Use of Computed Tomography Pulmonary Angiography in Emergency Departments: A Literature Review
Abstract
:1. Background
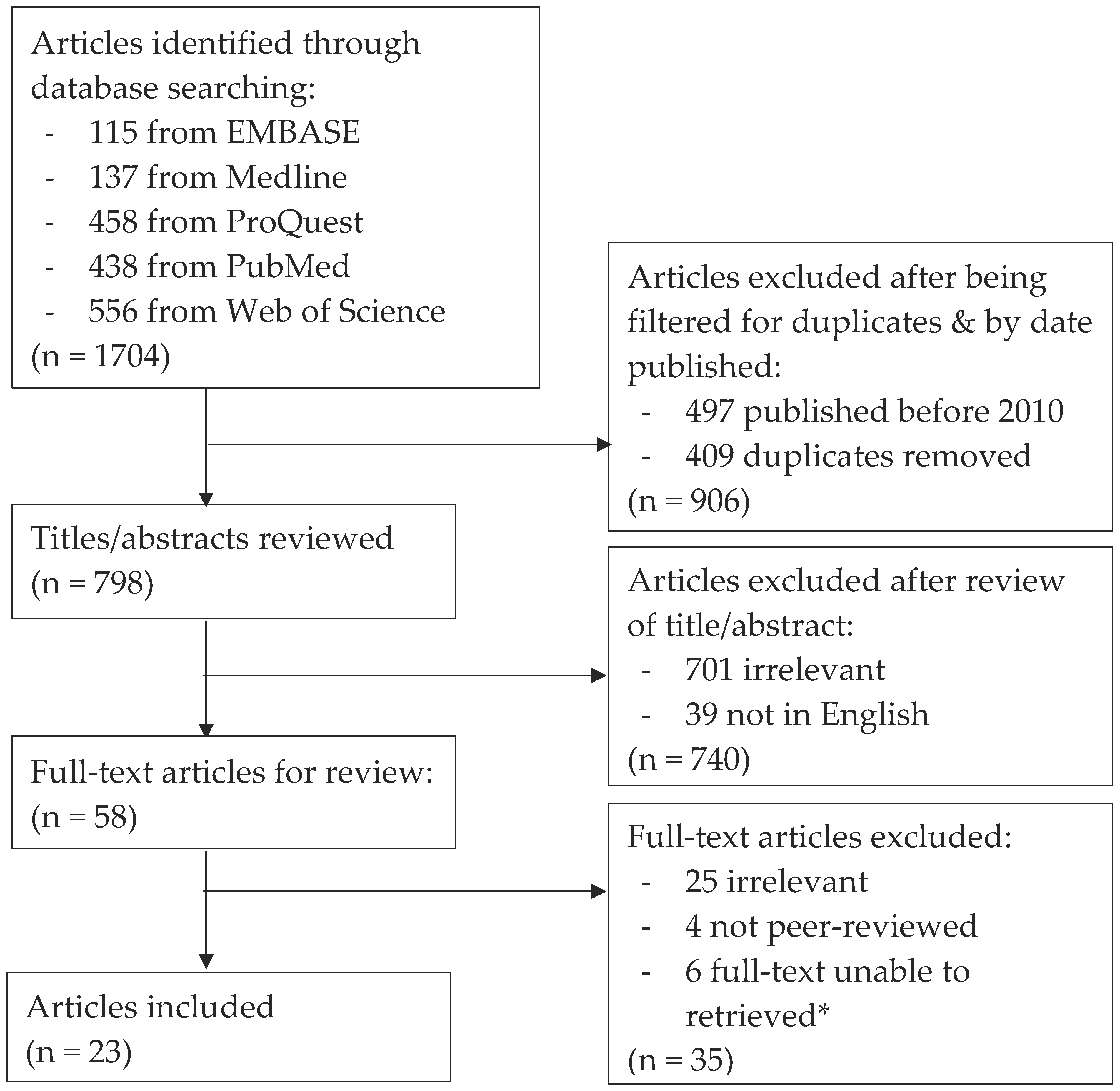
2. Search Strategy
3. Results
3.1. Use of CTPAs
3.2. Explanations for Overuse
3.3. Use of D-Dimer
3.4. Variability between Clinicians
3.5. Strategies to Reduce Overuse
4. Discussion
5. Limitations
6. Implications for Practice
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Author(s) | Year | Country | Study Design | n | CTPA Positivity Yield Pre-Intervention | Intervention (If Applicable) | CTPA Positivity Yield Post-Intervention | Potentially Avoidable CTPAs |
|---|---|---|---|---|---|---|---|---|
| Al Dandan et al. | 2020 | Saudi Arabia | Retrospective Observational Study | 353 | – | – | 18.7% | – |
| Anjum et al. | 2019 | Canada | Retrospective Cohort Study | 1708 | – | – | 13.6% | – |
| Booker et al. | 2017 | United States of America | Retrospective Review | 412 | 8.7% | Clinician Education | 9.2% | – |
| Buchanan et al. | 2017 | United States of America | Prospective Observational Study | 3024 | – | – | – | – |
| Chen et al. | 2015 | Canada | Cross-Sectional Retrospective Study | 835 | – | – | 17.8% | – |
| Crichlow et al. | 2012 | United States of America | Prospective Cohort Study | 152 | – | – | 11.8% | 9.2% |
| Ferguson, Low & Fung | 2019 | Canada | Retrospective Cohort Study | 510 | 5.9% | Clinical Practice Guidelines | 15.0% | – |
| Gyftopoulos et al. | 2018 | United States of America | Semi-Structured Interviews | 33 | – | – | – | – |
| Kanaan et al. | 2013 | United States of America | Retrospective Review | 200 | 8.0% | Clinician Education | 10.0% | – |
| Kindermann et al. | 2014 | United States of America | Retrospective Review | 12,883 1,2 | – | – | 5.9% * | – |
| Kline et al. | 2020 | United States of America | Retrospective Review | 42,267 1 | – | – | 3.0% ^ | – |
| Mountain et al. | 2016 | Australia | Retrospective Review | 7077 | – | – | 14.6% | – |
| Osman et al. | 2018 | United States of America | Retrospective Review | 295 | – | – | 5.4% | – |
| Parikh et al. | 2015 | United States of America | Retrospective Review | 196 | – | – | 10.7% | – |
| Perelas et al. | 2015 | United States of America | Retrospective Review | 646 | – | – | 9.4% | 49.5% |
| Raja et al. | 2012 | United States of America | Retrospective Review | 6838 | 5.8% | Clinical Practice Guidelines | 9.8% | – |
| Rohacek et al. | 2012 | Switzerland | Prospective Cohort Study including a Survey | 328 | 14.5% | Clinical Practice Guidelines Pre-Ordering Questionnaire | 19.2% | – |
| Salehi et al. | 2020 | Canada | Retrospective Observational Study | 2788 | – | – | 10.3% | – |
| Shujaat, Shapiro & Eden | 2013 | United States of America | Retrospective Review | 231 | – | – | 20.7% | – |
| Simon et al. | 2019 | United States of America | Retrospective Review | 212 | – | – | 8.5% | 8.7% |
| Stojanovska et al. | 2015 | United States of America | Prospective Cohort Study | 602 | – | – | 10.0% | – |
| Venkatesh et al. | 2012 | United States of America | Prospective Observational Study | 5940 | – | – | – | 32% |
| Yan et al. | 2017 | United States of America | Retrospective Cohort Study | 2993 | 4.2% | Clinical Practice Guidelines | 11.2% | 19.7% |
| Average CTPA Positivity Yield 3 | 11.74% | |||||||
References
- Salehi, L.; Phalpher, P.; Ossip, M.; Meaney, C.; Rahim, V.; Mercuri, M. Variability in practice patterns among emergency physicians in the evaluation of patients with a suspected diagnosis of pulmonary embolism. Emerg. Radiol. 2020, 27, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mountain, D.; Keijzersm, G.; Chu, K.; Joseph, A.; Read, C.; Blecher, G.; Jeremy Furyk, J.; Bharat, C.; Velusamy, K.; Munro, A.; et al. RESPECT-ED: Rates of Pulmonary Emboli (PE) and Sub-Segmental PE with Modern Computed Tomographic Pulmonary Angiograms in Emergency Departments: A Multi-Center Observational Study Finds Significant Yield Variation, Uncorrelated with Use or Small PE Rates. PLoS ONE 2016, 11, e0166483. [Google Scholar]
- Niemann, T.; Zbinden, I.; Roser, H.W.; Bremerich, J.; Remy-Jardin, M.; Bongartz, G. Computed tomography for pulmonary embolism: Assessment of a 1-year cohort and estimated cancer risk associated with diagnostic irradiation. Acta Radiol. 2013, 54, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Dobler, C.C. Overdiagnosis of pulmonary embolism: Definition, causes and implications. Breathe 2019, 15, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, N.; Morris, E.; Babb, J.; Wickstrom, M.; McMenamy, J.; Sharma, R.; Schwartz, D.; Lifshitz, M.; Kim, D. MDCT diagnosis of acute pulmonary embolism in the emergent setting. Emerg. Radiol. 2015, 22, 379–384. [Google Scholar] [CrossRef]
- Crichlow, A.; Cuker, A.; Mills, A.M. Overuse of computed tomography pulmonary angiography in the evaluation of patients with suspected pulmonary embolism in the emergency department. Acad. Emerg. Med. 2012, 19, 1219–1226. [Google Scholar] [CrossRef]
- Douma, R.A.; Le Gal, G.; Sohne, M.; Righini, M.; Kamphuisen, P.W.; Perrier, A.; Kruip, M.J.H.A.; Henri Bounameaux, H.; Büller, H.R.; Roy, P.-M. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: A retrospective analysis of three large cohorts. BMJ 2010, 340, c1475. [Google Scholar] [CrossRef] [Green Version]
- Kline, J.A.; Mitchell, A.M.; Kabrhel, C.; Richman, P.B.; Courtney, D.M. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J. Thromb. Haemost. 2004, 2, 1247–1255. [Google Scholar] [CrossRef]
- Choosing Wisely Australia: Recommendations from the Royal Australian and New Zealand College of Radiologists. 2015. Available online: https://www.choosingwisely.org.au/recommendations/ranzcr3 (accessed on 5 July 2021).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Booker, M.T.; Johnson, J.O. Optimizing CT pulmonary angiogram utilization in a community emergency department: A pre- and postintervention study. J. Am. Coll. Radiol. 2017, 14, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, J.A.; Garrett, J.S.; Sarmiento, E.J.; Strachan, C.C.; Courtney, D.M. Over-Testing for Suspected Pulmonary Embolism in American Emergency Departments: The Continuing Epidemic. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e005753. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Subedi, S.K.; Ahmed, A.; Khan, J.; Dawood, T.; Ríos-Bedoya, C.F.; Bachuwa, G. Computed tomography pulmonary angiography is overused to diagnose pulmonary embolism in the emergency department of academic community hospital. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perelas, A.; Dimou, A.; Saenz, A.; Rhee, J.H.; Teerapuncharoen, K.; Rowden, A.; Eiger, G. CT pulmonary angiography utilization in the emergency department: Diagnostic yield and adherence to current guidelines. Am. J. Med. Qual. 2015, 30, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, A.K.; Kline, J.A.; Courtney, D.M.; Camargo, C.A.; Plewa, M.C.; Nordenholz, K.E.; Moore, C.L.; Richman, P.B.; Smithline, H.A.; Daren, M.; et al. Evaluation of Pulmonary Embolism in the Emergency Department and Consistency with a National Quality Measure. Arch. Intern. Med. 2012, 17, 1028–1032. [Google Scholar] [CrossRef] [Green Version]
- Al Dandan, O.; Hassan, A.; Alnasr, A.; Al Gadeeb., M.; AbuAlola, H.; Alshahwan, S.; Shammari, M.A.; Alaa Alzaki, A. The use of clinical decision rules for pulmonary embolism in the emergency department: A retrospective study. Int. J. Emerg. Med. 2020, 13, 6. [Google Scholar] [CrossRef]
- Chen, Y.A.; Gray, B.G.; Bandiera, G.; Mackinnon, D.; Deva, D.P. Variation in the utilization and positivity rates of CT pulmonary angiography among emergency physicians at a tertiary academic emergency department. Emerg. Radiol. 2015, 22, 221–229. [Google Scholar] [CrossRef]
- Anjum, O.; Bleeker, H.; Ohle, R. Computed tomography for suspected pulmonary embolism results in a large number of non-significant incidental findings and follow-up investigations. Emerg. Radiol. 2019, 26, 29–35. [Google Scholar] [CrossRef]
- Rohacek, M.; Buatsi, J.; Szucs-Farkas, Z.; Kleim, B.; Zimmermann, H.; Exadaktylos, A.; Stoupis, C. Ordering CT pulmonary angiography to exclude pulmonary embolism: Defense versus evidence in the emergency room. Intensiv. Care Med. 2012, 38, 1345–1351. [Google Scholar] [CrossRef] [Green Version]
- Gyftopoulos, S.; Smith, S.W.; Simon, E.; Kuznetsova, M.; Horwitz, L.I.; Makarov, D.V. Qualitative Study to Understand Ordering of CT Angiography to Diagnose Pulmonary Embolism in the Emergency Room Setting. J. Am. Coll. Radiol. 2018, 15, 1276–1284. [Google Scholar] [CrossRef]
- Kindermann, D.R.; McCarthy, M.L.; Ding, R.; Frohna, W.J.; Hansen, J.; Maloy, K.; Milzman, D.P.; Pines, J.M. Emergency department variation in utilization and diagnostic yield of advanced radiography in diagnosis of pulmonary embolus. J. Emerg. Med. 2014, 46, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.; Low, G.; Fung, C. Retrospective Analysis of the Computed Tomography Pulmonary Angiogram Utilization Patterns in the Emergency Department. Can. Assoc. Radiol. J. 2019, 70, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.S.; Ip, I.K.; Prevedello, L.M.; Sodickson, A.D.; Farkas, C.; Zane, R.D.; Hanson, R.; Goldhaber, S.Z.; Gill, R.R.; Khorasani, R. Effect of computerized clinical decision support on the use and yield of CT pulmonary angiography in the emergency department. Radiology 2012, 262, 468–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, I.; Teeples, T.; Carlson, M.; Steenblik, J.; Bledsoe, J.; Madsen, T. Pulmonary embolism testing among emergency department patients who are pulmonary embolism rule-out criteria negative. Acad. Emerg. Med. 2017, 24, 1369–1376. [Google Scholar] [CrossRef] [Green Version]
- Dubin, J.; Rawal, H.; Ipaye, O.; Kalaria, A.; Vij, R.; Camire, L.; Weisman, D. Effects of Practice Site, Physician Experience, and Number of Tests Ordered Per Physician on the Diagnostic Yield of Computed Tomography Pulmonary Angiogram Ordered in the Emergency Department. Ann. Emerg. Med. 2019, 74, S12–S13. [Google Scholar] [CrossRef]
- Yan, Z.; Ip, I.K.; Raja, A.S.; Gupta, A.; Kosowsky, J.M.; Khorasani, R. Yield of CT Pulmonary Angiography in the Emergency Department When Providers Override Evidence-based Clinical Decision Support. Radiology 2017, 282, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Simon, E.; Miake-Lye, I.M.; Smith, S.W.; Swartz, J.L.; Horwitz, L.I.; Makarov, D.V.; Gyftopoulos, S. An Evaluation of Guideline-Discordant Ordering Behavior for CT Pulmonary Angiography in the Emergency Department. J. Am. Coll. Radiol. 2019, 16, 1064–1072. [Google Scholar] [CrossRef]
- Ross, J.; Santhirapala, R.; Macewen, C.; Coulter, A. Helping patients choose wisely. BMJ 2018, 361, k2585. [Google Scholar] [CrossRef]
- Olson, A.P.J.; Graber, M.L. Improving Diagnosis Through Education. Acad Med. 2020, 95, 1162–1165. [Google Scholar] [CrossRef]
- Stojanovska, J.; Carlos, R.C.; Kocher, K.E.; Nagaraju, A.; Guy, K.; Kelly, A.M.; Chughtai, A.R.; Kazerooni, E.A. CT Pulmonary Angiography: Using Decision Rules in the Emergency Department. J. Am. Coll. Radiol. 2015, 12, 1023–1029. [Google Scholar] [CrossRef]
- Baron, D.M.; Metnitz, P.G.H.; Rhodes, A.; Kozek-Langenecker, S.A. Clinical guidelines: How can we improve adherence and implementation? Eur. J. Anaesthesiol. 2017, 34, 329–331. [Google Scholar] [CrossRef] [PubMed]
| Concept 1 | Concept 2 | Concept 3 | |
|---|---|---|---|
| Key Concepts * | Overuse | CTPAs | Emergency Department |
| Free Text/Natural Language Terms | Over-use, over use, over used, use, utili#ation, overutili#ation, yield, diagnostic yield, positivity rate | CTPA, PCTA, CT pulmonary angiograms, CT pulmonary angiogram, CT pulmonary angiography, computed tomography pulmonary angiograms, computed tomography pulmonary angiogram, computed tomography pulmonary angiography | ED, emergency room. ER, accident & emergency, a & e, a&e |
| Controlled Vocabulary/Subject Terms | “Medical Overuse” | “Computed Tomography Angiography & Pulmonary Artery” | “Emergency Service, Hospital” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thurlow, L.E.; Van Dam, P.J.; Prior, S.J.; Tran, V. Use of Computed Tomography Pulmonary Angiography in Emergency Departments: A Literature Review. Healthcare 2022, 10, 753. https://doi.org/10.3390/healthcare10050753
Thurlow LE, Van Dam PJ, Prior SJ, Tran V. Use of Computed Tomography Pulmonary Angiography in Emergency Departments: A Literature Review. Healthcare. 2022; 10(5):753. https://doi.org/10.3390/healthcare10050753
Chicago/Turabian StyleThurlow, Lauren E., Pieter J. Van Dam, Sarah J. Prior, and Viet Tran. 2022. "Use of Computed Tomography Pulmonary Angiography in Emergency Departments: A Literature Review" Healthcare 10, no. 5: 753. https://doi.org/10.3390/healthcare10050753
APA StyleThurlow, L. E., Van Dam, P. J., Prior, S. J., & Tran, V. (2022). Use of Computed Tomography Pulmonary Angiography in Emergency Departments: A Literature Review. Healthcare, 10(5), 753. https://doi.org/10.3390/healthcare10050753









