Oral Hygiene and Periodontal Treatment Needs in Adult Patients with Cystic Fibrosis (CF)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. An Assessment of Oral Hygiene
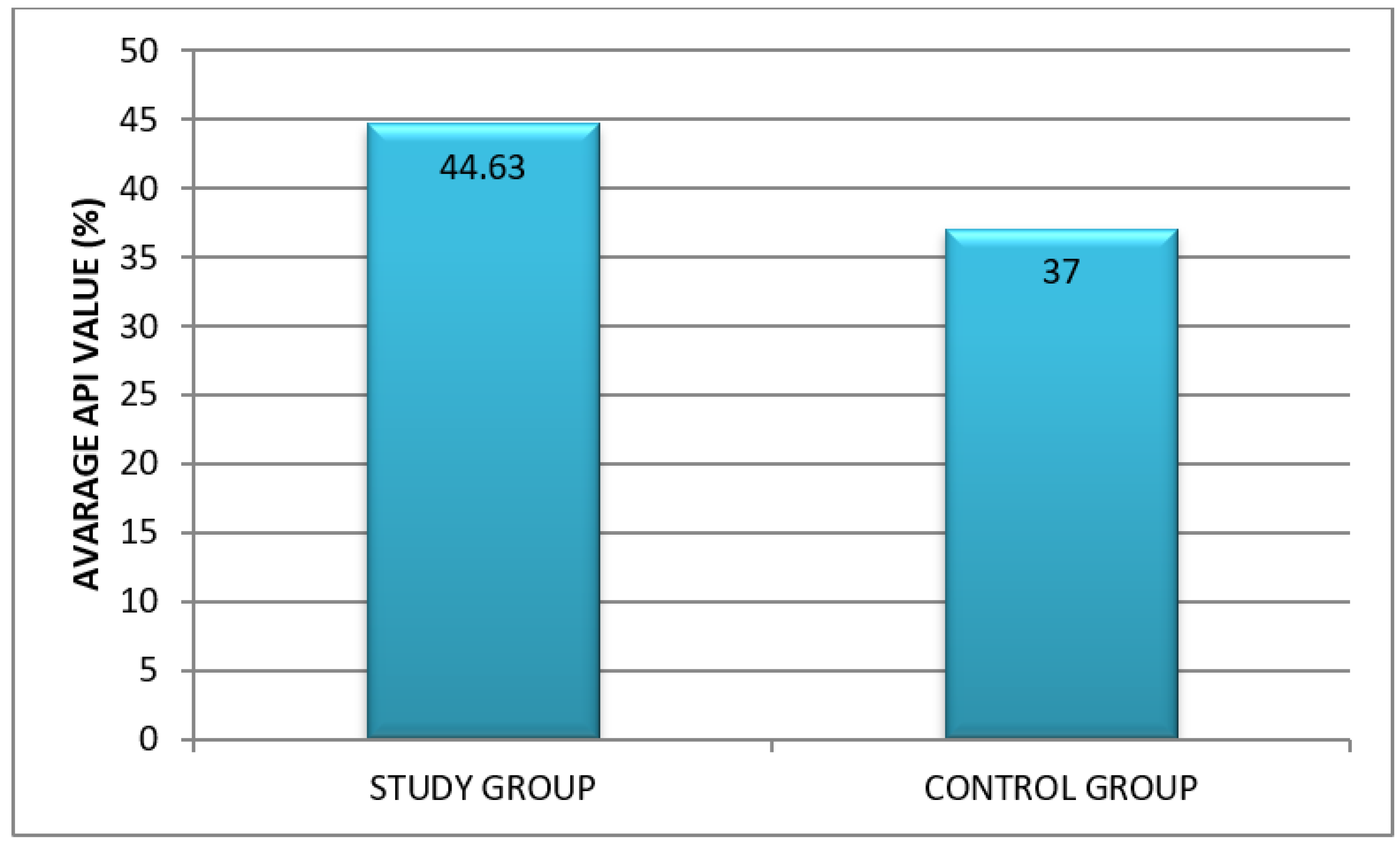
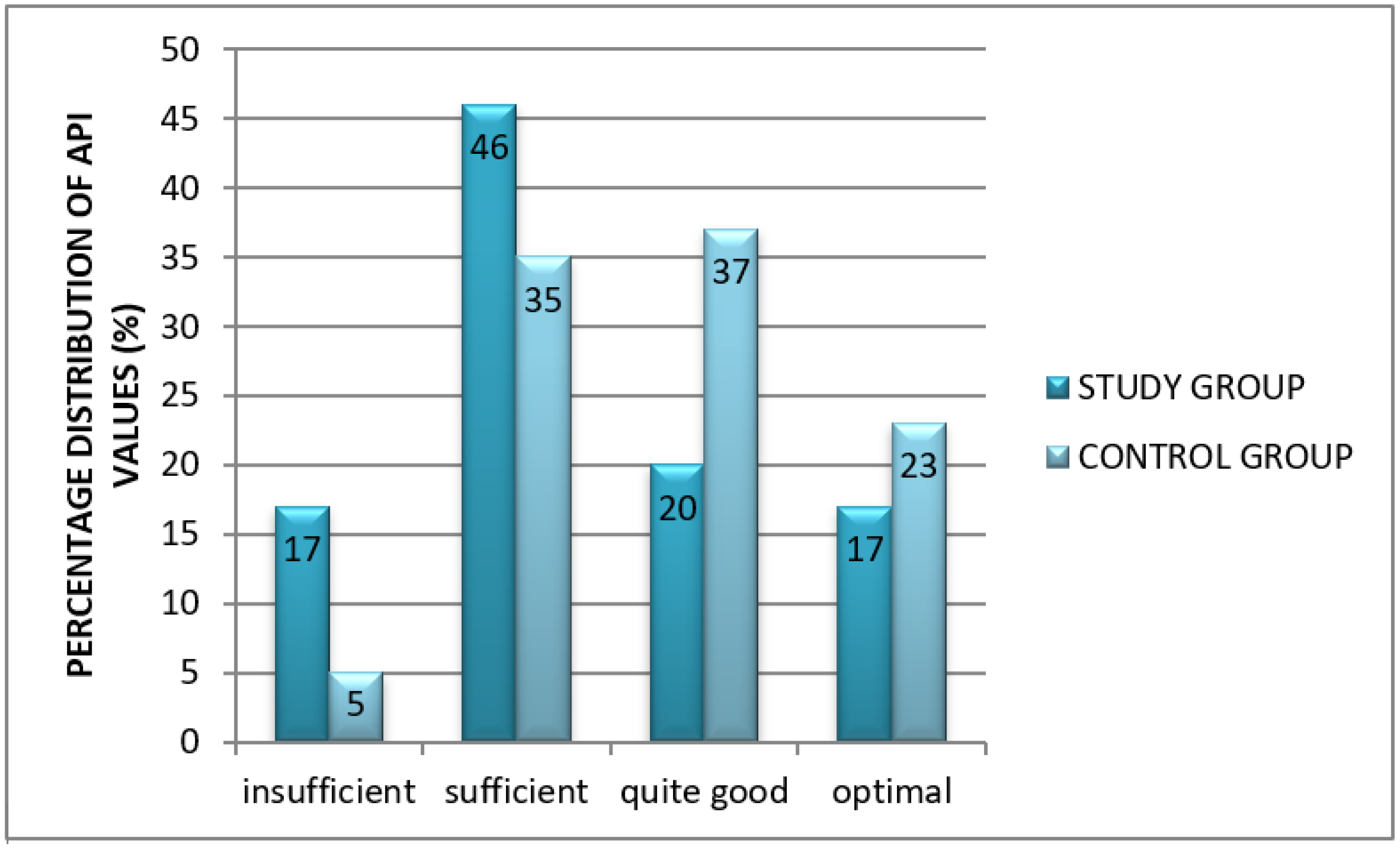
- Approximal Plaque Index (API) according to Lange. It allows for the assessment of oral hygiene in a large population and is useful in the comparative analysis against other clinical parameters. After the inspection of dental plaque using a probe, interdental hygiene was assessed. In quadrants 1 and 3, the presence of bacterial plaque was verified from the aspect of the oral cavity proper, while in quadrants 2 and 4—from the vestibular aspect. The sum of recorded interdental spaces with plaque (“+”) was divided by the sum of all inspected spaces and multiplied by 100 to obtain percentage values.API interpretation:
- 100–70%: insufficient (poor) oral hygiene;
- 70–40%: sufficient oral hygiene; improvement of oral hygiene is needed; 39–25%: quite good hygiene
- <25%: optimal conditions for protection against dental caries and periodontal diseases [12].
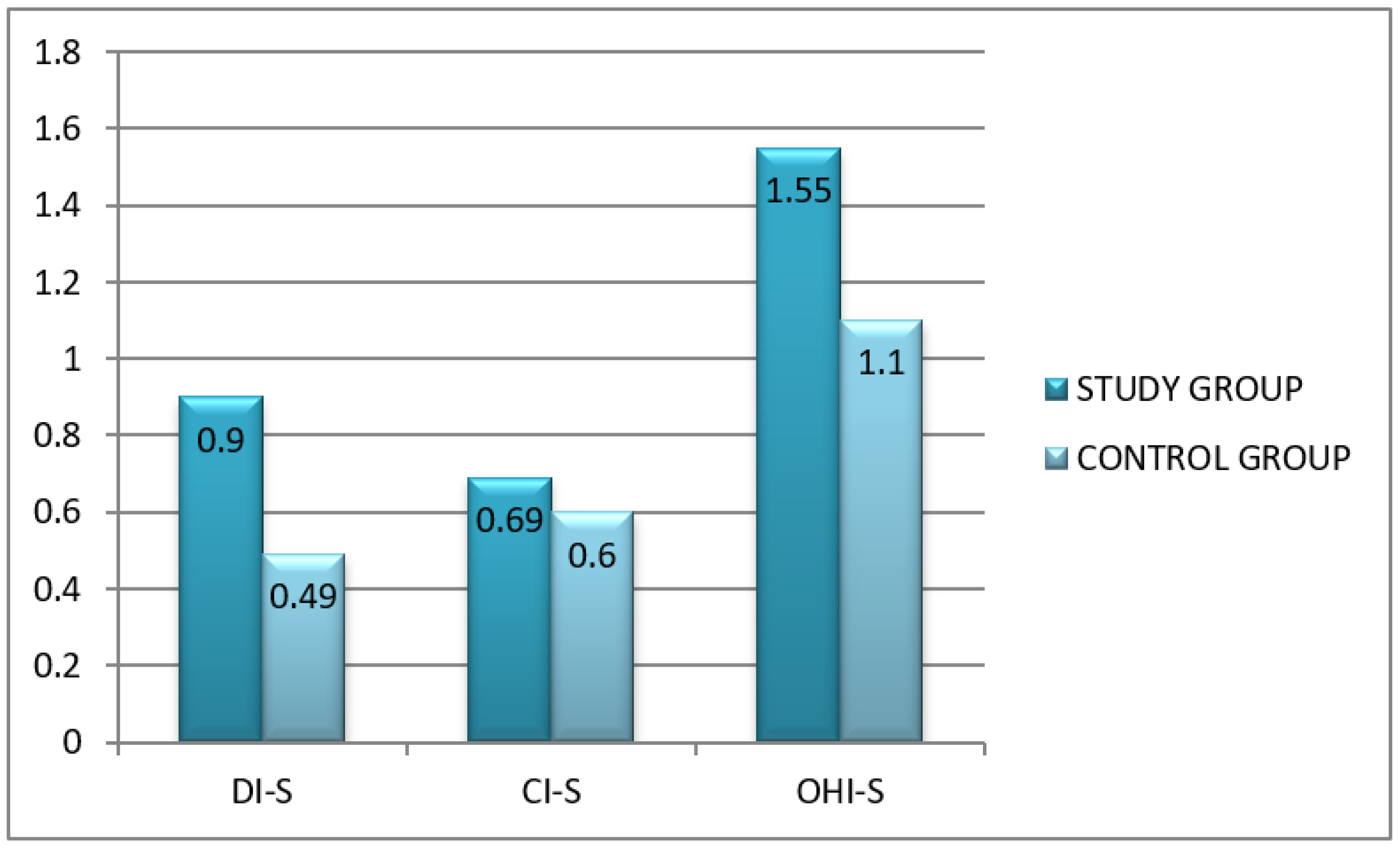
- The modified OHI-S according to Green and Vermillion (Simplified Oral Hygiene Index). This index determines both the amount (deposition rate) as well as the type of dental deposits as it consists of a simplified debris index (DI-S) and simplified calculus index (CI-S). Debris and calculus are assessed on 6 dental surfaces (6 teeth):
- -
- buccal surface of teeth 16 and 26
- -
- lingual surface of teeth 36 and 46
- -
- labial surface of tooth 11
- -
- lingual surface of tooth 31.
Criteria for classifying debris and calculus are the same:- 0—No debris or calculus present.
- 1—Supragingival debris or calculus covering not more than one third of the exposed tooth surface.
- 2—Supragingival debris or calculus covering more than one third but not more than two thirds of the exposed tooth surface or the presence of individual flecks of subgingival calculus.
- 3—Supragingival calculus covering more than two third of the exposed tooth surface or a continuous heavy band of subgingival calculus.
- 0—excellent hygiene
- 0.1–1.2—good hygiene
- 1.3–3.0—regular hygiene
- 3.1–6.0—poor hygiene [12].
2.2.2. Questionnaire
- When have you started to take care of oral cavity hygiene? (as a teenager, in my 20’s, in my 30’s, after 30 years of age)
- How long does it take you to brush your teeth? (1 min, 2 min, more than 2 min, I don’t know)
- What kind of toothbrush do you use? (ultra-soft, soft, medium, hard, electronic, manual)
- What type of the toothpaste do you use? (with fluoride in concentration 1000–1400 ppm, herbal, whitening, for dentinal hypersensitivity)
- Do you use any other additional products to maintain proper oral hygiene? (dental floss, toothpick, chewing gum, none)
- Do you use mouthwash? (yes, no)
- Do you brush your tongue? (yes, no)
- How often do you visit a dentist? (very rarely, only in case of pain, every 3–6 months)
- The Community Periodontal Index of Treatment Needs (CPITN) according to Ainamo. The examination was performed using a calibrated probe (WHO 621) with a blunt tip end allowing for the assessment of the depth of gingival crevices and pockets without traumatizing effects on the periodontium. The examination was performed in sextants, which correspond to the following dental segments: 17–14, 13–23, 24–27, 37–34, 33–43, 44–47. At least 2 teeth must be present in a sextant for it to be scored. If only 1 tooth is present in a sextant, it is included in the adjoining sextant.The clinical description of CPI codes is as follows:
- 0—Healthy;
- 1—Bleeding on probing;
- 2—Calculus detected or conditions promoting plaque retention, pockets < 3 mm;
- 3—Pockets 3.5–5.5 mm
- 4—Pockets > 6 mm.
Management according to the TN code:- CPI-0: TN-0 home care;
- CPI-1: TN-1 instructions on proper oral hygiene;
- CPI-2, CPI-3: TN-2 instructions on proper oral hygiene, professional scaling;
- CPI-4: TN-3 complex periodontal treatment [13].
2.2.3. Statistical Analysis
3. Results
3.1. General Characteristics of Study Participants
3.2. An Assessment of Oral Hygiene in Adult CF Patients
Oral Hygiene Indices
3.3. Questionnaire Results
3.4. Community Periodontal Index of Treatment Needs (CPITN)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcinkowski, A.; Ziebolz, M.; Kleibrink, B.E.; Weinreich, G.; Kamler, M.; Teschler, H.; Sommerwerck, U. Deficits in oral health behavior and oral health status in patients after lung transplantation. Clin. Respir. J. 2018, 12, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ning, W.; Wang, W.; Ziebolz, D.; Acharya, A.; Schmalz, G.; Zhao, J.; Huang, S.; Xiao, H. Oral Health-Related Quality of Life in Patients With Chronic Respiratory Diseases—Results of a Systematic Review. Front. Med. 2021, 8, 757739. [Google Scholar] [CrossRef] [PubMed]
- Herman, K.; Kowalczyk-Zając, M.; Pytrus, T. Oral cavity health among cystic fibrosis patients: Literature overview. Adv. Clin. Exp. Med. 2017, 26, 1147–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitch, A.E.; Rodgers, H.C. Cystic fibrosis. J. R. Coll. Physicians Edinb. 2013, 43, 144–150. [Google Scholar] [CrossRef]
- Dąbrowska, E.; Błahuszewska, K.; Minarowska, A.; Kaczmarski, M.; Niedźwiecka-Andrzejewicz, I.; Stokowska, W. Assessment of dental status and oral hygiene in the study population of cystic fibrosis patients in the Podlasie province. Adv. Med. Sci. 2006, 51, 100–103. [Google Scholar] [PubMed]
- Peker, S.; Mete, S.; Gokdemir, Y.; Karadag, B.; Kargul, B. Related factors of dental caries and molar incisor hypomineralisation in a group of children with cystic fibrosis. Eur. Arch. Paediatr. Den. 2014, 15, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Primosch, R.E. Tetracycline discoloration, enamel defects, and dental caries in patients with cystic fibrosis. Oral Surg. Oral Med. Oral Pathol. 1980, 50, 301–308. [Google Scholar] [CrossRef]
- Coffey, N.; O’Leary, F.; Burke, F.; Roberts, A.; Hayes, M. Periodontal and oral health status of people with Cystic Fibrosis: A systematic review. J. Dent. 2020, 103, 103509. [Google Scholar] [CrossRef]
- Narang, A.; Maguire, A.; Nunn, J.H.; Bush, A. Oral health and related factors in cystic fibrosis and other chronic respiratory disorders. Arch. Dis. Child. 2003, 88, 702–707. [Google Scholar] [CrossRef] [Green Version]
- Weerheijm, K.L.; Jalevik, B.; Alaluusua, S. Molar-incisor hypomineralisation. Caries Res. 2001, 35, 390–391. [Google Scholar] [CrossRef]
- Hildebrandt, T.; Zawilska, A.; Trzcionka, A.; Tanasiewicz, M.; Mazurek, H.; Świętochowska, E. Estimation of Proinflammatory Factors in the Saliva of Adult Patients with Cystic Fibrosis and Dental Caries. Medicina 2020, 56, 612. [Google Scholar] [CrossRef]
- Trzcionka, A.; Twardawa, H.; Mocny-Pachońska, K.; Tanasiewicz, A. Oral cavity status of long-term hemodialized patients vs. their socio-economic status. Med. Pr. 2020, 71, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Katarzyńska-Konwa, M.; Obersztyn, I.; Trzcionka, A.; Mocny-Pachońska, K.; Mosler, B.; Tanasiewicz, M. Oral status in pregnant women from post-industrial areas of upper Silesia in reference to occurrence of: Preterm labors, low birth weight and type of labor. Healthcare 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk-Kamieńska, T.; Borysewicz-Lewicka, M.; Śniatała, R.; Batura-Gabryel, H. Clinical evaluation of dental hard tissues in an adult population with cystic fibrosis. Pol. Arch. Intern. Med. 2019, 129, 725–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlaczyk-Kamieńska, T.; Śniatała, R.; Batura-Gabryel, H.; Borysewicz-Lewicka, M.; Cofta, S. Periodontal Status and Subgingival Biofilms in Cystic Fibrosis Adults. Pol. J. Microbiol. 2019, 68, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aps, J.K.; Van Maele, G.O.; Martens, L.C. Oral hygiene habits and oral health in cystic fibrosis. Eur. J. Paediatr. Dent. 2002, 3, 181–187. [Google Scholar]
- Aps, J.K.; Van Maele, G.O.; Martens, L.C. Caries experience and oral cleanliness in cystic fibrosis homozygotes and heterozygotes. Oral Surg. Oral Med. Oral Pathol. 2002, 93, 560–563. [Google Scholar] [CrossRef]
- Banach, J. Stan przyzębia oraz periodontologiczne potrzeby lecznicze dzieci i młodzieży w Polsce w latach 1987–1995. Przegl. Stomat. Wieku Rozw. 1996, 14, 34–38. [Google Scholar]
- Olejniczak, M.; Wierchoła, B.; Emerlich-Poplatek, K.; Adamowicz-Klepalska, B. Ekosystem jamy ustnej u chorych na mukowiscydozę, a stan uzębienia i stomatologiczne potrzeby lecznicze. Dent. Med. Probl. 2003, 40, 337–347. [Google Scholar]
- Olejniczak, M.; Emerlich-Poplatek, K.; Wierchoła, B.; Adamowicz-Klepalska, B. Stan przyzębia i periodontologiczne potrzeby lecznicze u chorych na mukowiscydozę. Dent. Med. Probl 2004, 41, 461–467. [Google Scholar]
- Page, R.C.; Eke, P. Case Definitions for Use in population-Based Surveillance of Periodontitis. J. Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef] [Green Version]
- Smyth, R.L.; Walters, S. Oral calorie supplements for cystic fibrosis. Cochrane Database Syst. Rev. 2012, 17, CD000406. [Google Scholar]
- Wotman, S.S. The occurrence of calculus in normal children, children with cystic fibrosis, and children with asthma. J. Periodontol. 1973, 44, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Kinirons, M.J. Increased salivary buffering in association with a low caries experience in children suffering from cystic fibrosis. J. Dent. Res. 1983, 62, 815–817. [Google Scholar] [CrossRef] [PubMed]
| Question | Answer | Group | χ2 | p | ||||
|---|---|---|---|---|---|---|---|---|
| Study | Control | |||||||
| N | % | N | % | |||||
| When have you started to take care of oral cavity hygiene | as a teenager | 33 | 82.5% | 31 | 77.5% | 0.313 | 0.576 | |
| in my 20’s | 7 | 17.5% | 9 | 22.5% | ||||
| How long does it take you to brush your teeth? | 1 min | 5 | 12.5% | 6 | 15,0% | 0.808 | 0.668 | |
| 2 min | 20 | 50.0% | 16 | 40.0% | ||||
| more than 2 min | 15 | 37.5% | 18 | 45.0% | ||||
| What kind of toothbrush do you use? | electronic | no | 35 | 87.5% | 32 | 80.0% | 0.827 | 0.363 |
| yes | 5 | 12.5% | 8 | 20.0% | ||||
| manual | no | 5 | 12.5% | 8 | 20.0% | 0.827 | 0.363 | |
| yes | 35 | 87.5% | 32 | 80.0% | ||||
| hard | no | 33 | 82.5% | 32 | 80.0% | 0.082 | 0.775 | |
| yes | 7 | 17.5% | 8 | 20.0% | ||||
| medium | no | 15 | 37.5% | 19 | 47.5% | 0.818 | 0.366 | |
| yes | 25 | 62.5% | 21 | 52.5% | ||||
| soft | no | 32 | 80.0% | 29 | 72.5% | 0.621 | 0.431 | |
| yes | 8 | 20.0% | 11 | 27.5% | ||||
| What type of the toothpaste do you use? | whitening | no | 28 | 70.00% | 27 | 67.5% | 0.058 | 0.809 |
| yes | 12 | 30.00% | 13 | 32.5% | ||||
| herbal | no | 37 | 92.5% | 35 | 87.5% | 0.556 | 0.456 | |
| yes | 3 | 7.5% | 5 | 12.5% | ||||
| for dentinal hypersensitivity | no | 37 | 92.5% | 37 | 92.5% | <0.001 | 1.000 | |
| yes | 3 | 7.5% | 3 | 7.5% | ||||
| with fluoride in concentration 1000–1400 ppm | no | 20 | 50.0% | 21 | 52.5% | 0.050 | 0.823 | |
| yes | 20 | 50.0% | 19 | 47.5% | ||||
| Do you use any other additional products to maintain proper oral hygiene? | none | 10 | 25.0% | 16 | 40.0% | 6.870 | 0.076 | |
| toothpick | 6 | 15.0% | 8 | 20.0% | ||||
| chewing gum | 9 | 22.5% | 11 | 27.5% | ||||
| dental floss | 15 | 37.5% | 5 | 12.5% | ||||
| Do you use mouthwash? | no | 27 | 67.5% | 32 | 80.0% | 1.614 | 0.204 | |
| yes | 13 | 32.5% | 8 | 20.0% | ||||
| Do you brush your tongue? | no | 17 | 42.5% | 13 | 32.5% | 0.853 | 0.356 | |
| yes | 23 | 57.5% | 27 | 67.5% | ||||
| How often do you visit a dentist? | very rarely | 3 | 7.5% | 8 | 20.0% | 6.247 | 0.044 | |
| only in case of pain | 17 | 42.5% | 22 | 55.0% | ||||
| regularly | 20 | 50.0% | 10 | 25.0% | ||||
| Dental Sextants | CPI-0 Healthy | CPI-1 Bleeding | CPI-2 Calculus | CPI-3 Pockets 4–5 mm | CPI-4 Pockets > 6 mm | Excluded |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| 17–14 | 17 (42.5%) | 18 (45%) | 4 (10%) | 0 (0%) | 0 (0%) | 1 (2.5%) |
| 13–23 | 26 (65%) | 11 (27.5%) | 2 (5%) | 0 (0%) | 0 (0%) | 1 (2.5%) |
| 24–27 | 16 (40%) | 19 (47.5%) | 4 (10%) | 0 (0%) | 0 (0%) | 1 (2.5%) |
| 34–37 | 18 (45%) | 7 (17.5%) | 11 (27.5%) | 3 (7.5%) | 0 (0%) | 1 (2.5%) |
| 43–33 | 9 (22.5%) | 10 (25%) | 16 (40%) | 4 (10%) | 0 (0%) | 1 (2.5%) |
| 47–44 | 18 (45%) | 7 (17.5%) | 12 (30%) | 2 (5%) | 0 (0%) | 1 (2.5%) |
| Dental Sextants | CPI-0 Healthy | CPI-1 Bleeding | CPI-2 Calculus | CP-3 Pockets 4–5 mm | CP-4 Pockets > 6 mm | Excluded |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| 17–14 | 19 (47.5%) | 15 (37.5%) | 6 (15%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 13–23 | 30 (75%) | 8 (20%) | 2 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 24–27 | 31 (77.5%) | 8 (20%) | 1 (2.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 34–37 | 30 (75%) | 8 (20%) | 2 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 43–33 | 13 (32.5%) | 14 (35%) | 13 (32.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 47–44 | 28 (70%) | 11 (27.5%) | 1 (2.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Periodontal Treatment Need Category | Study Group n = 40 | Control Group n = 40 | ||
|---|---|---|---|---|
| n | % | n | % | |
| TN-0 | 8 | 20.5 | 11 | 27.5 |
| TN-1 | 11 | 28.2 | 18 | 45 |
| TN-2 | 20 | 51.3 | 11 | 27.5 |
| TN-3 | 0 | 0 | 0 | 0 |
| Chi2 p = 0.092 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hildebrandt, T.; Świętochowska, E.; Trzcionka, A.; Zawilska, A.; Mazurek, H.; Mączkowiak, D.; Rahnama, M.; Tanasiewicz, M. Oral Hygiene and Periodontal Treatment Needs in Adult Patients with Cystic Fibrosis (CF). Healthcare 2022, 10, 766. https://doi.org/10.3390/healthcare10050766
Hildebrandt T, Świętochowska E, Trzcionka A, Zawilska A, Mazurek H, Mączkowiak D, Rahnama M, Tanasiewicz M. Oral Hygiene and Periodontal Treatment Needs in Adult Patients with Cystic Fibrosis (CF). Healthcare. 2022; 10(5):766. https://doi.org/10.3390/healthcare10050766
Chicago/Turabian StyleHildebrandt, Tomasz, Elżbieta Świętochowska, Agata Trzcionka, Anna Zawilska, Henryk Mazurek, Dagmara Mączkowiak, Mansur Rahnama, and Marta Tanasiewicz. 2022. "Oral Hygiene and Periodontal Treatment Needs in Adult Patients with Cystic Fibrosis (CF)" Healthcare 10, no. 5: 766. https://doi.org/10.3390/healthcare10050766
APA StyleHildebrandt, T., Świętochowska, E., Trzcionka, A., Zawilska, A., Mazurek, H., Mączkowiak, D., Rahnama, M., & Tanasiewicz, M. (2022). Oral Hygiene and Periodontal Treatment Needs in Adult Patients with Cystic Fibrosis (CF). Healthcare, 10(5), 766. https://doi.org/10.3390/healthcare10050766







