Evidence-Based Data Models for Return-to-Play Criteria after Anterior Cruciate Ligament Reconstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procedures
2.2. Statistical Analysis
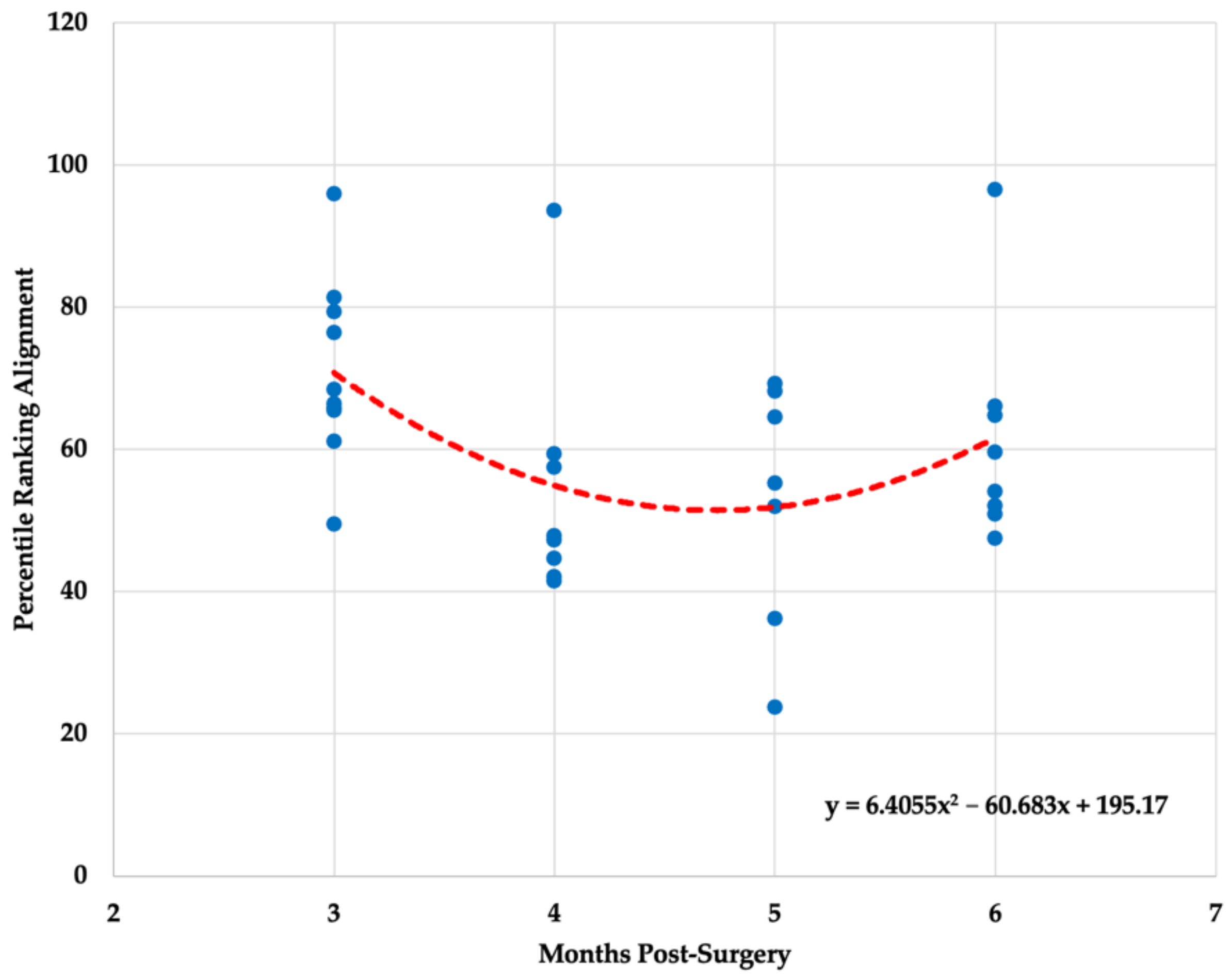
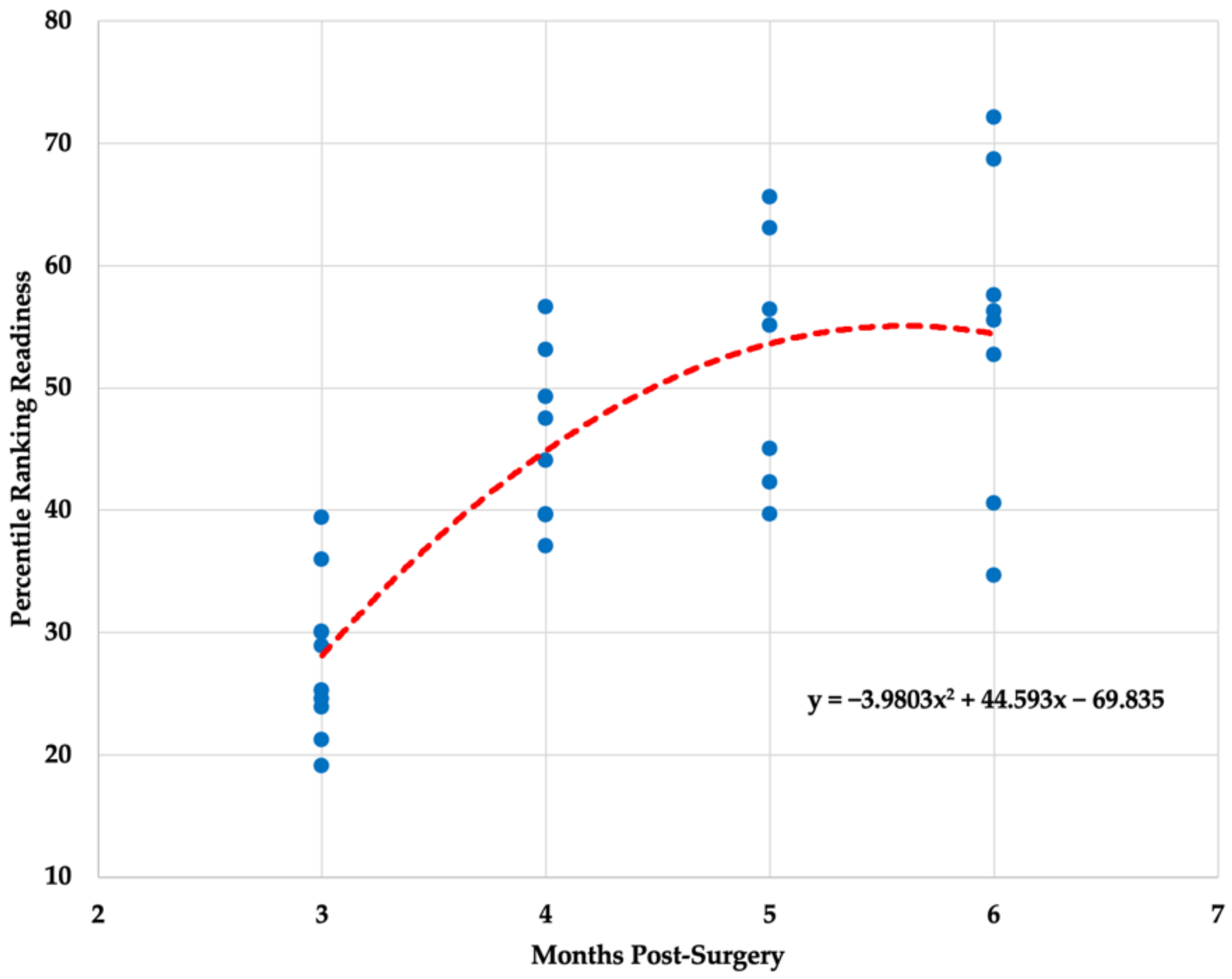
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bortone, I.; Moretti, L.; Bizzoca, D.; Caringella, N.; Delmedico, M.; Piazzolla, A.; Moretti, B. The importance of biomechanical assessment after Return to Play in athletes with ACL-Reconstruction. Gait Posture 2021, 88, 240–246. [Google Scholar] [PubMed]
- Flagg, K.Y.; Karavatas, S.G.; Thompson, S., Jr.; Bennett, C. Current criteria for return to play after anterior cruciate ligament reconstruction: An evidence-based literature review. Ann. Transl. Med. 2019, 7, S252. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.P.; de Carvalho, R.T.; Matsuda, M.M.; Filho, J.S.; Cohen, M. Common injuries in athletes’ knee: Experience of a specialized center. Acta Ortop. Bras. 2014, 22, 127–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, D.; LeBlanc, J.C.; Wooley, S.E.; Micheli, L.J.; Kramer, D.E. The effectiveness of a functional knee brace on joint-position sense in anterior cruciate ligament-reconstructed individuals. J. Sport Rehabil. 2016, 25, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Davies, G.J.; McCarty, E.; Provencher, M.; Manske, R.C. ACL return to sport guidelines and criteria. Curr. Rev. Musculoskelet. Med. 2017, 10, 307–314. [Google Scholar]
- Herbst, E.; Hoser, C.; Hildebrandt, C.; Raschner, C.; Hepperger, C.; Pointner, H.; Fink, C. Functional assessments for decision-making regarding return to sports following ACL reconstruction. Part II: Clinical application of a new test battery. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1283–1291. [Google Scholar]
- Webster, K.E.; Feller, J.A.; Leigh, W.B.; Richmond, A.K. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2014, 42, 641–647. [Google Scholar] [CrossRef]
- Paterno, M.V.; Thomas, S.; VanEtten, K.T.; Schmitt, L.C. Confidence, ability to meet return to sport criteria, and second ACL injury risk associations after ACL-reconstruction. J. Orthop. Res. 2022, 40, 182–190. [Google Scholar] [CrossRef]
- Buckthorpe, A.; Fizziero, A.; Roi, G.S. Update on functional recovery process for the injured athlete: Return to sport continuum redefined. Br. J. Sports Med. 2019, 53, 265–267. [Google Scholar] [CrossRef]
- Bien, D.P.; Dubuque, T.J. Considerations for late stage ACL rehabilitation and return to sport to limit re-injury risk and maximize athletic performance. Int. J. Sports Phys. Ther. 2015, 10, 256. [Google Scholar]
- Goerger, B.M.; Marshall, S.W.; Beutler, A.I.; Blackburn, J.T.; Wilckens, J.H.; Padua, D.A. Anterior cruciate ligament injury alters preinjury lower extremity biomechanics in the injured and uninjured leg: The JUMP-ACL study. Br. J. Sports Med. 2015, 49, 188–195. [Google Scholar]
- Kuenze, C.M.; Hertel, J.; Weltman, A.; Diduch, D.; Saliba, S.A.; Hart, J.M. Persistent neuromuscular and corticomotor quadriceps asymmetry after anterior cruciate ligament reconstruction. J. Athl. Train. 2015, 50, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Knezevic, O.M.; Mirkov, D.M.; Kadija, M.; Nedeljkovic, A.; Jaric, S. Asymmetries in explosive strength following anterior cruciate ligament reconstruction. Knee 2014, 21, 1039–1045. [Google Scholar]
- Van Melick, N.; van Cingel, R.E.H.; Brooijmans, F.; Neeter, C.; van Tienen, T.; Hullegie, W.; Nijhuis-van der Sanden, M.W.G. Evidence-based clinical practice update: Practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br. J. Sports Med. 2016, 50, 1506–1515. [Google Scholar]
- Harsted, S.; Holsgaard-Larsen, A.; Hestbaek, L.; Boyle, E.; Lauridsen, H.H. Concurrent validity of lower extremity kinematics and jump characteristics captured in pre-school children by a markerless 3D motion capture system. Chiropr. Man. Ther. 2019, 27, 39. [Google Scholar]
- Sandau, M.; Koblauch, H.; Moeslund, T.B.; Aanaes, H.; Alkjaer, T.; Simonsen, E.B. Markerless motion capture can provide reliable 3D gait kinematics in the sagittal and frontal plane. Med. Eng. Phys. 2014, 36, 1168–1175. [Google Scholar]
- Corazza, S.; Mundermann, L.; Chaudhari, A.M.; Demattio, T.; Cobelli, C.; Andriacchi, T.P. A markerless motion capture system to study musculoskeletal biomechanics: Visual hull and simulated annealing approach. Ann. Biomed. Eng. 2006, 34, 1019–1029. [Google Scholar] [CrossRef]
- Perrott, M.A.; Pizzari, T.; Cook, J.; McClelland, J.A. Comparison of lower limb and trunk kinematics between markerless and marker-based motion capture systems. Gait Posture 2017, 52, 57–61. [Google Scholar] [CrossRef]
- Cabarkapa, D.; Fry, A.C.; Mosier, E.M. Validity of 3-D markerless motion capture system for assessing basketball dunk kinetics—A case study. Sport J. 2020. Available online: http://thesportjournal.org/article/tag/basketball/ (accessed on 11 May 2022).
- Cabarkapa, D.; Whetstone, J.M.; Patterson, A.M.; Mosier, E.M.; Cabarkapa, D.V.; Fry, A.C. Relationship between health-related physical fitness parameters and functional movement screening scores acquired from a three-dimensional markerless motion capture system. Int. J. Environ. Res. Public Health 2022, 19, 4551. [Google Scholar] [CrossRef]
- Kaplan, Y.; Witvrouw, E. When is it safe to return to sport after ACL reconstruction? Reviewing the criteria. Sports Health 2019, 11, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Orishimo, K.F.; Kremenic, I.J.; Mullaney, M.J.; McHugh, M.P.; Nicholas, S.J. Adaptations in single-leg hop biomechanics following anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- De Fontenay, B.P.; Argaud, S.; Blache, Y.; Monteil, K. Motion alterations after anterior cruciate ligament reconstruction: Comparison of the injured and uninjured lower limbs during a single-legged jump. J. Athl. Train. 2014, 49, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuenze, C.; Collins, K.; Pfeiffer, K.A.; Lisee, C. Assessing physical activity after ACL injury: Moving beyond return to sport. Sports Health 2022, 14, 197–204. [Google Scholar]

| Specific Body Movement | Description of Movement |
|---|---|
| Bilateral Squat | Start with feet forward and shoulder-distance apart, and while holding a light bar directly above the head, lower body downward as far as possible. |
| Right Leg (Unilateral) Squat | Start by raising left foot off the ground and while balancing on the right leg, lower body down as far as possible on the standing leg and return to the starting position. |
| Left Leg (Unilateral) Squat | Start by raising right foot off the ground and while balancing on the left leg, lower body down as far as possible on the standing leg and return to the starting position. |
| Right Leg Lateral Lunge | Start by taking two large steps to your left within the capture space. Push off with your left leg and bound as far to your right side. Land on your right leg and immediately push off in the opposite direction to reach the starting position. |
| Left Leg Lateral Lunge | Start by taking two large steps to your right within the capture space. Push off with your right leg and bound as far to your left side. Land on your left leg and immediately push off in the opposite direction to reach the starting position. |
| Right Unilateral Jump | Start with feet forward, legs straight and arms extended backwards as far as possible, raise left leg off ground then jump as high as possible off right leg. |
| Left Unilateral Jump | Start with feet forward, legs straight and arms extended backwards as far as possible, raise right leg off ground then jump as high as possible off left leg. |
| Five Hops Right Leg | Start with feet forward, lift left leg to a near 90-degree angle, then jump as high as possible off right leg, five consecutive times. |
| Five Hops Left Leg | Start with feet forward, right left leg to a near 90-degree angle, then jump as high as possible off left leg, five consecutive times. |
| Time Post ACLR | Mobility (%) | Alignment (%) | Readiness (%) |
|---|---|---|---|
| Three Months | 3.79 ± 2.81 (74.02) | 70.96 ± 12.80 (18.04) | 27.85 ± 6.36 (22.84) |
| Four Months | 24.86 ± 12.75 * (51.29) | 54.20 ± 17.23 (31.79) | 45.88 ± 6.96 * (15.17) |
| Five Months | 39.19 ± 19.59 * (49.98) | 52.72 ± 17.18 (32.58) | 52.46 ± 10.25 * (19.53) |
| Six Months | 50.43 ± 32.53 * (64.61) | 61.42 ± 15.65 (25.47) | 54.78 ± 12.61 * (23.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daggett, M.C.; Witte, K.A.; Cabarkapa, D.; Cabarkapa, D.V.; Fry, A.C. Evidence-Based Data Models for Return-to-Play Criteria after Anterior Cruciate Ligament Reconstruction. Healthcare 2022, 10, 929. https://doi.org/10.3390/healthcare10050929
Daggett MC, Witte KA, Cabarkapa D, Cabarkapa DV, Fry AC. Evidence-Based Data Models for Return-to-Play Criteria after Anterior Cruciate Ligament Reconstruction. Healthcare. 2022; 10(5):929. https://doi.org/10.3390/healthcare10050929
Chicago/Turabian StyleDaggett, Matthew C., Kevin A. Witte, Dimitrije Cabarkapa, Damjana V. Cabarkapa, and Andrew C. Fry. 2022. "Evidence-Based Data Models for Return-to-Play Criteria after Anterior Cruciate Ligament Reconstruction" Healthcare 10, no. 5: 929. https://doi.org/10.3390/healthcare10050929
APA StyleDaggett, M. C., Witte, K. A., Cabarkapa, D., Cabarkapa, D. V., & Fry, A. C. (2022). Evidence-Based Data Models for Return-to-Play Criteria after Anterior Cruciate Ligament Reconstruction. Healthcare, 10(5), 929. https://doi.org/10.3390/healthcare10050929







