgood-Schlatter disease. Acta Orthop. Scand. 2003, 74, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Smillie, I. The effect of tibial torsion of the pathology of the knee. J. Bone Jt. Surgery. Br. 1981, 63-B, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Osgood-Schlatter’s disease: Etiology and treatment. Clin. Orthop. Relat. Res. 1969, 62, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Jibri, Z.; Jamieson, P.; Rakhra, K.S.; Sampaio, M.L.; Dervin, G. Patellar maltracking: An update on the diagnosis and treatment strategies. Insights Imaging 2019, 14, 65. [Google Scholar] [CrossRef]
- Green, D.W.; Sidharthan, S.; Schlichte, L.M.; Aitchison, A.H.; Mintz, D.N. Increased Posterior Tibial Slope in Patients with Osgood-Schlatter Disease: A New Association. Am. J. Sports Med. 2020, 48, 642–646. [Google Scholar] [CrossRef]
- Pan, T.; Mun, F.; Martinazzi, B.; King, T.S.; Petfield, J.L.; Hennrikus, W.L. The posterior tibial slope and Insall–Salvati index in operative and nonoperative adolescent athletes with Osgood-Schlatter disease. Arch. Orthop. Trauma. Surg. 2022, 25. online ahead of print. [Google Scholar] [CrossRef]
- Seyfettinoğlu, F.; Köse, Ö.; Oğur, H.U.; Tuhanioğlu, Ü.; Çiçek, H.; Acar, B. Is There a Relationship between Patellofemoral Alignment and Osgood-Schlatter Disease? A Case-Control Study. J. Knee Surg. 2020, 1, 67–72. [Google Scholar] [CrossRef]
- Aparicio, G.; Abril, J.C.; Calvo, E.; Alvarez, L. Radiologic Study of Patellar Height in Osgood-Schlatter Disease. J. Pediatr. Orthop. 1997, 17, 63–66. [Google Scholar] [CrossRef]
- Léonard, J.C.; Albecq, J.F.; Leclet, H.; Morin, C. Complications de la maladie d’Osgood-Schlatter: Les pièges d’une maladie répu tée banale. Sci. Sport 1995, 10, 95–101. [Google Scholar] [CrossRef]
- Lancourt, J.; Cristini, J. Patella alta and patella infera. Their etiological role in patellar dislocation, chondromalacia, and apophy sitis of the tibial tubercle. J. Bone Jt. Surg. Am. 1975, 57, 1112–1115. [Google Scholar] [CrossRef]
- Hall, R.; Foss, K.B.; Hewett, T.E.; Myer, G.D. Sport Specialization’s Association with an Increased Risk of Developing Anterior Knee Pain in Adolescent Female Athletes. J. Sport Rehabil. 2015, 24, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, E.D.; Ramamurti, P.; Stake, S.; Stadecker, M.; Rana, S.; Oetgen, M.E.; Young, M.L.; Martin, B.D. Posterior Tibial Slope is Increased in Patients with Tibial Tubercle Fractures and Osgood-Schlatter Disease. J. Pediatr. Orthop. 2021, 1, e411–e416. [Google Scholar] [CrossRef]
- Launay, F. Sports-related overuse injuries in children. Orthop. Traumatol. Surg. Res. 2015, 101, S139–S147. [Google Scholar] [CrossRef]
- Ross, M.D.; Villard, D.; Hopmans, J.; Simunek, J. Disability levels of college-aged men with a history of Osgood-Schlatter disease. J. Strength Cond. Res. 2003, 17, 659–663. [Google Scholar] [CrossRef]
- Smida, M.; Kandara, H.; Jlalia, Z.; Saied, W. Pathophysiology of Osgood-Schlatter Disease: Does Vitamin D have a Role? Vitam. Miner. 2018, 7, e97027. [Google Scholar] [CrossRef]
- Sevenler, D.; Buckley, M.R.; Kim, G.; van der Meulen, M.C.; Cohen, I.; Bonassar, L.J. Spatial periodicity in growth plate shear mechanical properties is disrupted by vitamin D deficiency. J. Biomech. 2013, 21, 1597–1603. [Google Scholar] [CrossRef][Green Version]
- Cahalan, R.; Purtill, H.; O’Sullivan, P.; O’Sullivan, K. A Cross-Sectional Study of Elite Adult Irish Dancers: Biopsychosocial Traits, Pain, and Injury. J. Dance Med. Sci. 2015, 19, 31–43. [Google Scholar] [CrossRef]
- Cahalan, R.; Bargary, N.; O’Sullivan, K. Pain and Injury in Elite Adolescent Irish Dancers: A Cross-Sectional Study. J. Dance Med. Sci. 2018, 15, 91–99. [Google Scholar] [CrossRef]
- Nur, S.I.; Siti, S.M.; Wan, A.W. An active boy with bilateral knee pain. Malays. Fam Physician 2019, 30, 26–28. [Google Scholar]
- Sailly, M.; Whiteley, R.; Johnson, A. Doppler ultrasound and tibial tuberosity maturation status predicts pain in adolescent male athletes with Osgood-Schlatter’s disease: A case series with comparison group and clinical interpretation. Br. J. Sports Med. 2013, 47, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Guldhammer, C.; Rathleff, M.S.; Jensen, H.P.; Holden, S. Long-term Prognosis and Impact of Osgood-Schlatter Disease 4 Years After Diagnosis: A Retrospective Study. Orthop. J. Sports Med. 2019, 31, 2325967119878136. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.O.; Toprak, U.; Baltaci, G.; Yosmaoglu, B.; Ozer, H. Long-term functional and sonographic outcomes in Osgood-Schlatter disease. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1131–1139. [Google Scholar] [CrossRef]
- Ishida, K.; Kuroda, R.; Sato, K.; Iguchi, T.; Doita, M.; Kurosaka, M.; Yamamoto, T. Infrapatellar Bursal Osteochondromatosis Associated with Unresolved Osgood-Schlatter Disease: A case report. J. Bone Jt. Surg. 2005, 87, 2780–2783. [Google Scholar] [CrossRef]
- Blankstein, A.; Cohen, I.; Heim, M.; Salai, M.; Chechick, A.; Ganel, A.; Diamant, L. Ultrasonography as a diagnostic modality in Osgood-Schlatter disease: A clinical study and review of the literature. Arch. Orthop. Trauma. Surg. 2001, 121, 536–539. [Google Scholar] [CrossRef]
- Hanada, M.; Takahashi, M.; Matsuyama, Y. Relationship between the clinical findings and radiographic severity in Osgood-Schlatter disease. Open Access J. Sports Med. 2012, 9, 17–20. [Google Scholar] [CrossRef]
- Circi, E.; Atalay, Y.; Beyzadeoglu, T. Treatment of Osgood-Schlatter disease: Review of the literature. Musculoskelet. Surg. 2017, 101, 195–200. [Google Scholar] [CrossRef]
- Mebis, W.; Jager, T.; Van Hedent, E. Intratendinous Patellar Ganglion Cyst with Coexistant Osgood Schlatter Disease. J. Belg. Soc. Radiol. 2016, 27, 86. [Google Scholar] [CrossRef]
- Czyrny, Z. Osgood-Schlatter disease in ultrasound diagnostics—A pictorial essay. Med. Ultrason. 2010, 12, 323–335. [Google Scholar]
- Circi, E.; Beyzadeoglu, T. Results of arthroscopic treatment in unresolved Osgood-Schlatter disease in athletes. Int. Orthop. 2017, 41, 351–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirano, A.; Fukubayashi, T.; Ishii, T.; Ochiai, N. Magnetic resonance imaging of Osgood-Schlatter disease: The course of the disease. Skelet. Radiol. 2002, 31, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Capitani, G.; Sehnem, E.; Rosa, C.; Matos, F.; Reis, V.M.; Neves, E.B. Osgood-schlatter Disease Diagnosis by Algometry and Infrared Thermography. Open Sports Sci. J. 2017, 10, 223–228. [Google Scholar] [CrossRef]
- Rathleff, M.S.; Winiarski, L.M.; Krommes, K.; Graven-Nielsen, T.; Hölmich, P.; Olesen, J.L.; Holden, S.; Thorborg, K. Activity Modification and Knee Strengthening for Osgood-Schlatter Disease: A Prospective Cohort Study. Orthop. J. Sports Med. 2020, 6, 2325967120911106. [Google Scholar] [CrossRef]
- Nührenbörger, C.; Gaulrapp, H. Morbus Osgood Schaltter. Sport Orthop. Traumatol. 2018, 34, 393–395. [Google Scholar] [CrossRef]
- Yen, Y.-M. Assessment and Treatment of Knee Pain in the Child and Adolescent Athlete. Pediatr. Clin. N. Am. 2014, 61, 1155–1173. [Google Scholar] [CrossRef]
- Gerulis, V.; Kalesinskas, R.; Pranckevicius, S.; Birgeris, P. Importance of conservative treatment and physical load restriction to the course of Osgood-Schlatter’s disease. Medicina 2004, 40, 363–369. [Google Scholar]
- Herrero-Morín, J.D.; Fernández-González, N.; Gutiérrez-Díez, C.; Pérez-Menéndez, M.T.; Fernández-Fernández, E.M. Enferme dad de Osgood-Schlatter en un adolescente deportista. Arch. Argent. Pediatr. 2017, 115, 445–448. [Google Scholar]
- Morris, E. Acupuncture in Osgood-Schlatter disease. BMJ Case Rep. 2016, 8, bcr2015214129. [Google Scholar] [CrossRef]
- Ladenhauf, H.N.; Seitlinger, G.; Green, D.W. Osgood-Schlatter disease: A 2020 update of a common knee condition in children. Curr. Opin. Pediatr. 2020, 32, 107–112. [Google Scholar] [CrossRef]
- Chaudhari, A.M.W.; VAN Horn, M.R.; Monfort, S.M.; Pan, X.; Oñate, J.A.; Best, T.M. Reducing Core Stability Influences Lower Extremity Biomechanics in Novice Runners. Med. Sci. Sports Exerc. 2020, 52, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Kimura, Y.; Fujita, Y.; Ishibashi, Y. Core-Muscle Training and Neuromuscular Control of the Lower Limb and Trunk. J. Athl. Train. 2019, 54, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, C.; Appenzeller-Herzog, C.; Faude, O. A systematic review on conservative treatment options for OSGOOD-Schlatter disease. Phys. Ther. Sport 2021, 49, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Rostron, P.K.; Calver, R.F. Subcutaneous atrophy following methyl prednisolone injection in Osgood-Schlatter epiphysitis. J. Bone Jt. Surg. 1979, 61, 627–628. [Google Scholar] [CrossRef]
- Wise, K.; Warren, D.; Diaz, L. Unilateral striae distensae of the knee after a steroid injection for the treatment of Os-good-Schlatter disease. Dermatol. Online J. 2016, 15, qt9g62f74c. [Google Scholar] [CrossRef]
- Bloom, O.J.; Mackler, L. Clinical inquires. What is the best treatment for Osgood-Schlatter disease? J. Fam. Pract. 2004, 53, 153–156. [Google Scholar]
- Weiler, R.; Ingram, M.; Wolman, R. 10-minute consultation. Osgood-Schlatter disease. BMJ 2011, 343, d4534. [Google Scholar] [CrossRef]
- Clark, S.; Jones, M.W.; Choudhury, R.R.; Smith, E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J. Accid. Emerg. Med. 1995, 12, 300–301. [Google Scholar] [CrossRef]
- Bandak, E.; Overgaard, A.F.; Kristensen, L.E.; Ellegaard, K.; Guldberg-Møller, J.; Bartholdy, C.; Hunter, D.J.; Altman, R.D.; Christensen, R.; Bliddal, H.; et al. Exercise therapy and patient education versus intra-articular saline injections in the treatment of knee osteoarthritis: An evidence-based protocol for an open-label randomised controlled trial (the DISCO trial). Trials 2021, 6, 18. [Google Scholar] [CrossRef]
- Zhang, W.; Robertson, J.; Jones, A.C.; Dieppe, P.A.; Doherty, M. The placebo effect and its determinants in osteoarthritis: Meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2008, 67, 1716–1723. [Google Scholar] [CrossRef]
- Altman, R.D.; Devji, T.; Bhandari, M.; Fierlinger, A.; Niazi, F.; Christensen, R. Clinical benefit of intra-articular saline as a com parator in clinical trials of knee osteoarthritis treatments: A systematic review and meta-analysis of randomized trials. Semin. Arthritis Rheum. 2016, 46, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E. Towards reaching consensus on hyaluronic acid efficacy in knee osteoarthritis. Clin. Rheumatol. 2019, 38, 2881–2883. [Google Scholar] [CrossRef] [PubMed]
- Topol, G.A.; Podesta, L.A.; Reeves, K.D.; Raya, M.F.; Fullerton, B.D.; Yeh, H.-W. Hyperosmolar Dextrose Injection for Recalcitrant Osgood-Schlatter Disease. Pediatrics 2011, 128, e1121–e1128. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tu, X.; Tu, Z. Hyperosmolar dextrose injection for Osgood-Schlatter disease: A double-blind, randomized controlled trial. Arch. Orthop. Trauma. Surg. 2021, 21. online ahead of print. [Google Scholar] [CrossRef]
- Danneberg, D.-J. Successful Treatment of Osgood-Schlatter Disease with Autologous-Conditioned Plasma in Two Patients. Joints 2017, 24, 191–194. [Google Scholar] [CrossRef][Green Version]
- Cairns, G.; Owen, T.; Kluzek, S.; Thurley, N.; Holden, S.; Rathleff, M.S.; Dean, B.J.F. Therapeutic interventions in children and adolescents with patellar tendon related pain: A systematic review. BMJ Open Sport Exerc. Med. 2018, 13, e000383. [Google Scholar] [CrossRef]
- Nakase, J.; Oshima, T.; Takata, Y.; Shimozaki, K.; Asai, K.; Tsuchiya, H. No superiority of dextrose injections over placebo injections for Osgood-Schlatter disease: A prospective randomized double-blind study. Arch. Orthop. Trauma. Surg. 2020, 140, 197–202. [Google Scholar] [CrossRef]
- Lohrer, H.; Nauck, T.; Schöll, J.; Zwerver, J.; Malliaropoulos, N. Extracorporeal shock wave therapy for patients suffering from recalcitrant Osgood-Schlatter disease. Sportverletz. Sportschaden 2012, 26, 218–222. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, M.J.; Kim, W.J.; Ha, J.K.; Kim, J.G. Correlation between Magnetic Resonance Imaging Characteristics of the Patellar Tendon and Clinical Scores in Osgood-Schlatter Disease. Knee Surg. Relat. Res. 2016, 28, 62–67. [Google Scholar] [CrossRef]
- Trail, I.A. Tibial Sequestrectomy in the Management of Osgood-Schlatter Disease. J. Pediatr. Orthop. 1988, 8, 554–557. [Google Scholar] [CrossRef]
- Frey, S.; Hosalkar, H.; Cameron, D.B.; Heath, A.; Horn, B.D.; Ganley, T.J. Tibial tuberosity fractures in adolescents. J. Child. Orthop. 2008, 2, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Narayan, N.; Mitchell, P.; Latimer, M.D. Complete resolution of the symptoms of refractory Osgood-Schlatter disease following percutaneous fixation of the tibial tuberosity. BMJ Case Rep. 2015, 12, bcr2014206734. [Google Scholar] [CrossRef] [PubMed]
- Beyzadeoglu, T.; Inan, M.; Bekler, H.; Altintas, F. Arthroscopic Excision of an Ununited Ossicle Due to Osgood-Schlatter Disease. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 1081–1083. [Google Scholar] [CrossRef]
- Binazzi, R.; Felli, L.; Vaccari, V.; Borelli, P. Surgial treatment of unresolved Osgood-Schlatter lesion. Clin. Orthop. Relat. Res. 1993, 289, 202–204. [Google Scholar]
- Flowers, M.J.; Bhadreshwa, D.R. Tibial tuberosity excision for symptomatic Osgood-Schlatter diseas. J. Pediatr. Orthop. 1995, 15, 292–297. [Google Scholar] [CrossRef]
- Pihlajamäki, H.K.; Visuri, T.I. Long-Term Outcome After Surgical Treatment of Unresolved Osgood-Schlatter Disease in Young Men. J. Bone Jt. Surg. 2010, 92, 258–264. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbi, F.; Matas, S.; Álvarez-Herms, J.; Sitko, S.; Baiget, E.; Reverter-Masia, J.; López-Laval, I. Osgood-Schlatter Disease: Appearance, Diagnosis and Treatment: A Narrative Review. Healthcare 2022, 10, 1011. https://doi.org/10.3390/healthcare10061011
Corbi F, Matas S, Álvarez-Herms J, Sitko S, Baiget E, Reverter-Masia J, López-Laval I. Osgood-Schlatter Disease: Appearance, Diagnosis and Treatment: A Narrative Review. Healthcare. 2022; 10(6):1011. https://doi.org/10.3390/healthcare10061011
Chicago/Turabian StyleCorbi, Francisco, Sergi Matas, Jesús Álvarez-Herms, Sebastian Sitko, Ernest Baiget, Joaquim Reverter-Masia, and Isaac López-Laval. 2022. "Osgood-Schlatter Disease: Appearance, Diagnosis and Treatment: A Narrative Review" Healthcare 10, no. 6: 1011. https://doi.org/10.3390/healthcare10061011
APA StyleCorbi, F., Matas, S., Álvarez-Herms, J., Sitko, S., Baiget, E., Reverter-Masia, J., & López-Laval, I. (2022). Osgood-Schlatter Disease: Appearance, Diagnosis and Treatment: A Narrative Review. Healthcare, 10(6), 1011. https://doi.org/10.3390/healthcare10061011







