Ankle–Brachial Index Predicts Long-Term Renal Outcomes in Acute Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
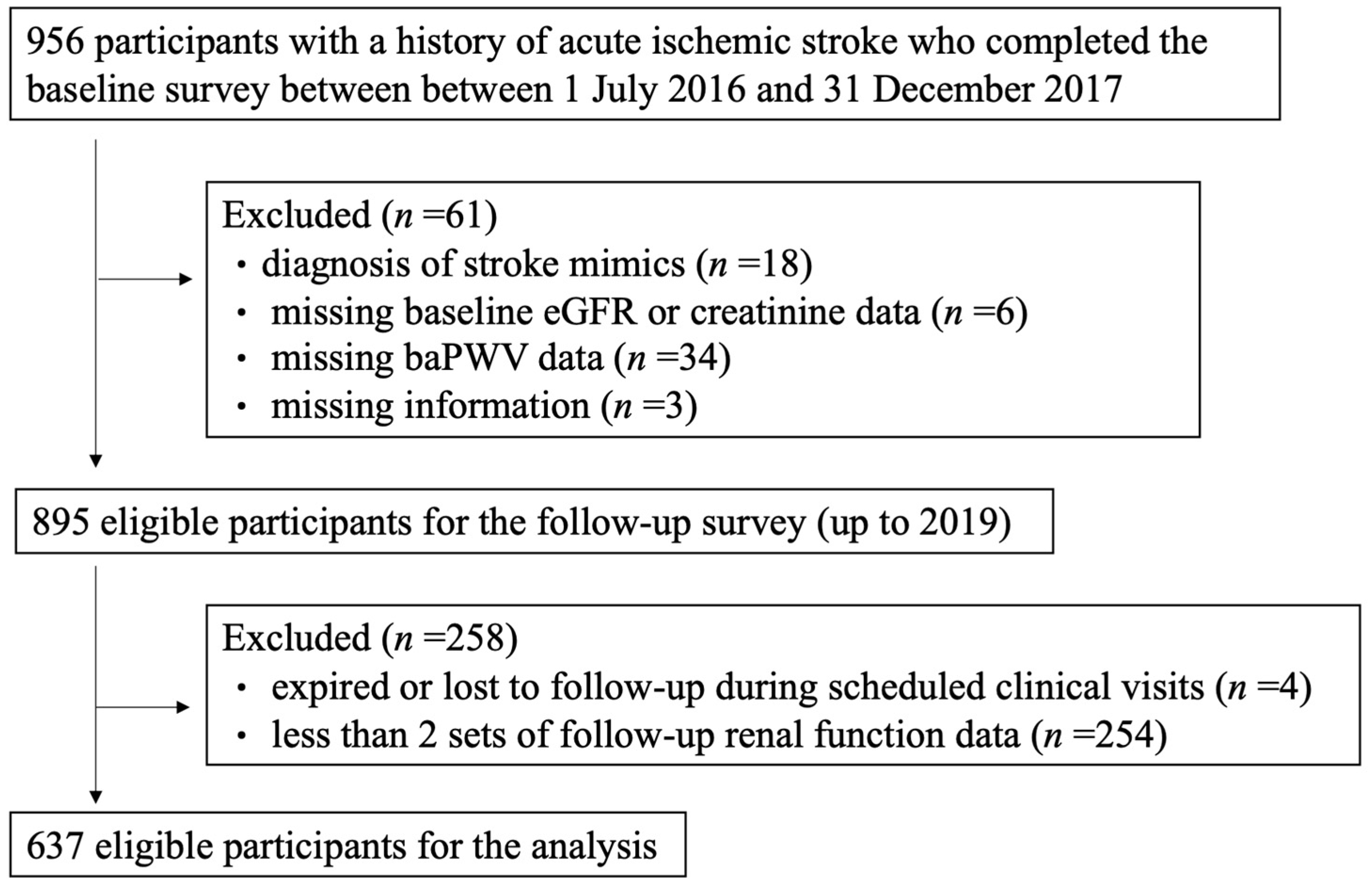
2.1. Study Design and Dataset
2.2. Baseline Survey
2.3. Outcome Definition and Statistical Analysis
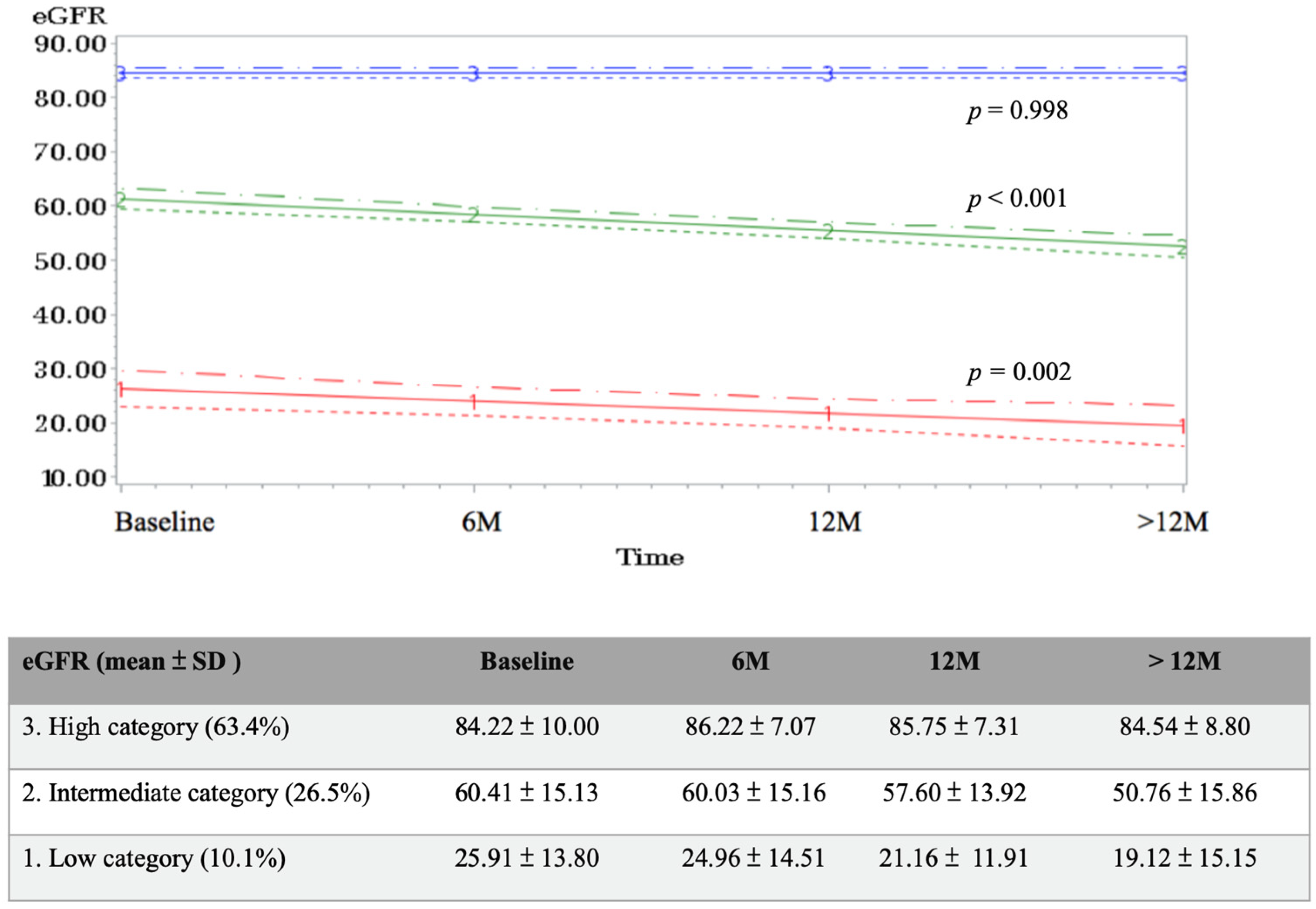
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bragg-Gresham, J.; Balkrishnan, R.; Bhave, N.; Dietrich, X.; Ding, Z.; Eggers, P.W.; et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2019, 73, A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, A.; Gioberge, S.; Moeller, S.; Brown, G. ESRD patients in 2004: Global overview of patient numbers, treatment modalities and associated trends. Nephrol. Dial. Transplant. 2005, 20, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chwojnicki, K.; Król, E.; Wierucki, Ł.; Kozera, G.; Sobolewski, P.; Nyka, W.M.; Zdrojewski, T. Renal Dysfunction in Post-Stroke Patients. PLoS ONE 2016, 11, e0159775. [Google Scholar] [CrossRef]
- Dai, L.; Golembiewska, E.; Lindholm, B.; Stenvinkel, P. End-Stage Renal Disease, Inflammation and Cardiovascular Outcomes. Contrib. Nephrol. 2017, 191, 32–43. [Google Scholar] [CrossRef]
- Vidt, D.G. Inflammation in renal disease. Am. J. Cardiol. 2006, 97, 20a–27a. [Google Scholar] [CrossRef]
- Ishikawa, J.; Tamura, Y.; Hoshide, S.; Eguchi, K.; Ishikawa, S.; Shimada, K.; Kario, K. Low-grade inflammation is a risk factor for clinical stroke events in addition to silent cerebral infarcts in Japanese older hypertensives: The Jichi Medical School ABPM Study, wave 1. Stroke 2007, 38, 911–917. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Tripepi, G. Traditional and emerging cardiovascular risk factors in end-stage renal disease. Kidney Int. 2003, 63, S105–S110. [Google Scholar] [CrossRef]
- Klag, M.J.; Whelton, P.K.; Randall, B.L.; Neaton, J.D.; Brancati, F.L.; Ford, C.E.; Shulman, N.B.; Stamler, J. Blood pressure and end-stage renal disease in men. N. Engl. J. Med. 1996, 334, 13–18. [Google Scholar] [CrossRef]
- Levin, A.; Djurdjev, O.; Barrett, B.; Burgess, E.; Carlisle, E.; Ethier, J.; Jindal, K.; Mendelssohn, D.; Tobe, S.; Singer, J.; et al. Cardiovascular disease in patients with chronic kidney disease: Getting to the heart of the matter. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2001, 38, 1398–1407. [Google Scholar] [CrossRef]
- Bleyer, A.J.; Shemanski, L.R.; Burke, G.L.; Hansen, K.J.; Appel, R.G. Tobacco, hypertension, and vascular disease: Risk factors for renal functional decline in an older population. Kidney Int. 2000, 57, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.A.; Yang, C.Y.; Yang, W.C.; Chen, J.Y.; Ng, Y.Y.; Li, S.Y.; Liu, W.S.; Cheng, S.T.; Wang, Y.J.; Lin, C.C. Ankle-brachial index is a powerful predictor of renal outcome and cardiovascular events in patients with chronic kidney disease. Sci. World J. 2012, 2012, 238494. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, P.A.; Maloberti, A.; Micale, M.; Pagliarin, F.; Antonazzo, I.C.; Mazzaglia, G.; Giannattasio, C.; Mantovani, L.G. Costs and effects of cardiovascular risk reclassification using the ankle-brachial index (ABI) in addition to the Framingham risk scoring in women. Atherosclerosis 2021, 317, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): Executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J. Am. Coll. Cardiol. 2006, 47, 1239–1312. [Google Scholar] [CrossRef]
- Abboud, H.; Monteiro Tavares, L.; Labreuche, J.; Arauz, A.; Bryer, A.; Lavados, P.M.; Massaro, A.; Munoz Collazos, M.; Steg, P.G.; Yamout, B.I.; et al. Impact of Low Ankle-Brachial Index on the Risk of Recurrent Vascular Events. Stroke 2019, 50, 853–858. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Low, L.P.; Tuta, S.; Kozak, J. Ankle-brachial index and extent of atherothrombosis in 8891 patients with or at risk of vascular disease: Results of the international AGATHA study. Eur. Heart J. 2006, 27, 1861–1867. [Google Scholar] [CrossRef]
- Chen, S.C.; Chang, J.M.; Liu, W.C.; Tsai, Y.C.; Tsai, J.C.; Hsu, P.C.; Lin, T.H.; Lin, M.Y.; Su, H.M.; Hwang, S.J.; et al. Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 724–732. [Google Scholar] [CrossRef]
- Ninomiya, T.; Kojima, I.; Doi, Y.; Fukuhara, M.; Hirakawa, Y.; Hata, J.; Kitazono, T.; Kiyohara, Y. Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general Japanese population: The Hisayama Study. J. Hypertens. 2013, 31, 477–483; discussion 483. [Google Scholar] [CrossRef]
- Ai, Z.S.; Li, J.; Liu, Z.M.; Fan, H.M.; Zhang, D.F.; Zhu, Y.; Zhang, L.J.; Zhu, W.Q.; Bao, Y. Reference value of brachial-ankle pulse wave velocity for the eastern Chinese population and potential influencing factors. Braz. J. Med. Biol. Res. 2011, 44, 1000–1005. [Google Scholar] [CrossRef]
- Chung, C.M.; Yang, T.Y.; Lin, Y.S.; Chang, S.T.; Hsiao, J.F.; Pan, K.L.; Jang, S.J.; Hsu, J.T. Relation of arterial stiffness assessed by brachial-ankle pulse wave velocity to complexity of coronary artery disease. Am. J. Med. Sci. 2014, 348, 294–299. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [CrossRef] [PubMed]
- Lin, J.S.; Olson, C.M.; Johnson, E.S.; Whitlock, E.P. The ankle-brachial index for peripheral artery disease screening and cardiovascular disease prediction among asymptomatic adults: A systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Yamashina, A.; Arai, T.; Hirose, K.; Koji, Y.; Chikamori, T.; Hori, S.; Yamamoto, Y.; Doba, N.; Hinohara, S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—A survey of 12517 subjects. Atherosclerosis 2003, 166, 303–309. [Google Scholar] [CrossRef]
- Yamashina, A.; Tomiyama, H.; Takeda, K.; Tsuda, H.; Arai, T.; Hirose, K.; Koji, Y.; Hori, S.; Yamamoto, Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens. Res. 2002, 25, 359–364. [Google Scholar] [CrossRef]
- Jones, B.L.; Nagin, D.S.; Roeder, K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociol. Methods Res. 2001, 29, 374–393. [Google Scholar] [CrossRef]
- George, B.; Seals, S.; Aban, I. Survival analysis and regression models. J. Nucl. Cardiol. 2014, 21, 686–694. [Google Scholar] [CrossRef]
- Chang, W.X.; Asakawa, S.; Toyoki, D.; Nemoto, Y.; Morimoto, C.; Tamura, Y.; Ota, T.; Shibata, S.; Fujigaki, Y.; Shen, Z.Y.; et al. Predictors and the Subsequent Risk of End-Stage Renal Disease-Usefulness of 30% Decline in Estimated GFR over 2 Years. PLoS ONE 2015, 10, e0132927. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; Perkovic, V.; de Zeeuw, D. Is doubling of serum creatinine a valid clinical ‘hard’ endpoint in clinical nephrology trials? Nephron Clin. Pract. 2011, 119, c195–c199; discussion c199. [Google Scholar] [CrossRef]
- Murray, C.J.; Lopez, A.D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997, 349, 1498–1504. [Google Scholar] [CrossRef]
- Kumai, Y.; Kamouchi, M.; Hata, J.; Ago, T.; Kitayama, J.; Nakane, H.; Sugimori, H.; Kitazono, T. Proteinuria and clinical outcomes after ischemic stroke. Neurology 2012, 78, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Ovbiagele, B.; Bath, P.M.; Cotton, D.; Sha, N.; Diener, H.C. Low glomerular filtration rate, recurrent stroke risk, and effect of renin-angiotensin system modulation. Stroke 2013, 44, 3223–3225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldstein, L.B.; Adams, R.; Alberts, M.J.; Appel, L.J.; Brass, L.M.; Bushnell, C.D.; Culebras, A.; Degraba, T.J.; Gorelick, P.B.; Guyton, J.R.; et al. Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council: Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guideline. Stroke 2006, 37, 1583–1633. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Reilly, M.; Yang, W.; Chen, J.; Go, A.S.; Lash, J.P.; Rahman, M.; DeFilippi, C.; Gadegbeku, C.; Kanthety, R.; et al. Risk factors for coronary artery calcium among patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study). Am. J. Cardiol. 2012, 110, 1735–1741. [Google Scholar] [CrossRef]
- Hung, P.H.; Huang, Y.T.; Hsiao, C.Y.; Sung, P.S.; Guo, H.R.; Tsai, K.J. Young stroke patients are at high risk for subsequent end-stage renal disease: A population-based observational study. Nephrol. Dial. Transplant. 2014, 29, 873–878. [Google Scholar] [CrossRef]
- Arroyo, D.; Betriu, A.; Valls, J.; Gorriz, J.L.; Pallares, V.; Abajo, M.; Gracia, M.; Valdivielso, J.M.; Fernandez, E. Factors influencing pathological ankle-brachial index values along the chronic kidney disease spectrum: The NEFRONA study. Nephrol. Dial. Transplant. 2017, 32, 513–520. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Murray, G.D.; Butcher, I.; Heald, C.L.; Lee, R.J.; Chambless, L.E.; Folsom, A.R.; Hirsch, A.T.; Dramaix, M.; deBacker, G.; et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA 2008, 300, 197–208. [Google Scholar] [CrossRef]
- Iwakiri, T.; Sato, Y.; Matsuura, Y.; Hatakeyama, K.; Marutsuka, K.; Yamashita, A.; Fujimoto, S.; Kitamura, K.; Asada, Y. Association between renal vasculature changes and generalized atherosclerosis: An autopsy survey. J. Atheroscler. Thromb. 2014, 21, 99–107. [Google Scholar] [CrossRef][Green Version]
- Asmar, R.; Benetos, A.; Topouchian, J.; Laurent, P.; Pannier, B.; Brisac, A.M.; Target, R.; Levy, B.I. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension 1995, 26, 485–490. [Google Scholar] [CrossRef]
| Enrolled Subjects (n = 637) a | |||||||
|---|---|---|---|---|---|---|---|
| ABI > 0.9 | ABI ≤ 0.9 | p | baPWV < 14 | baPWV ≥ 14 | p | ||
| n | 524 | 113 | 44 | 559 | |||
| Age (mean ± SD) | [years] | 66.7 ± 12.8 | 75.4 ± 13.2 | <0.001 | 56.4 ± 19.5 | 68.9 ± 12.3 | <0.001 |
| Male | n [%] | 323 (61.6) | 66 (58.4) | 0.594 | 28 (63.64) | 345 (61.72) | 0.927 |
| BMI (mean ± SD) | [kg/m2] | 24.5 ± 4.0 | 22.9 ± 4.3 | <0.001 | 24.0 ± 4.4 | 24.3 ± 4.1 | 0.977 |
| Hyperlipidemia | n [%] | 308 (58.8) | 68 (60.2) | 0.866 | 24 (54.6) | 334 (59.8) | 0.605 |
| Heart disease | n [%] | 36 (6.9) | 13 (11.5) | 0.138 | 8 (18.2) | 92 (16.5) | 0.932 |
| Hypertension | n [%] | 340 (65.4) | 88 (77.9) | 0.014 | 17 (38.6) | 389 (70.1) | <0.001 |
| Diabetes Mellitus | n [%] | 168 (32.31) | 39 (34.5) | 0.732 | 7 (15.9) | 189 (34.1) | 0.021 |
| ABI ≤ 0.9 | n [%] | - | - | - | 10 (22.7) | 92 (16.5) | 0.390 |
| baPWV ≥ 1.4 | n [%] | 467 (93.2) | 92 (90.2) | 0.390 | - | - | - |
| eGFR (mean ± SD) | [mL/min/1.73 m2] | 73.7 ± 21.5 | 64.3 ± 23.2 | <0.001 | 76.1 ± 19.6 | 72.1 ± 22.0 | 0.205 |
| IC vs. HC | LC vs. HC | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
| Age | 1.05 (1.03–1.06) | <0.001 | 1.05 (1.03–1.07) | <0.001 * | 1.04 (1.02–1.06) | 0.001 | 1.03 (1.01–1.06) | 0.017 * |
| Male | 0.95 (0.66–1.37) | 0.772 | 1.13 (0.65–1.95) | 0.672 | ||||
| BMI | 1.01 (0.96–1.05) | 0.774 | 0.96 (0.89–1.02) | 0.191 | ||||
| Borderline ABI (0.91~0.99) | 1.17 (0.69–1.98) | 0.560 | 0.79 (0.44–1.41) | 0.421 | 2.12 (1.05–4.29) | 0.037 | 1.55 (0.71–3.40) | 0.274 |
| Abnormal ABI (≤0.90) | 1.94 (1.21–3.08) | 0.006 | 1.25 (0.73–2.15) | 0.413 | 3.15 (1.67–5.96) | <0.001 | 2.40 (1.16–4.95) | 0.019 * |
| baPWV ≥ 1.4 | 1.31 (0.63–2.75) | 0.469 | 1.60 (0.47–5.42) | 0.447 | ||||
| Hyperlipidemia | 0.90 (0.63–1.30) | 0.585 | 0.92 (0.54–1.57) | 0.757 | ||||
| Heart disease | 1.46 (0.75–2.85) | 0.263 | 1.00 (0.45–2.23) | 0.995 | 2.47 (1.10–5.58) | 0.029 | 1.24 (0.44–3.51) | 0.690 |
| Smoking | 0.51 (0.25–1.05) | 0.067 | 0.36 (0.08–1.61) | 0.183 | ||||
| Hypertension | 1.30 (0.88–1.91) | 0.189 | 1.77 (0.96–3.26) | 0.069 | ||||
| Diabetes Mellitus | 0.92(0.63–1.36) | 0.683 | 1.43 (0.83–2.46) | 0.193 | ||||
| Poor discharge mRS (≥2) | 1.73 (1.14–2.60) | 0.009 | 1.26 (0.81–1.97) | 0.303 | 1.63 (0.89–2.99) | 0.113 | 1.09 (0.57–2.08) | 0.802 |
| A 30% Decline in eGFR | Serum Creatinine Doubling | ESRD | ||||
|---|---|---|---|---|---|---|
| Variables | Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) |
| Age | 1.04 (1.02–1.06) | 1.03 (1.01–1.06) * | 1.04 (1.01–1.07) | 1.02 (0.99–1.05) | 1.05 (1.01–1.09) | 1.04 (1.00–1.08) * |
| Male | 0.77 (0.50–1.18) | - | 0.99 (0.51–1.91) | - | 1.36 (0.56–3.28) | - |
| BMI | 0.99 (0.93–1.04) | - | 0.94 (0.87–1.03) | - | 0.97 (0.88–1.08) | - |
| Borderline ABI (0.91~0.99) | 1.33 (0.72–2.46) | 1.15 (0.60–2.22) | 2.09 (0.86–5.09) | 1.98 (0.78–4.98) | 1.06 (0.24–4.68) | 0.91 (0.21–4.04) |
| Abnormal ABI (≤0.90) | 2.24 (1.35–3.71) | 1.90 (1.09–3.34) * | 4.35 (2.15–8.80) | 3.60 (1.64–7.91) * | 4.04 (1.56–10.45) | 3.28 (1.23–8.74) * |
| baPWV ≥ 1.4 | 0.99 (0.40–2.46) | - | 0.54 (0.19–1.53) | - | 0.59 (0.14–2.56) | - |
| Hyperlipidemia | 0.89 (0.60–1.31) | - | 1.08 (0.55–2.11) | - | 1.17 (0.50–2.73) | - |
| Heart disease | 0.95 (0.47–1.92) | - | 0.65 (0.20–2.14) | - | 1.03 (0.30–3.52) | - |
| Smoking | 0.84 (0.37–1.94) | - | 1.01 (0.33–3.09) | - | 0.99 (0.27–3.70) | - |
| Hypertension | 1.47 (0.91–2.39) | - | 1.65 (0.80–3.42) | - | 1.36 (0.56–3.30) | - |
| Diabetes Mellitus | 1.66 (1.08–2.55) | 1.61 (1.00–2.60) * | 1.51 (0.79–2.88) | - | 1.40 (0.62–3.16) | - |
| Poor discharge mRS (≥2) | 2.02 (1.18–3.44) | 1.45 (0.84–2.52) | 2.57 (1.12–5.90) | 1.74 (0.73–4.12) | 1.21 (0.52–2.84) | - |
| (1) eGFR Decline > 30% | |||||
| ABI group | p Value | ||||
| ABI > 1.1 (n = 172) | 0.9 < ABI ≤ 1.1 (n = 351) | 0.7 < ABI ≤ 0.9 (n = 72) | ABI ≤ 0.7 (n = 41) | ||
| n (%) | n (%) | n (%) | n (%) | ||
| No | 150 (87.21) | 297 (84.62) | 55 (76.39) | 30 (73.17) | 0.048 |
| Yes | 22 (12.79) | 54 (15.38) | 17 (23.61) | 11 (26.83) | |
| (2) SerumCreatinineDoubling | |||||
| ABI group | p Value | ||||
| ABI > 1.1 (n = 172) | 0.9 < ABI ≤ 1.1 (n = 351) | 0.7 < ABI ≤ 0.9 (n = 72) | ABI ≤ 0.7 (n = 41) | ||
| n (%) | n (%) | n (%) | n (%) | ||
| No | 165 (95.93) | 335 (95.44) | 64 (88.89) | 34 (82.93) | 0.004 |
| Yes | 7 (4.07) | 16 (4.56) | 8 (11.11) | 7 (17.07) | |
| (3) The Occurrence of ESRD | |||||
| ABI group | p Value | ||||
| ABI > 1.1 (n = 171) | 0.9 < ABI ≤ 1.1 (n = 339) | 0.7 < ABI ≤ 0.9 (n = 68) | ABI ≤ 0.7 (n = 39) | ||
| n (%) | n (%) | n (%) | n (%) | ||
| No | 164 (95.91) | 329 (97.05) | 64 (94.12) | 36 (92.31) | 0.268 |
| Yes | 7 (4.09) | 10 (2.95) | 4 (5.88) | 3 (7.69) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-L.; Chang, Y.-M.; Liu, C.-H.; Su, H.-C.; Sung, P.-S.; Lin, S.-H.; Chen, C.-H. Ankle–Brachial Index Predicts Long-Term Renal Outcomes in Acute Stroke Patients. Healthcare 2022, 10, 913. https://doi.org/10.3390/healthcare10050913
Lee T-L, Chang Y-M, Liu C-H, Su H-C, Sung P-S, Lin S-H, Chen C-H. Ankle–Brachial Index Predicts Long-Term Renal Outcomes in Acute Stroke Patients. Healthcare. 2022; 10(5):913. https://doi.org/10.3390/healthcare10050913
Chicago/Turabian StyleLee, Tsung-Lin, Yu-Ming Chang, Chi-Hung Liu, Hui-Chen Su, Pi-Shan Sung, Sheng-Hsiang Lin, and Chih-Hung Chen. 2022. "Ankle–Brachial Index Predicts Long-Term Renal Outcomes in Acute Stroke Patients" Healthcare 10, no. 5: 913. https://doi.org/10.3390/healthcare10050913
APA StyleLee, T.-L., Chang, Y.-M., Liu, C.-H., Su, H.-C., Sung, P.-S., Lin, S.-H., & Chen, C.-H. (2022). Ankle–Brachial Index Predicts Long-Term Renal Outcomes in Acute Stroke Patients. Healthcare, 10(5), 913. https://doi.org/10.3390/healthcare10050913






