Effects of Regular Low-Level Alcohol Consumption in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Beverages
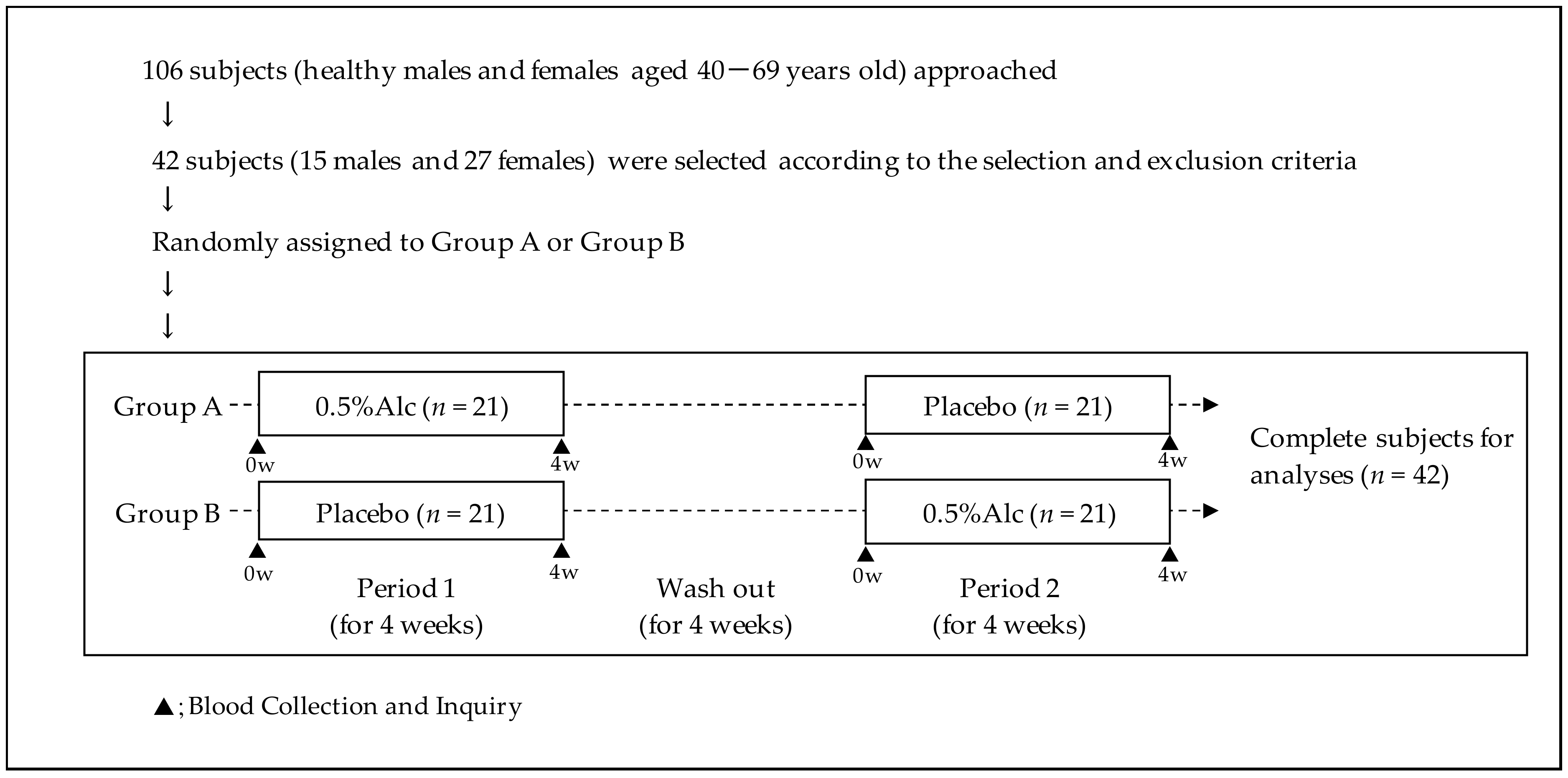
2.3. Study Design
2.4. Blood Analyses and Subjective Assessments
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shield, K.D.; Parry, C.; Rehm, J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2014, 35, 155–171. [Google Scholar]
- Holman, C.D.; English, D.R.; Milne, E.; Winter, M.G. Meta-analysis of alcohol and all-cause mortality: A validation of NHMRC recommendations. Med. J. Aust. 1996, 164, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Tsugane, S.; Fahey, M.T.; Sasaki, S.; Baba, S. Alcohol consumption and all-cause and cancer mortality among middle-aged Japanese men: Seven-year follow-up of the JPHC study Cohort I. Am. J. Epidemiol. 1999, 150, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.; Taylor, B.; Irving, H.; Roerecke, M.; Baliunas, D.; Mohapatra, S.; Rehm, J. Alcohol consumption and the risk of morbidity and mortality for different stroke types-a systematic review and meta-analysis. BMC Public Health 2010, 10, 258. [Google Scholar] [CrossRef]
- Ronksley, P.E.; Brien, S.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ 2011, 342, d671. [Google Scholar] [CrossRef]
- Nova, E.; Baccan, G.C.; Veses, A.; Zapatera, B.; Marcos, A. Potential health benefits of moderate alcohol consumption: Current perspectives in research. Proc. Nutr. Soc. 2012, 71, 307–315. [Google Scholar] [CrossRef]
- Boden, J.M.; Fergusson, D.M. Alcohol and depression. Addiction 2011, 106, 906–914. [Google Scholar] [CrossRef]
- Gea, A.; Beunza, J.J.; Estruch, R.; Sánchez-Villegas, A.; Salas-Salvadó, J.; Buil-Cosiales, P.; Gómez-Gracia, E.; Covas, M.-I.; Corella, D.; Fiol, M.; et al. Alcohol intake, wine consumption and the development of depression: The PREDIMED study. BMC Med. 2013, 11, 192. [Google Scholar] [CrossRef]
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.M.; Tymeson, H.D.; Venkateswaran, V.; Tapp, A.D.; Forouzanfar, M.H.; Salama, J.S.; et al. Alcohol use and burden for 195 countries and territories 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, S.; Lichtenstein, A.H.; Chen, S.; Na, M.; Veldheer, S.; Xing, A.; Wang, Y.; Wu, S.; et al. Alcohol consumption and risk of cardiovascular disease, cancer and mortality: A prospective cohort study. Nutr. J. 2021, 20, 13. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Costanzo, S.; Bonaccio, M.; McElduff, P.; Linneberg, A.; Salomaa, V.; Männistö, S.; Moitry, M.; Ferrières, J.; Dallongeville, J.; et al. Alcohol intake and total mortality in 142 960 individuals from the MORGAM Project: A population-based study. Addiction 2022, 117, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.G.; Agapito, V.V.; Obeso, A.; Prieto-Lloret, J.; Bustamante, R.; Castañeda, J.; Agapito, T.; Gonzalez, C. Moderate ethanol ingestion, redox status, and cardiovascular system in the rat. Alcohol 2011, 45, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Osaki, A.; Okazaki, Y.; Kimoto, A.; Izu, H.; Kato, N. Beneficial effect of low dose of ethanol on liver function and serum urate in rats fed a high-fat diet. J. Nutr. Sci. Vitaminol. 2014, 60, 408–412. [Google Scholar] [CrossRef]
- Kimoto, A.; Izu, H.; Fu, C.; Suidasari, S.; Kato, N. Effects of low dose of ethanol on the senescence score, brain function and gene expression in senescence-accelerated mice 8 (SAMP8). Exp. Ther. Med. 2017, 14, 1433–1440. [Google Scholar] [CrossRef]
- Yang, Y.; Takahara, K.; Kumrungsee, T.; Kimoto, A.; Shimamoto, F.; Kato, N. Consumption of Low-Dose of Ethanol Suppresses Colon Tumorigenesis in 1, 2-Dimethylhydrazine-Treated Rats. J. Nutr. Sci. Vitaminol. 2019, 65, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yang, Y.; Kumrungsee, T.; Kimoto, A.; Izu, H.; Kato, N. Low-dose ethanol has impacts on plasma levels of metabolites relating to chronic disease risk in SAMP8 mice. J. Nutr. Sci. Vitaminol. 2020, 66, 553–560. [Google Scholar] [CrossRef]
- Oshima, S.; Haseba, T.; Masuda, C.; Kakimi, E.; Sami, M.; Kanda, T.; Ohno, Y. Individual differences in blood alcohol concentrations after moderate drinking are mainly regulated by gastric emptying rate together with ethanol distribution volume. FNS 2012, 3, 732–737. [Google Scholar] [CrossRef][Green Version]
- Diener, E.; Chan, M.Y. Happy people live longer: Subjective well-being contributes to health and longevity. Appl. Psychol. Health Well-Being 2011, 3, 1–43. [Google Scholar] [CrossRef]
- Dupuy, H.J. Self-representations of general psychological well-being of American adults. In Proceedings of the Meeting of the American Public Health Association, Los Angeles, CA, USA, 16 October 1978. [Google Scholar]
- Nakayama, T.; Toyoda, H.; Ohno, K.; Yoshiike, N.; Futagami, T. Validity, reliability and acceptability of the Japanese version of the General Well-Being Schedule (GWBS). Qual. Life Res. 2000, 9, 529–539. [Google Scholar] [CrossRef]
- Nakayama, T. Validity, reliability and acceptability of the Japanese version of the General Well-Being Schedule. J. Health Welfare Stat. (Kousei no Shihyou) 2002, 49, 8–18. (In Japanese) [Google Scholar]
- Goldberg, D.P.; Blackwell, B. Psychiatric illness in general practice: A detailed study using a new method of case identification. Br. Med. J. 1970, 2, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Ohkawa, M. Development of the Japanese version of the Pittsburgh Sleep Quality Index. Jpn. J. Psychiatry Treat. (Seishinka Chiryogaku) 1998, 13, 755–763. (In Japanese) [Google Scholar]
- Lelbach, W.K. Liver damage in chronic alcoholism: Results of a clinical, clinical-chemical and bioptic-histological study in 526 alcoholic patients during a low-calorie diet in an open drinking sanatorium. Acta Hepatosplenol. 1966, 13, 321–349. [Google Scholar]
- Arteel, G.E. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 2003, 124, 778–790. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Rad. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef]
- Harjumäki, R.; Pridgeon, C.S.; Ingelman-Sundberg, M. CYP2E1 in alcoholic and non-alcoholic liver injury. Roles of ROS, reactive intermediates and lipid overload. Int. J. Mol. Sci. 2021, 22, 8221. [Google Scholar] [CrossRef]
- Nieradko-Iwanicka, B. The role of alcohol consumption in pathogenesis of gout. Crit. Rev. Food Sci. Nutr. 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef]
- Isakadze, N.; Soliman, E.Z.; Vaccarino, V.; Whang, W.; Lampert, R.; Bremner, J.D.; Shah, A.J. Association of positive well-being with reduced cardiac repolarization abnormalities in the First National Health and Nutrition Examination Survey. Int. J. Cardiol. 2018, 265, 246–250. [Google Scholar] [CrossRef]
- Diener, E.; Lucas, R.E.; Oishi, S.; Hall, N.; Donnellan, M.B. Advances and open questions in the science of subjective well-being. Collabra Psychol. 2018, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Alexander, G.; Berman, N.; Salehian, B.; Davidson, T.; McDonald, V.; Steiner, B.; Hull, L.; Callegari, C.; Swerdloff, R.S. Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. J. Clin. Endocrinol. Metab. 1996, 81, 3578–3583. [Google Scholar] [PubMed]
- Akerman, J.; Kovac, J.R.; Lipshultz, L.I. Testosterone therapy improves well being and psychological health. Curr. Opin. Urol. 2017, 27, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.J.; Nolan, J.J.; Nelson, J.C.; Yen, S.S. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J. Clin. Endocrinol. Metab. 1994, 78, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Brooke, A.M.; Kalingag, L.A.; Miraki-Moud, F.; Camacho-Hubner, C.; Maher, K.T.; Walker, D.M.; Hinson, J.P.; Monson, J.P. Dehydroepiandrosterone improves psychological well-being in male and female hypopituitary patients on maintenance growth hormone replacement. J. Clin. Endocrinol. Metab. 2006, 91, 3773–3779. [Google Scholar] [CrossRef]
- Stafford, M.; Ben-Shlomo, Y.; Cooper, C.; Gale, C.; Gardner, M.P.; Geoffroy, M.C.; Power, C.; Kuh, D.; Cooper, R. Diurnal cortisol and mental well-being in middle and older age: Evidence from four cohort studies. BMJ Open 2017, 7, e016085. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.C.; Glasper, E.R.; Detillion, C.E. Social modulation of stress responses. Physiol. Behav. 2003, 79, 399–407. [Google Scholar] [CrossRef]
- Ylikahri, R.H.; Huttunen, M.O.; Härkönen, M. Hormonal changes during alcohol intoxication and withdrawal. Pharmacol. Biochem. Behav. 1980, 13, 131–137. [Google Scholar] [CrossRef]
- Sarkola, T.; Eriksson, C.P. Testosterone increases in men after a low dose of alcohol. Alcohol. Clin. Exp. Res. 2003, 27, 682–685. [Google Scholar] [CrossRef]
- Eriksson, C.J.; Fukunaga, T.; Lindman, R. Sex hormone response to alcohol. Nature 1994, 369, 711. [Google Scholar] [CrossRef]
- Zakhari, S. Alcohol metabolism and epigenetics changes. Alcohol Res. 2013, 35, 6–16. [Google Scholar] [PubMed]
- Orpana, A.K.; Orava, M.M.; Vihko, R.K.; Härkönen, M.; Eriksson, C.P. Ethanol-induced inhibition of testosterone biosynthesis in rat Leydig cells: Role of L-glutamate and pyruvate. J. Steroid Biochem. 1990, 36, 473–478. [Google Scholar] [CrossRef]
- Bolour, S.; Braunstein, G. Testosterone therapy in women: A review. Int. J. Impot. Res. 2005, 17, 399–408. [Google Scholar] [CrossRef]
- Miller, M.B.; Freeman, L.; Curtis, A.F.; Boissoneault, J.; McCrae, C.S. Sleep Health and Alcohol Use. Neurol. Modul. Sleep 2020, 27, 255–264. [Google Scholar] [CrossRef]
- Omachi, T.A. Measuring sleep in rheumatologic diseases: The ESS, FOSQ, ISI, and PSQI. Arthritis Care Res. 2011, 63, S287–S296. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Mean (SD) |
|---|---|
| Age (y) | 52.3 (7.3) |
| Male, n = 15 | 53.1 (7.9) |
| Female, n = 27 | 51.8 (7.0) |
| Height (cm) | 164.3 (8.9) |
| Male, n = 15 | 173.1 (6.5) |
| Female, n = 27 | 159.4 (5.7) |
| Body weight (kg) | 55.3 (8.5) |
| Male, n = 15 | 62.2 (7.8) |
| Female, n = 27 | 51.5 (6.2) |
| Body mass index (kg/m2) | 20.4 (2.2) |
| Male, n = 15 | 20.7 (2.1) |
| Female, n = 27 | 20.2 (2.2) |
| Systolic blood pressure (mmHg) | 113 (16) |
| Diastolic blood pressure (mmHg) | 75 (9) |
| Hemoglobin (g/dL) | 13.7 (1.3) |
| Hematocrit (%) | 42.4 (3.6) |
| Platelet count (104/μL) | 24.1 (5.5) |
| White blood cell count (104/μL) | 5269 (1306) |
| Red blood cell count (104/μL) | 448 (34) |
| Parameter | Study Group | Before Consumption | After 4 Weeks | p a | Change from | p b |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| AST (IU) | Placebo, n = 41 | 21 (5) | 21 (5) | 0.965 | −0 (4) | 0.059 † |
| 0.5%Alc, n = 41 | 22 (9) | 20 (5) | 0.034 * | −2 (6) | ||
| ALT (U/L) | Placebo, n = 41 | 19 (10) | 19 (11) | 0.838 | −0 (7) | 0.064 † |
| 0.5%Alc, n = 41 | 22 (17) | 18 (10) | 0.033 * | −4 (10) | ||
| LDH (U/L) | Placebo, n = 41 | 173 (26) | 172 (23) | 0.576 | −1 (14) | 0.340 |
| 0.5%Alc, n = 41 | 173 (27) | 169 (25) | 0.013 * | −4 (10) | ||
| GGT (U/L) | Placebo, n = 41 | 21 (11) | 20 (9) | 0.400 | −1 (6) | 0.718 |
| 0.5%Alc, n = 41 | 22 (14) | 22 (12) | 0.453 | −1 (5) | ||
| ALP (U/L) | Placebo, n = 41 | 72 (20) | 71 (20) | 0.154 | −1 (6) | 0.270 |
| 0.5%Alc, n = 41 | 72 (21) | 73 (22) | 0.603 | 1 (10) | ||
| Total bilirubin (mg/dL) | Placebo, n = 41 | 0.7 (0.3) | 0.7 (0.2) | 0.704 | −0.0 (0.2) | 0.184 |
| 0.5%Alc, n = 41 | 0.7 (0.3) | 0.6 (0.2) | 0.130 | −0.1 (0.2) | ||
| Creatinine (mg/dL) | Placebo, n = 41 | 0.7 (0.2) | 0.7 (0.2) | 0.140 | −0.0 (0.0) | 0.322 |
| 0.5%Alc, n = 41 | 0.7 (0.2) | 0.7 (0.1) | 0.944 | −0.0 (0.0) | ||
| BUN (mg/dL) | Placebo, n = 41 | 13.9 (2.9) | 13.4 (3.5) | 0.264 | −0.5 (2.9) | 0.150 |
| 0.5%Alc, n = 41 | 13.7 (3.2) | 14.2 (3.3) | 0.301 | 0.6 (3.4) | ||
| Glucose (mg/dL) | Placebo, n = 41 | 88 (7) | 89 (7) | 0.886 | 0 (8) | 0.837 |
| 0.5%Alc, n = 41 | 89 (6) | 90 (9) | 0.661 | 1 (8) | ||
| LDL-C (mg/dL) | Placebo, n = 41 | 125 (23) | 122 (20) | 0.179 | −3 (13) | 0.113 |
| 0.5%Alc, n = 41 | 120 (22) | 123 (20) | 0.237 | 2 (12) | ||
| HDL-C (mg/dL) | Placebo, n = 41 | 71 (14) | 72 (14) | 0.703 | 0 (6) | 0.276 |
| 0.5%Alc, n = 41 | 69 (13) | 71 (15) | 0.077 † | 2 (7) | ||
| Triglyceride (mg/dL) | Placebo, n = 41 | 73 (33) | 72 (28) | 0.722 | −1 (21) | 0.416 |
| 0.5%Alc, n = 41 | 77 (37) | 72 (41) | 0.235 | −5 (27) | ||
| Uric acid (mg/dL) | Placebo, n = 41 | 4.7 (1.1) | 4.7 (1.1) | 0.740 | 0.0 (0.5) | 0.303 |
| 0.5%Alc, n = 41 | 4.6 (1.3) | 4.8 (1.3) | 0.062 † | 0.2 (0.5) | ||
| Total protein (g/dL) | Placebo, n = 41 | 7.3 (0.4) | 7.1 (0.4) | 0.006 * | −0.1 (0.3) | 0.283 |
| 0.5%Alc, n = 41 | 7.2 (0.3) | 7.1 (0.3) | 0.116 | −0.1 (0.2) | ||
| Albumin (g/dL) | Placebo, n = 41 | 4.4 (0.3) | 4.4 (0.3) | 0.474 | −0.0 (0.2) | 0.700 |
| 0.5%Alc, n = 41 | 4.4 (0.3) | 4.3 (0.2) | 0.183 | −0.0 (0.2) |
| Subjective | Study Group | Before Consumption | After 4 Weeks | Change from | p a |
|---|---|---|---|---|---|
| Parameter | Baseline | ||||
| GWBS | Placebo, n = 42 | 54.5 (13.5) | 67.0 (17.5) | 12.0 (13.0) | 0.206 |
| 0.5%Alc, n = 42 | 55.0 (19.5) | 70.0 (14.8) | 15.0 (16.5) | ||
| Stratified analyses | |||||
| Placebo, n = 30 | 53.0 (11.8) | 62.5 (10.5) | 11.5 (11.8) | 0.041 * | |
| 0.5%Alc, n = 30 | 49.5 (15.0) | 67.0 (16.8) | 16.0 (19.5) | ||
| PSQI | Placebo, n = 42 | 5.0 (4.5) | 4.0 (3.0) | −1.0 (2.0) | 1.000 |
| 0.5%Alc, n = 42 | 5.0 (5.0) | 4.0 (3.0) | −1.0 (1.8) | ||
| Parameter | Study Group | Before Consumption | After 4 Weeks | Change from | p a |
|---|---|---|---|---|---|
| Baseline | |||||
| Free testosterone (pg/mL) | Placebo, n = 41 | 4.8 (5.7) | 4.9 (6.0) | 0.14 (2.0) | 0.973 |
| 0.5%Alc, n = 41 | 4.6 (5.3) | 4.9 (5.7) | 0.15 (1.8) | ||
| Stratified analyses | |||||
| Placebo, n = 30 | 4.8 (6.0) | 4.5 (5.7) | −0.4 (1.2) | 0.051 † | |
| 0.5%Alc, n = 30 | 4.3 (5.3) | 4.7 (5.8) | 0.2 (2.0) | ||
| DHEA-S (μg/dL) | Placebo, n = 41 | 129 (60) | 126 (63) | −3 (25) | 0.903 |
| 0.5%Alc, n = 41 | 127 (59) | 123 (58) | −4 (31) | ||
| Stratified analyses | |||||
| Placebo, n = 30 | 118 (62) | 110 (59) | −8 (23) | 0.579 | |
| 0.5%Alc, n = 30 | 111 (54) | 107 (54) | −4 (30) | ||
| Cortisol (μg/dL) | Placebo, n = 41 | 7.4 (1.8) | 7.0 (2.4) | −0.3 (2.5) | 0.131 |
| 0.5%Alc, n = 41 | 7.0 (1.8) | 7.5 (2.6) | 0.5 (2.6) | ||
| Stratified analyses | |||||
| Placebo, n = 30 | 7.4 (1.9) | 7.1 (2.5) | −0.3 (2.5) | 0.142 | |
| 0.5%Alc, n = 30 | 6.7 (1.5) | 7.4 (2.7) | 0.6 (2.4) | ||
| ACTH (pg/mL) | Placebo, n = 41 | 19.9 (11.9) | 19.6 (11.5) | −0.3 (8.2) | 0.687 |
| 0.5%Alc, n = 41 | 19.5 (11.0) | 19.8 (12.1) | 0.3 (5.3) | ||
| Stratified analyses | |||||
| Placebo, n = 30 | 19.6 (12.1) | 19.8 (12.7) | 0.2 (8.6) | 0.872 | |
| 0.5%Alc, n = 30 | 17.9 (10.8) | 18.6 (11.8) | 0.4 (4.2) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshima, S.; Shiiya, S.; Kato, Y. Effects of Regular Low-Level Alcohol Consumption in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Healthcare 2022, 10, 882. https://doi.org/10.3390/healthcare10050882
Oshima S, Shiiya S, Kato Y. Effects of Regular Low-Level Alcohol Consumption in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Healthcare. 2022; 10(5):882. https://doi.org/10.3390/healthcare10050882
Chicago/Turabian StyleOshima, Shunji, Sachie Shiiya, and Yasuhito Kato. 2022. "Effects of Regular Low-Level Alcohol Consumption in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Crossover Study" Healthcare 10, no. 5: 882. https://doi.org/10.3390/healthcare10050882
APA StyleOshima, S., Shiiya, S., & Kato, Y. (2022). Effects of Regular Low-Level Alcohol Consumption in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Healthcare, 10(5), 882. https://doi.org/10.3390/healthcare10050882






