Impact of System and Diagnostic Errors on Medical Litigation Outcomes: Machine Learning-Based Prediction Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Source and Study Population
2.3. Outcomes
2.4. Variables and Definitions
2.5. Statistical Analysis
2.6. Performance Metrics and Feature Importance
3. Results
3.1. Machine Learning
3.2. Indemnity Costs
4. Discussion
4.1. Strengths
4.2. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosma, E.; Veen, E.J.; Roukema, J.A. Incidence, Nature and Impact of Error in Surgery. Br. J. Surg. 2011, 98, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.; Bhome, R.; Somani, B. National Trends and Cost of Litigation in UK National Health Service (NHS): A Specialty-Specific Analysis from the Past Decade. Scott. Med. J. 2021, 66, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Menon, N.K.; Shanafelt, T.D.; Sinsky, C.A.; Linzer, M.; Carlasare, L.; Brady, K.J.S.; Stillman, M.J.; Trockel, M.T. Association of Physician Burnout with Suicidal Ideation and Medical Errors. JAMA Netw. Open. 2020, 3, e2028780. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Snyder, A.; Kachalia, A.; Flanders, S.; Saint, S.; Chopra, V. Malpractice Claims Related to Diagnostic Errors in the Hospital. BMJ Qual. Saf. 2017, 27, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Watari, T.; Shibata, A.; Noda, T.; Ozaki, T. The Impact of System and Diagnostic Errors for Medical Litigation Outcomes in Orthopedic Surgery. J. Orthop. Sci. 2021; in press. [Google Scholar] [CrossRef]
- Studdert, D.M.; Mello, M.M.; Gawande, A.A.; Gandhi, T.K.; Kachalia, A.; Yoon, C.; Puopolo, A.L.; Brennan, T.A. Claims, Errors, and Compensation Payments in Medical Malpractice Litigation. N. Engl. J. Med. 2006, 354, 2024–2033. [Google Scholar] [CrossRef] [Green Version]
- Graber, M.L.; Franklin, N.; Gordon, R. Diagnostic Error in Internal Medicine. Arch. Intern. Med. 2005, 165, 1493–1499. [Google Scholar] [CrossRef]
- Watari, T. Malpractice Claims of Internal Medicine Involving Diagnostic and System Errors in Japan. Intern. Med. 2021, 60, 2919–2925. [Google Scholar] [CrossRef]
- Singh, H.; Schiff, G.D.; Graber, M.L.; Onakpoya, I.; Thompson, M.J. The Global Burden of Diagnostic Errors in Primary Care. BMJ Qual. Saf. 2017, 26, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Mello, M.M.; Frakes, M.D.; Blumenkranz, E.; Studdert, D.M. Malpractice Liability and Health Care Quality: A Review. JAMA 2020, 323, 352–366. [Google Scholar] [CrossRef]
- Tang, O.Y.; Hartnett, D.A.; Hays, S.B.; Syed, S.; Daniels, A.H. Determinants of Brain Tumor Malpractice Litigation Outcome and Indemnity Payments: A 29-Year Nationwide Analysis. Neurosurg. Focus 2020, 49, E21. [Google Scholar] [CrossRef] [PubMed]
- Rynecki, N.D.; Coban, D.; Gantz, O.; Gupta, R.; Ayyaswami, V.; Prabhu, A.V.; Ruskin, J.; Lin, S.S.; Beebe, K.S. Medical Malpractice in Orthopedic Surgery: A Westlaw-Based Demographic Analysis. Orthopedics 2018, 41, e615–e620. [Google Scholar] [CrossRef] [PubMed]
- Bors-Koefoed, R.; Zylstra, S.; Resseguie, L.J.; Ricci, B.A.; Kelly, E.E.; Mondor, M.C. Statistical Models of Outcome in Malpractice Lawsuits Involving Death or Neurologically Impaired Infants. J. Matern. Fetal Med. 1998, 7, 124–131. Available online: https://pubmed.ncbi.nlm.nih.gov/9642609/ (accessed on 17 February 2022). [CrossRef] [PubMed]
- Lage-Freitas, A.; Allende-Cid, H.; Santana, O.; Oliveira-Lage, L. Predicting Brazilian Court Decisions. PeerJ Comput. Sci. 2022, 8, e904. [Google Scholar] [CrossRef]
- Sert, M.F.; Yıldırım, E.; Haşlak, İ. Using Artificial Intelligence to Predict Decisions of the Turkish Constitutional Court. Soc. Sci. Comput. Rev. 2021. [Google Scholar] [CrossRef]
- Song, X.; Liu, X.; Liu, F.; Wang, C. Comparison of Machine Learning and Logistic Regression Models in Predicting Acute Kidney Injury: A Systematic Review and Meta-Analysis. Int. J. Med. Inform. 2021, 151, 104484. [Google Scholar] [CrossRef]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A Systematic Review Shows No Performance Benefit of Machine Learning over Logistic Regression for Clinical Prediction Models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, N.S.; Stason, W.B. Managing Quality in Hospital Practice. Int. J. Qual. Health Care. 1998, 10, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Watari, T.; Tokuda, Y.; Mitsuhashi, S.; Otuki, K.; Kono, K.; Nagai, N.; Onigata, K.; Kanda, H. Factors and Impact of Physicians’ Diagnostic Errors in Malpractice Claims in Japan. PLoS ONE 2020, 15, e0237145. [Google Scholar] [CrossRef]
- What Is Diagnostic Error? Society to Improve Diagnosis in Medicine. Available online: https://www.improvediagnosis.org/what-is-diagnostic-error/ (accessed on 11 May 2022).
- Stone, M. Cross-Validatory Choice and Assessment of Statistical Predictions. J. R. Stat. Soc. B 1974, 36, 111–133. [Google Scholar] [CrossRef]
- Cox, D.R. The Regression Analysis of Binary Sequences. J. R. Stat. Soc. Ser. B (Methodol.) 1958, 20, 215–232. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees. Classification and Regression Trees; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Kopitar, L.; Kocbek, P.; Cilar, L.; Sheikh, A.; Stiglic, G. Early Detection of Type 2 Diabetes Mellitus Using Machine Learning-Based Prediction Models. Sci. Rep. 2020, 10, 11981. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.M.; Erion, G.G.; Lee, S.I. Consistent Individualized Feature Attribution for Tree Ensembles. arXiv 2018, arXiv:1802.03888. [Google Scholar]
- Kuno, T.; Sahashi, Y.; Kawahito, S.; Takahashi, M.; Iwagami, M.; Egorova, N.N. Prediction of in-Hospital Mortality with Machine Learning for COVID-19 Patients Treated with Steroid and Remdesivir. J. Med. Virol. 2021, 94, 958–964. [Google Scholar] [CrossRef]
- Osawa, I.; Goto, T.; Yamamoto, Y.; Tsugawa, Y. Machine-Learning-Based Prediction Models for High-Need High-Cost Patients Using Nationwide Clinical and Claims Data. NPJ Digit. Med. 2020, 3, 148. [Google Scholar] [CrossRef]
- Matulis, J.C.; Kok, S.N.; Dankbar, E.C.; Majka, A.J. A Survey of Outpatient Internal Medicine Clinician Perceptions of Diagnostic Error. Diagnosis 2020, 7, 107–114. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Defroda, S.F.; Naqvi, S.J.; Eltorai, A.E.M.; Hartnett, D.; Ruddell, J.H.; Born, C.T.; Daniels, A.H. Malpractice Litigation Following Traumatic Fracture. J. Bone Jt. Surg. Am. 2019, 101, e27. [Google Scholar] [CrossRef]
- Sasao, S.; Hiyama, T.; Tanaka, S.; Mukai, S.; Yoshihara, M.; Chayama, K. Medical Malpractice Litigation in Gastroenterological Practice in Japan: A 22-yr Review of Civil Court Cases. Am. J. Gastroenterol. 2006, 101, 1951–1953. [Google Scholar] [CrossRef]
- Sato, T.; Okada, S.; Nitta, K. Deliberation Process Support System for Citizen Judge Trial Based on Structure of Factors. In Symposium on Artificial Intelligence; International, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 155–169. [Google Scholar]
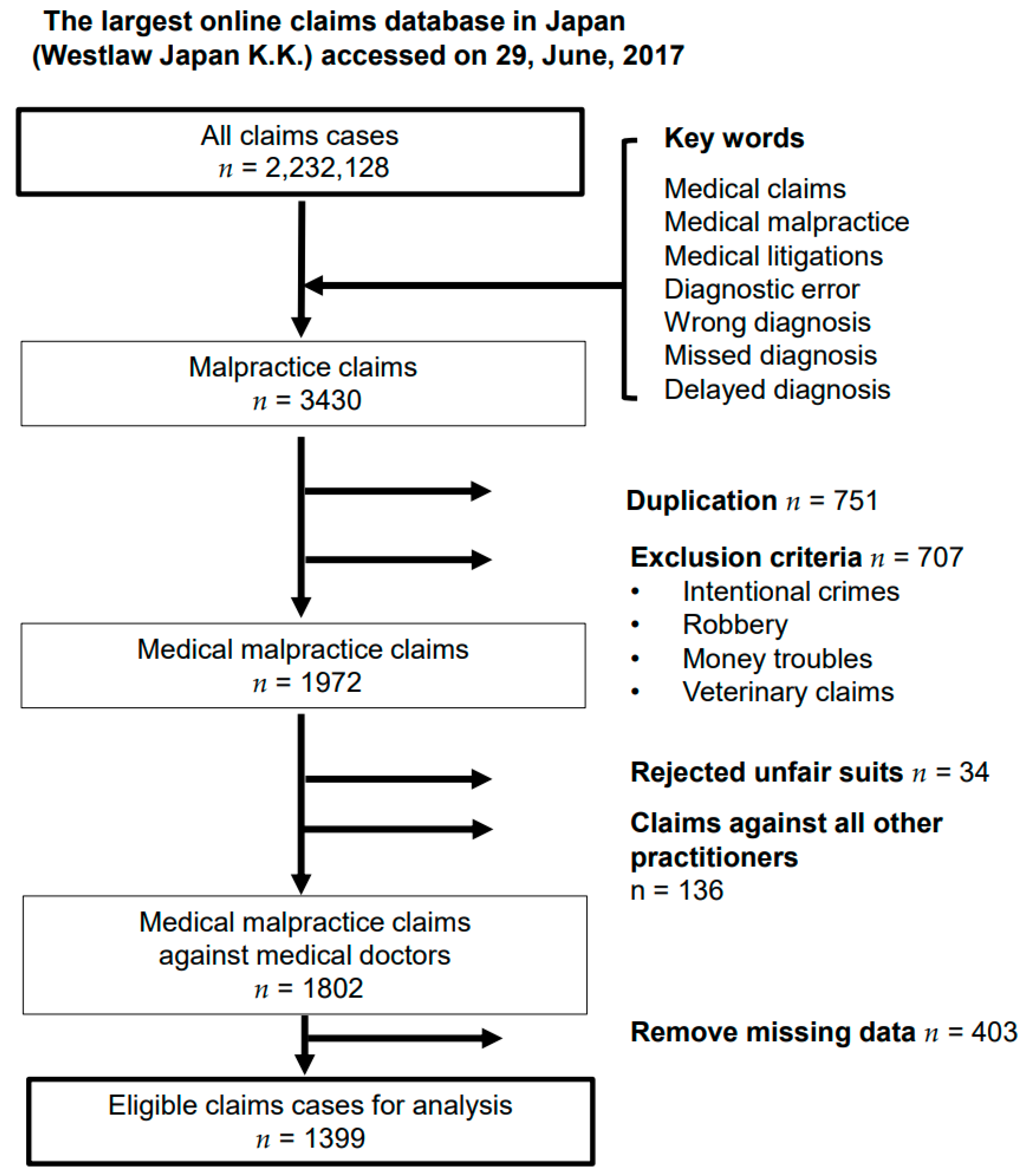
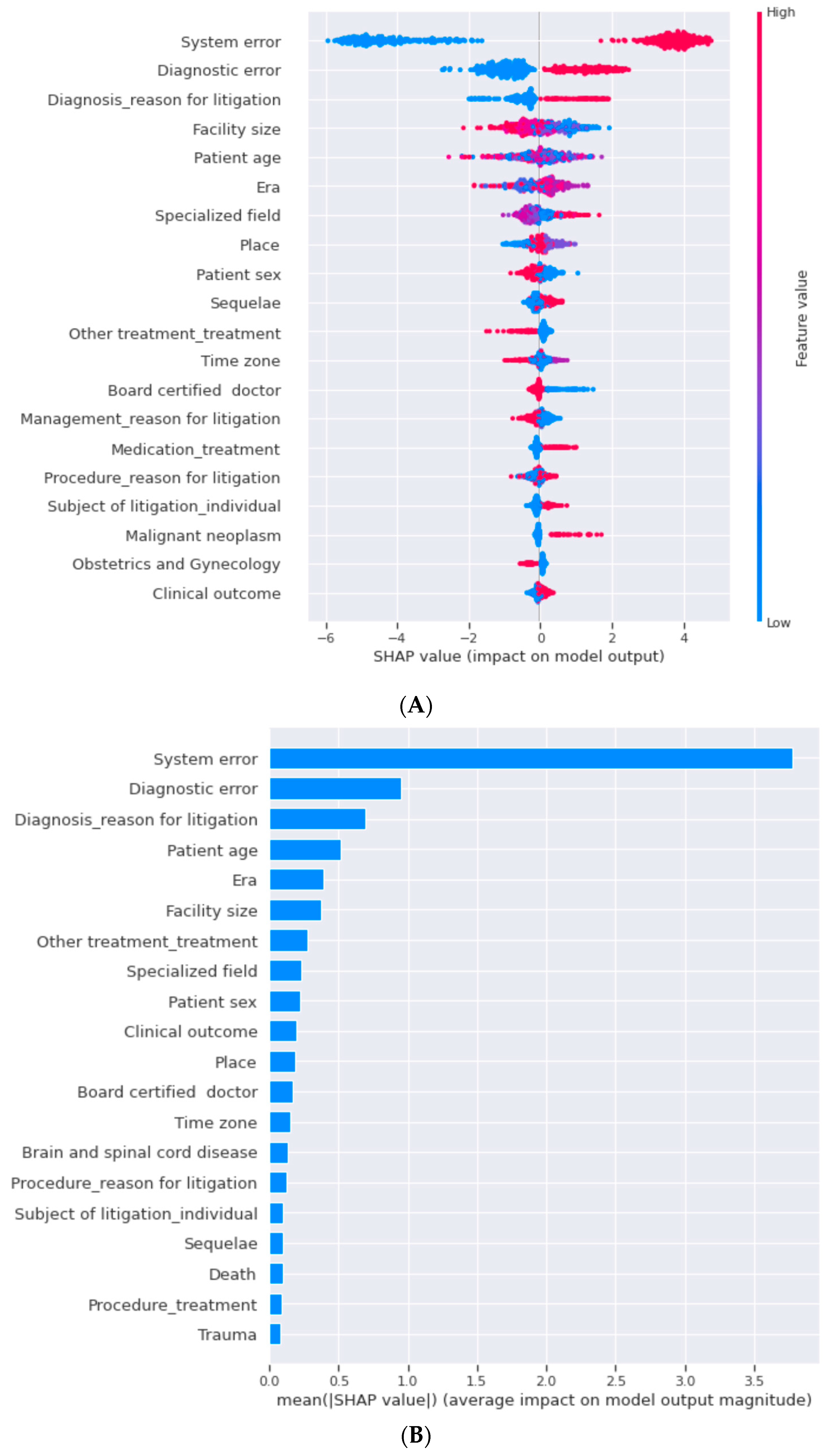
| Demographics/Characteristics | Reason for Litigation | |||
|---|---|---|---|---|
| without Procedures or Surgery | Procedures or Surgery | |||
| Total | Outpatient | Inpatient | ||
| (n = 1399) | (n = 368) | (n = 424) | (n = 583) | |
| Patient sex, male, n (%) | 736 (52.6) | 200 (53.4) | 237 (55.9) | 284 (48.7) |
| Patient age, median (IQR) | 33 (9–54) | 38 (20–54) | 27 (0–55) | 34 (6–52) |
| Adjusted total billing amount ($), median (IQR) | 460,149 (202,106–799,794) | 428,908 (194,291–716,004) | 485,750 (241,197–777,529) | 468,779 (191,367–877,166) |
| Subject of litigation, individual medical doctor, n (%) | 394 (28.2) | 137 (37.2) | 88 (20.8) | 162 (27.8) |
| Duration of claim (years), median (IQR) | 7 (5–10) | 6 (5–9) | 7 (5–11) | 7 (5–10) |
| Accepted claim, n (%) | 764 (54.6) | 196 (53.3) | 231 (54.5) | 327 (56.1) |
| Adjusted median indemnity ($), median (IQR) | 236,017 (56,784–504,513) | 157,069 (33,867–432,290) | 265,011 (72,347–532,206) | 220,008 (59,826–517,553) |
| Clinical outcome | ||||
| Deaths, n (%) | 785 (56.1) | 232 (63.0) | 261 (61.6) | 273 (46.8) |
| Sequelae, n (%) | 554 (39.6) | 113 (30.7) | 151 (35.6) | 286 (49.1) |
| Full recovery, n (%) | 60 (5.3) | 23 (6.3) | 12 (2.8) | 24 (4.1) |
| Accepted (n = 764) | Rejected (n = 635) | p-Value | ||
|---|---|---|---|---|
| Patient sex, male, n (%) | 395 (51.7) | 341 (53.7) | 0.485 | |
| Patient age, median (IQR) | 32 (11–53) | 34 (7.5–56) | 0.625 | |
| Initial diagnoses (Top 5 involved in malpractice claims), n (%) | ||||
| Malignant neoplasm (n = 115) | 60 (7.9) | 55 (8.7) | 0.625 | |
| Neonatal disease (n = 110) | 66 (8.6) | 44 (6.9) | 0.273 | |
| Trauma (n = 109) | 64 (8.4) | 45 (7.1) | 0.423 | |
| Procedure and postoperative complications (n = 67) | 42 (5.5) | 25 (3.9) | 0.208 | |
| Acute coronary syndrome (n = 66) | 37 (4.8) | 29 (4.6) | 0.899 | |
| Specialty, n (%) | ||||
| Surgical specialties | 439 (57.5) | 335 (52.8) | 0.084 | |
| Non-surgical specialties | 202 (26.4) | 202 (31.8) | 0.028 | |
| Place, n (%) | ||||
| Outpatient office | 145 (19.0) | 132 (20.8) | 0.419 | |
| Emergency room | 51 (6.7) | 40 (6.3) | 0.828 | |
| Ward | 231 (30.2) | 193 (30.4) | 0.953 | |
| Operation room | 327 (42.8) | 256 (40.3) | 0.355 | |
| Facility size, n (%) | ||||
| Clinic | 223 (29.2) | 137 (21.6) | 0.001 | |
| Small hospital (<200 beds) | 166 (21.7) | 110 (17.3) | 0.043 | |
| Medium hospital (200–399 beds) | 264 (34.6) | 243 (38.3) | 0.163 | |
| Large (>400 beds) or university hospital | 111 (14.5) | 145 (22.8) | <0.001 | |
| Time, n (%) | ||||
| Day time | 480 (62.8) | 379 (59.7) | 0.247 | |
| Night shift | 121 (15.8) | 83 (13.1) | 0.149 | |
| Error type, n (%) | ||||
| System error | 634 (83.0) | 127 (20.0) | <0.001 | |
| Diagnostic error | 377 (49.3) | 205 (32.3) | <0.001 | |
| Subject of litigation, n (%) | ||||
| Individual medical doctor | 238 (31.2) | 156 (24.6) | 0.007 | |
| Group or hospital | 548 (71.7) | 491 (77.3) | 0.02 | |
| Era * | 4/11/60/135/174/311/65/4 | 0/6/67/162/114/197/85/4 | NA | |
| Clinical outcome, n (%) | ||||
| Deaths | 411 (53.8) | 374 (58.9) | 0.058 | |
| Sequelae | 321 (42.0) | 233 (36.7) | 0.048 | |
| Full recovery | 32 (4.2) | 28 (4.4) | 0.895 | |
| LightGBM | Decision Tree | Random Forest | Logistic Model | ||||
|---|---|---|---|---|---|---|---|
| Accuracy | 0.839 | (0.838–0.841) | 0.825 | (0.823–0.826) | 0.832 | (0.831–0.834) | 0.826 (0.825–0.827) |
| Precision | 0.811 | (0.808–0.813) | 0.787 | (0.781–0.794) | 0.810 | (0.808–0.813) | 0.810 (0.809–0.811) |
| Recall | 0.924 | (0.819–0.928) | 0.935 | (0.920–0.950) | 0.907 | (0.901–0.913) | 0.893 (0.894–0.891) |
| F1 score | 0.863 | (0.864–0.862) | 0.853 | (0.850–0.856) | 0.855 | (0.853–0.857) | 0.849 (0.848–0.850) |
| AUC | 0.894 | (0.893–0.895) | 0.874 | (0.872–0.876) | 0.894 | (0.893–0.896) | 0.881 (0.881–0.882) |
| Factors | n (%) | Indemnity ($), Median (IQR) | Total Indemnity ($) | Proportion of All Total Indemnity in Each Group (%) |
|---|---|---|---|---|
| All Cases (n = 764) | ||||
| System error | 634 (82.9) | 212,971 (53,651–450,478) | 201,959,117 | 82.5 |
| Diagnostic error | 377 (49.3) | 248,534 (59,279–507,662) | 133,875,865 | 54.6 |
| Reason for litigation: diagnosis | 186 (24.3) | 202,639 (67,344–482,423) | 60,084,309 | 24.5 |
| Facility size (medium hospital) | 264 (34.5) | 237,931 (67,344–482,423) | 86,910,186 | 35.5 |
| Patient age (age 0) | 134 (17.5) | 349,625 (126,867–727, 673) | 59,320,694 | 24.2 |
| Subgroups | ||||
| Outpatient (n = 196) | ||||
| System error | 108 (55.1) | 82,920 (28,562–346,612) | 26,098,691 | 46.6 |
| Diagnostic error | 150 (76.5) | 206,364 (39,476–463,340) | 49,727,644 | 88.9 |
| Patient age (age 0) | 10 (5.1) | 95,216 (27,170–804,262) | 4,101,603 | 7.3 |
| Era (1991–1999) | 85 (43.3) | 184,002 (31,533–499,561) | 28,974,661 | 51.8 |
| Treatment: other treatments | 55 (28.0) | 59,279 (31,676–271,776) | 12,369,668 | 22.1 |
| Inpatient (n = 231) | ||||
| System error | 209 (90.4) | 242,469 (73,847–492,951) | 70,966,535 | 91.0 |
| Facility size (medium hospital) | 100 (43.2) | 269,137 (89,484–513,205) | 32,763,778 | 42.0 |
| Era (1991–1999) | 89 (38.5) | 300,128 (57,342–531,114) | 31,572,902 | 40.4 |
| Diagnostic error | 109 (47.1) | 297,139 (103,474–565,210) | 42,819,638 | 54.9 |
| Sequence | 138 (59.7) | 237,485 (62,423–408,069) | 39,358,055 | 50.4 |
| Procedures or surgery (n = 327) | ||||
| System error | 309 (94.4) | 206,791 (68,185–478,915) | 102,194,174 | 94.4 |
| Facility size (medium hospital) | 113 (34.5) | 254,086 (108,194–643,171) | 25,272,862 | 23.3 |
| Patient age (age 0) | 60 (18.3) | 481,070 (278,112–833,305) | 32,018,881 | 29.6 |
| Diagnostic error | 112 (34.2) | 249,226 (76,811–486,514) | 40,354,005 | 37.3 |
| Era (1991–1999) | 134 (40.9) | 283,772 (77,425–620,218) | 52,708,845 | 48.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, N.; Sukegawa, S.; Watari, T. Impact of System and Diagnostic Errors on Medical Litigation Outcomes: Machine Learning-Based Prediction Models. Healthcare 2022, 10, 892. https://doi.org/10.3390/healthcare10050892
Yamamoto N, Sukegawa S, Watari T. Impact of System and Diagnostic Errors on Medical Litigation Outcomes: Machine Learning-Based Prediction Models. Healthcare. 2022; 10(5):892. https://doi.org/10.3390/healthcare10050892
Chicago/Turabian StyleYamamoto, Norio, Shintaro Sukegawa, and Takashi Watari. 2022. "Impact of System and Diagnostic Errors on Medical Litigation Outcomes: Machine Learning-Based Prediction Models" Healthcare 10, no. 5: 892. https://doi.org/10.3390/healthcare10050892
APA StyleYamamoto, N., Sukegawa, S., & Watari, T. (2022). Impact of System and Diagnostic Errors on Medical Litigation Outcomes: Machine Learning-Based Prediction Models. Healthcare, 10(5), 892. https://doi.org/10.3390/healthcare10050892






