The Effects of High-Intensity Functional Training on Cognition in Older Adults with Cognitive Impairment: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Assessment of Methodological Quality
3. Results
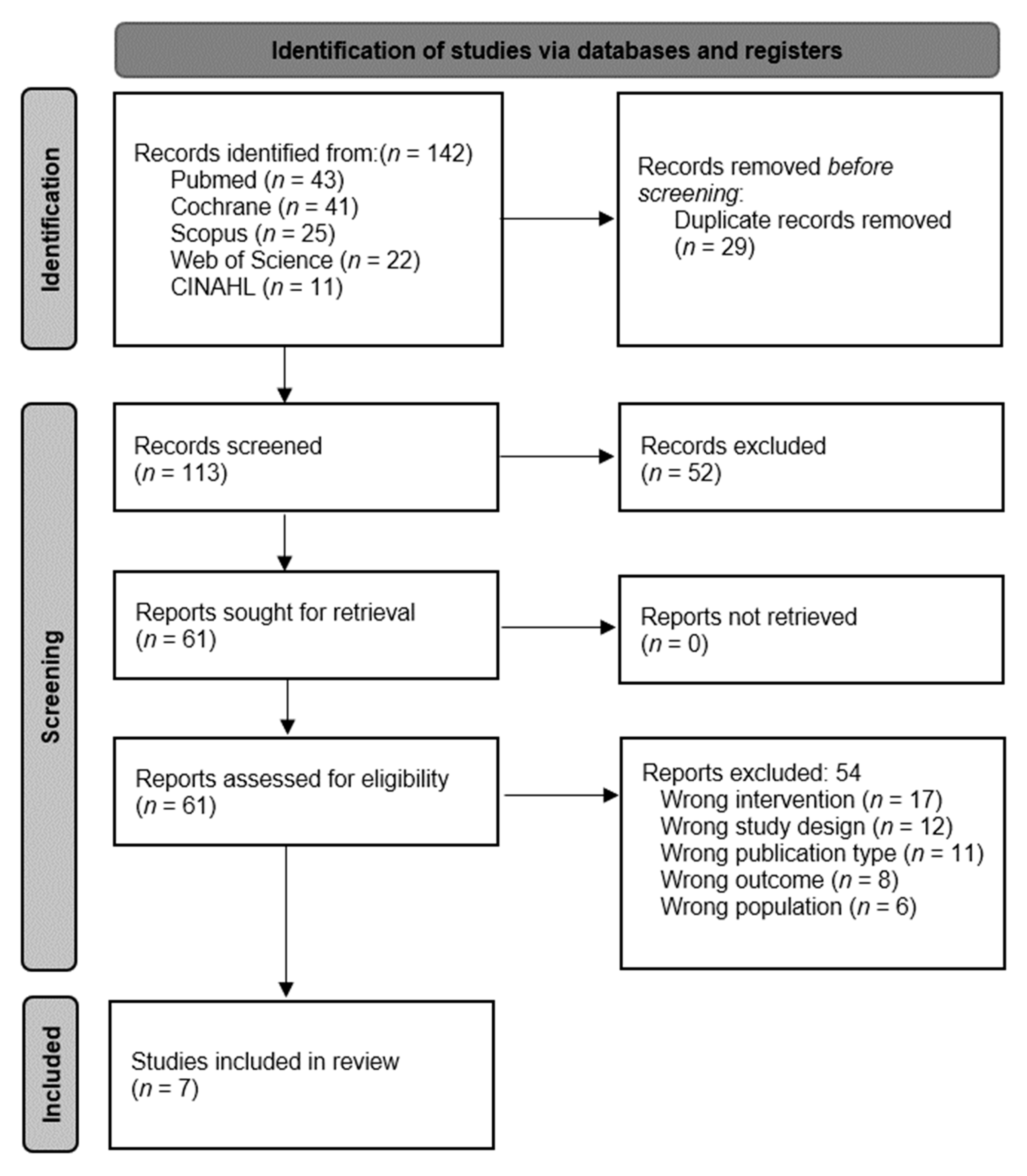
3.1. Selection of the Studies
3.2. Methodological Quality
3.3. Characteristics of the Studies
3.4. Outcomes
3.5. Study Intervention
3.6. Study Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franco-Martin, M.; Vidales, E.P.; Palau, F.G.; Navarro, M.B.; Solis, A. Influencia del ejercicio físico en la prevención del deterioro cognitivo en las personas mayores: Revisión sistemática. Rev. Neurol. 2013, 56, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, J.D.J.; Martínez-Amat, A.; De La Torre-Cruz, M.J.; Fábrega-Cuadros, R.; Díaz, D.C.; Aibar-Almazán, A.; Achalandabaso-Ochoa, A.; Hita-Contreras, F. Suspension Training HIIT Improves Gait Speed, Strength and Quality of Life in Older Adults. Int. J. Sports Med. 2019, 40, 116–124. [Google Scholar] [CrossRef]
- Tseng, C.-N.; Gau, B.-S.; Lou, M.-F. The Effectiveness of Exercise on Improving Cognitive Function in Older People: A systematic review. J. Nurs. Res. 2011, 19, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Valecchi, D.; Bacci, D.; Abbate, R.; Gensini, G.F.; Casini, A.; Macchi, C. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J. Intern. Med. 2011, 269, 107–117. [Google Scholar] [CrossRef]
- Kirk-Sanchez, N.; McGough, E. Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 2014, 9, 51–62. [Google Scholar] [CrossRef] [Green Version]
- de Asteasu, M.L.S.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Casas-Herrero; Izquierdo, M. Role of physical exercise on cognitive function in healthy older adults: A systematic review of randomized clinical trials. Ageing Res. Rev. 2017, 37, 117–134. [Google Scholar] [CrossRef]
- Sugano, K.; Yokogawa, M.; Yuki, S.; Dohmoto, C.; Yoshita, M.; Hamaguchi, T.; Yanase, D.; Iwasa, K.; Komai, K.; Yamada, M. Effect of Cognitive and Aerobic Training Intervention on Older Adults with Mild or No Cognitive Impairment: A Derivative Study of the Nakajima Project. Dement. Geriatr. Cogn. Disord. Extra 2012, 2, 69–80. [Google Scholar] [CrossRef]
- Lautenschlager, N.T.; Cox, K.; Cyarto, E.V. The influence of exercise on brain aging and dementia. Biochim. Biophys. Acta 2012, 1822, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Foster, P.P.; Rosenblatt, K.P.; Kuljiš, R.O. Exercise-Induced Cognitive Plasticity, Implications for Mild Cognitive Impairment and Alzheimer’s Disease. Front. Neurol. 2011, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- López, M.D.; Zamarrón, M.D.; Fernández-Ballesteros, R. Asociación entre la realización de ejercicio e indicadores de funcionamiento físico y cognitivo. Comparativa de resultados en función de la edad. Rev. Española Geriatría Gerontol. 2011, 46, 15–20. [Google Scholar] [CrossRef]
- Peixoto, R.P.; Trombert, V.; Poncet, A.; Kizlik, J.; Gold, G.; Ehret, G.; Trombetti, A.; Reny, J.-L. Feasibility and safety of high-intensity interval training for the rehabilitation of geriatric inpatients (HIITERGY) a pilot randomized study. BMC Geriatr. 2020, 20, 197. [Google Scholar] [CrossRef] [PubMed]
- Knowles, A.-M.; Herbert, P.; Easton, C.; Sculthorpe, N.; Grace, F.M. Impact of low-volume, high-intensity interval training on maximal aerobic capacity, health-related quality of life and motivation to exercise in ageing men. AGE 2015, 37, 25. [Google Scholar] [CrossRef] [Green Version]
- Kliszczewicz, B.; Williamson, C.; Bechke, E.; McKenzie, M.; Hoffstetter, W. Autonomic response to a short and long bout of high-intensity functional training. J. Sports Sci. 2018, 36, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-Intensity Functional Training (HIFT): Definition and Research Implications for Improved Fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, K.M.; Patel, P.M.; O’Neal, J.L.; Heinrich, B.S. High-intensity compared to moderate-intensity training for exercise initiation, enjoyment, adherence, and intentions: An intervention study. BMC Public Health 2014, 14, 789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.; Knapp, K.; Lackie, A.; Lewry, C.; Horvey, K.; Benko, C.; Trinh, J.; Butcher, S. Multimodal high-intensity interval training increases muscle function and metabolic performance in females. Appl. Physiol. Nutr. Metab. 2015, 40, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.; Sales, A.; Carlson, L.; Steele, J. A comparison of the motivational factors between CrossFit participants and other resistance exercise modalities: A pilot study. J. Sports Med. Phys. Fit. 2017, 57, 1227–1234. [Google Scholar] [CrossRef]
- Quiles, J.M.; Klemp, A.; Dolan, C.; Maharaj, A.; Huang, C.-J.; Khamoui, A.V.; Trexler, E.T.; Whitehurst, M.; Zourdos, M.C. Impact of resistance training program configuration on the circulating brain-derived neurotrophic factor response. Appl. Physiol. Nutr. Metab. 2019, 45, 667–674. [Google Scholar] [CrossRef]
- Chen, H.-T.; Chung, Y.-C.; Chen, Y.-J.; Ho, S.-Y.; Wu, H.-J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef]
- Ben-Zeev, T.; Okun, E. High-Intensity Functional Training: Molecular Mechanisms and Benefits. NeuroMolecular Med. 2021, 23, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zeev, T.; Hirsh, T.; Weiss, I.; Gornstein, M.; Okun, E. The Effects of High-intensity Functional Training (HIFT) on Spatial Learning, Visual Pattern Separation and Attention Span in Adolescents. Front. Behav. Neurosci. 2020, 14, 577390. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J. Functional high-intensity exercise is more effective in acutely increasing working memory than aerobic walking: An exploratory randomized, controlled trial. Sci. Rep. 2020, 10, 12335. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ariza, A.; Suárez-Manzano, S.; López-Serrano, S.; Martínez-López, E.J. The effect of cooperative high-intensity interval training on creativity and emotional intelligence in secondary school: A randomised controlled trial. Eur. Phys. Educ. Rev. 2017, 25, 355–373. [Google Scholar] [CrossRef]
- Costigan, S.A.; Eather, N.; Plotnikoff, R.; Hillman, C.; Lubans, D. High-Intensity Interval Training for Cognitive and Mental Health in Adolescents. Med. Sci. Sports Exerc. 2016, 48, 1985–1993. [Google Scholar] [CrossRef]
- Eather, N.; Riley, N.; Miller, A.; Smith, V.; Poole, A.; Vincze, L.; Morgan, P.J.; Lubans, D. Efficacy and feasibility of HIIT training for university students: The Uni-HIIT RCT. J. Sci. Med. Sport 2019, 22, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.-Y.; Chen, F.-T.; Hsieh, S.-S.; Kao, S.-C.; Chen, A.-G.; Hung, T.-M.; Chang, Y.-K. The Effect of Acute High-Intensity Interval Training on Executive Function: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 3593. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Dias, R.; Neiva, H.; Marinho, D.; Marques, M.; Sousa, A.; Loureiro, V.; Loureiro, N. High-Intensity Interval Training upon Cognitive and Psychological Outcomes in Youth: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 5344. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Macedo, L.G.; Elkins, M.; Maher, C.; Moseley, A.M.; Herbert, R.; Sherrington, C. There was evidence of convergent and construct validity of Physiotherapy Evidence Database quality scale for physiotherapy trials. J. Clin. Epidemiol. 2010, 63, 920–925. [Google Scholar] [CrossRef]
- Moseley, A.M.; Rahman, P.; Wells, G.A.; Zadro, J.; Sherrington, C.; Toupin-April, K.; Brosseau, L. Agreement between the Cochrane risk of bias tool and Physiotherapy Evidence Database (PEDro) scale: A meta-epidemiological study of randomized controlled trials of physical therapy interventions. PLoS ONE 2019, 14, e0222770. [Google Scholar] [CrossRef] [PubMed]
- Olivo, S.A.; Macedo, L.; Gadotti, I.C.; Fuentes, J.; Stanton, T.; Magee, D.J. Scales to Assess the Quality of Randomized Controlled Trials: A Systematic Review. Phys. Ther. 2008, 88, 156–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.A.F.; Gates, N.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C.; et al. The Study of Mental and Resistance Training (SMART) Study—Resistance Training and/or Cognitive Training in Mild Cognitive Impairment: A Randomized, Double-Blind, Double-Sham Controlled Trial. J. Am. Med Dir. Assoc. 2014, 15, 873–880. [Google Scholar] [CrossRef]
- Gbiri, C.A.O.; Amusa, B.F. Progressive task-oriented circuit training for cognition, physical functioning and societal participation in individuals with dementia. Physiother. Res. Int. 2020, 25, e1866. [Google Scholar] [CrossRef]
- Lamb, S.E.; Sheehan, B.; Atherton, N.; Nichols, V.; Collins, H.; Mistry, D.; Dosanjh, S.; Slowther, A.M.; Khan, I.; Petrou, S.; et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: Randomised controlled trial. BMJ 2018, 361, k1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littbrand, H.; Carlsson, M.; Lundin-Olsson, L.; Lindelöf, N.; Håglin, L.; Gustafson, Y.; Rosendahl, E. Effect of a High-Intensity Functional Exercise Program on Functional Balance: Preplanned Subgroup Analyses of a Randomized Controlled Trial in Residential Care Facilities. J. Am. Geriatr. Soc. 2011, 59, 1274–1282. [Google Scholar] [CrossRef]
- Telenius, E.W.; Engedal, K.; Bergland, A. Effect of a High-Intensity Exercise Program on Physical Function and Mental Health in Nursing Home Residents with Dementia: An Assessor Blinded Randomized Controlled Trial. PLoS ONE 2015, 10, e0126102. [Google Scholar] [CrossRef] [Green Version]
- Telenius, E.W.; Engedal, K.; Bergland, A. Long-term effects of a 12 weeks high-intensity functional exercise program on physical function and mental health in nursing home residents with dementia: A single blinded randomized controlled trial. BMC Geriatr. 2015, 15, 158. [Google Scholar] [CrossRef] [Green Version]
- Toots, A.; Littbrand, H.; Boström, G.; Hörnsten, C.; Holmberg, H.; Lundin-Olsson, L.; Lindelöf, N.; Nordström, P.; Gustafson, Y.; Rosendahl, E. Effects of Exercise on Cognitive Function in Older People with Dementia: A Randomized Controlled Trial. J. Alzheimer’s Dis. 2017, 60, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Gates, N.J.; Valenzuela, M.; Sachdev, P.S.; Singh, N.A.; Baune, B.T.; Brodaty, H.; Suo, C.; Jain, N.; Wilson, G.C.; Wang, Y.; et al. Study of Mental Activity and Regular Training (SMART) in at risk individuals: A randomised double blind, sham controlled, longitudinal trial. BMC Geriatr. 2011, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Toots, A.; Littbrand, H.; Lindelöf, N.; Wiklund, R.; Holmberg, H.; Nordström, P.; Lundin-Olsson, L.; Gustafson, Y.; Rosendahl, E. Effects of a High-Intensity Functional Exercise Program on Dependence in Activities of Daily Living and Balance in Older Adults with Dementia. J. Am. Geriatr. Soc. 2016, 64, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Luxton, N.; Alison, J.A.; Wu, J.; Mackey, M.G. Relationship between field walking tests and incremental cycle ergometry in COPD. Respirology 2008, 13, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Khandker, R.; Black, C.; Pike, J.; Husbands, J.; Ambegaonkar, B.; Jones, E. The relationship between Mini-Mental State Examination (MMSE) & Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) using real world data in US & Europe (P5. 178). Neurology 2018, 90, P5.178. Available online: http://n.neurology.org/content/90/15_Supplement/P5.178.abstract (accessed on 2 February 2022).
- Levine, S.Z.; Yoshida, K.; Goldberg, Y.; Samara, M.; Cipriani, A.; Efthimiou, O.; Iwatsubo, T.; Leucht, S.; Furukawa, T.A. Linking the Mini-Mental State Examination, the Alzheimer’s Disease Assessment Scale–Cognitive Subscale and the Severe Impairment Battery: Evidence from individual participant data from five randomised clinical trials of donepezil. Évid. Based Ment. Health 2021, 24, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Richens, B.; Cleather, D. The relationship between the number of repetitions performed at given intensities is different in endurance and strength trained athletes. Biol. Sport 2014, 31, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimano, T.; Kraemer, W.J.; Spiering, B.A.; Volek, J.S.; Hatfield, D.L.; Silvestre, R.; Vingren, J.L.; Fragala, M.S.; Maresh, C.M.; Fleck, S.J.; et al. Relationship Between the Number of Repetitions and Selected Percentages of One Repetition Maximum in Free Weight Exercises in Trained and Untrained Men. J. Strength Cond. Res. 2006, 20, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Willardson, J.M.; Burkett, L.N. A Comparison of 3 Different Rest Intervals on the Exercise Volume Completed During a Workout. J. Strength Cond. Res. 2005, 19, 23–26. [Google Scholar] [CrossRef]
- Vincent, K.R.; Vasilopoulos, T.; Montero, C.; Vincent, H.K. Eccentric and Concentric Resistance Exercise Comparison for Knee Osteoarthritis. Med. Sci. Sports Exerc. 2019, 51, 1977–1986. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: Progression and exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 674–688. Available online: https://journals.lww.com/acsm-msse/Fulltext/2004/04000/Fundamentals_of_Resistance_Training__Progression.17.aspx (accessed on 2 February 2022). [CrossRef]
- Souron, R.; Nosaka, K.; Jubeau, M. Changes in central and peripheral neuromuscular fatigue indices after concentric versus eccentric contractions of the knee extensors. Eur. J. Appl. Physiol. 2018, 118, 805–816. [Google Scholar] [CrossRef]
- Balsalobre-Fernández, C.; Cordón; Unquiles, N.; Muñoz-García, D. Movement velocity in the chair squat is associated with measures of functional capacity and cognition in elderly people at low risk of fall. PeerJ 2018, 6, e4712. [Google Scholar] [CrossRef] [PubMed]
- Kan, B.; Speelman, C.; Nosaka, K. Cognitive demand of eccentric versus concentric cycling and its effects on post-exercise attention and vigilance. Eur. J. Appl. Physiol. 2019, 119, 1599–1610. [Google Scholar] [CrossRef]
- Peterson, M.D.; Pistilli, E.; Haff, G.G.; Hoffman, E.; Gordon, P.M. Progression of volume load and muscular adaptation during resistance exercise. Eur. J. Appl. Physiol. 2011, 111, 1063–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, C.-K.; Lam, F.M.H.; Chung, R.C.; Pang, M.Y. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: A systematic review. J. Physiother. 2020, 66, 9–18. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Park, H.; Park, J.H.; Park, K.W.; Lee, K.; Kim, S.; Chae, K.; Park, M.H.; Koh, S.-H.; Na, H.R. Effects of Multicomponent Exercise on Cognitive Function in Elderly Korean Individuals. J. Clin. Neurol. 2020, 16, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.E.; Rutkowsky, J.; Bodine, S.; Rutledge, J.C. The Potential Mechanisms of Exercise-induced Cognitive Protection: A Literature Review. Curr. Pharm. Des. 2018, 24, 1827–1831. [Google Scholar] [CrossRef]
- Russ, J.; Weyh, C.; Pilat, C. High-intensity exercise programs in people with dementia—A systematic review and meta-analysis. Ger. J. Exerc. Sport Res. 2021, 51, 4–16. [Google Scholar] [CrossRef]
- Law, L.L.F.; Mok, V.C.T.; Yau, M.M.K. Effects of functional tasks exercise on cognitive functions of older adults with mild cognitive impairment: A randomized controlled pilot trial. Alzheimer’s Res. Ther. 2019, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Lindelöf, N.; Lundin-Olsson, L.; Skelton, D.A.; Lundman, B.; Rosendahl, E. Experiences of older people with dementia participating in a high-intensity functional exercise program in nursing homes: “While it’s tough, it’s useful”. PLoS ONE 2017, 12, e0188225. [Google Scholar] [CrossRef]
- Sondell, A.; Rosendahl, E.; Gustafson, Y.; Lindelöf, N.; Littbrand, H. The Applicability of a High-Intensity Functional Exercise Program Among Older People With Dementia Living in Nursing Homes. J. Geriatr. Phys. Ther. 2019, 42, E16–E24. [Google Scholar] [CrossRef]
- Mavros, Y.; Gates, N.; Wilson, G.C.; Jain, N.; Bs, J.M.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C.; et al. Mediation of Cognitive Function Improvements by Strength Gains After Resistance Training in Older Adults with Mild Cognitive Impairment: Outcomes of the Study of Mental and Resistance Training. J. Am. Geriatr. Soc. 2017, 65, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.; Markwick, A.; De Jager, C.; Zamboni, G.; Wilcock, G.; Rothwell, P. Differences in Cognitive Profile between TIA, Stroke and Elderly Memory Research Subjects: A Comparison of the MMSE and MoCA. Cerebrovasc. Dis. 2012, 34, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jin, X.; Chen, B.; Xue, F.; Niu, H.; Guo, R.; Chen, Z.; Zheng, H.; Wang, L.; Zhang, Y. Comparison of the Mini-Mental State Examination and Montreal Cognitive Assessment executive subtests in detecting post-stroke cognitive impairment. Geriatr. Gerontol. Int. 2017, 17, 2329–2335. [Google Scholar] [CrossRef]
- Tangen, G.G.; Engedal, K.; Bergland, A.; Moger, T.A.; Mengshoel, A.M. Relationships Between Balance and Cognition in Patients With Subjective Cognitive Impairment, Mild Cognitive Impairment, and Alzheimer Disease. Phys. Ther. 2014, 94, 1123–1134. [Google Scholar] [CrossRef] [Green Version]
- De Andrade, L.P.; Gobbi, L.T.B.; Coelho, F.G.M.; Christofoletti, G.; Costa, J.L.R.; Stella, F. Benefits of Multimodal Exercise Intervention for Postural Control and Frontal Cognitive Functions in Individuals with Alzheimer’s Disease: A Controlled Trial. J. Am. Geriatr. Soc. 2013, 61, 1919–1926. [Google Scholar] [CrossRef]
- Xiao, T.; Yang, L.; Smith, L.; Loprinzi, P.D.; Veronese, N.; Yao, J.; Zhang, Z.; Yu, J.J. Correlation Between Cognition and Balance Among Middle-Aged and Older Adults Observed Through a Tai Chi Intervention Program. Front. Psychol. 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
| Databases | Search Strategy | Limits | Filter |
|---|---|---|---|
| MEDLINE Pubmed | (“high intensity functional training” OR “intensive functional exercise” OR “high intensity functional motor training” OR “intensive functional training” OR “high intensity functional exercise” OR “intermittent exercise” OR “circuit training” OR “interval exercise” OR “intensive functional motor training” OR “HIFT”) AND (“cognitive impairment” OR “dementia”) AND (“older adults” OR “older” OR “elder” OR “elderly” OR “older people” OR “elderly people” OR “aged” OR “geriatric” OR “senior”) | Published date: 2011–2021; Clinical study | 43 |
| Cochrane | Published date: 2011–2021; Trial | 41 | |
| Scopus | Published date: 2011–2021; Article; Humans. | 25 | |
| Web of Science | Published date: 2011–2021; Articles | 22 | |
| CINAHL | Published date: 2011–2021; Randomized controlled trial | 11 |
| Items Authorship | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fiatarone et al., 2014 [33] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Lamb, et al., 2018 [35] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Littbrand, et al., 2011 [36] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Gbiri et al., 2020 [34] | Y | Y | N | Y | N | N | Y | N | Y | Y | Y | 6 |
| Telenius, et al., 2015 [37] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Telenius, et al., 2015 [38] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Toots et al., 2017 [39] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Author | Sample (I/C) | Age | Intervention | Intensity | Control | Measuring Instrument | Assessments | Results |
|---|---|---|---|---|---|---|---|---|
| Fiatarone et al., 2014 [33] | 73/27 | 55–89 | EG1: CT and Progressive HIFT. EG2: HIFT and SCOG. EG3: CT and SPEX | 15–18 on the Borg Scale and 80% RM | SCOG and SPEX | ADAS-Cog | T0 = Baseline T1 = 6 months T2 = 18 months | 6 months intervention of a HIFT program improves global cognition compared to sham exercise (p < 0.05); this benefit persisted for 18 months (p = 0.08). |
| Lamb, et al., 2018 [35] | 329/165 | 77 ± 7.9 | HIFT | RPE adapted for use by people with dementia 20 RM 12 RM | Usual Physical Activity of the Participant. | MMSE ADAS-Cog | T0 = Baseline T1 = 6 months T2 = 12 months | 4 months intervention of HIFT that includes aerobic and strength exercise has negative effects on the cognitive impairment in people with mild to moderate dementia (adjusted mean difference −0.6; 95% confidence interval −1.6 to 0.4; p = 0.24). Cognitive impairment declined over the 12-month follow-up in both trial arms; (adjusted mean difference −1.4, 95% confidence interval −2.6 to −0.2) p = 0.03. |
| Littbrand, et al., 2011 [36] | 91/100 | 85.3 ± 6.1 | HIFT | 8–12 RM | Occupational Therapist Exercise Program developed exclusively for this study. | MMSE Berg Scale | T0 = Baseline T1 = 3 months T2 = 6 months | No significant differences were found between the groups after the intervention. After 3 months p = 5.28 and after 6 months p = 0.47. |
| Gbiri et al., 2020 [34] | 16/15 | 69.6 ± 3.4 | Progressive HIFT | 80% RM | Basic Home Exercise Program. | MMSE ADAS-Cog | T0 = Baseline T1 = 6 weeks T2 = 12 weeks | Progressive HIFT improves cognitive function. MMSE (mean rank) between baseline and post 6-week interventions: 3.56 for experimental group and 1.20 for control group- p = 0.022. MMSE between 6-week and 12-week of intervention 3.75 (experimental group), 1.87 (control group) p = 0.000. |
| Telenius, et al., 2015 [37] | 87/83 | 86.7 ± 7.4 | HIFT | 12 RM | Light physical activity in sitting. | MMSE CDR Berg Scale | T0 = Baseline T1 = 12 weeks | No significant changes in the MMSE (p = 0.69, effect size 0.1) |
| Telenius, et al., 2015 [38] | 87/81 | 86.9 ±7.4 | HIFT | 12 RM | Light physical activity, reading, playing games, listening to music and conversations | MMSE CDR Berg Scale | T0 = Baseline T1 = 3 months T2 = 6 months | Post-intervention measures showed no significant differences between groups p = 0.492. |
| Toots et al., 2017 [39] | 93/93 | 85.1 ± 7.1 | HIFT | 8–12 RM | While seated they sang, listened to music or readings, and/or looked at pictures and objects concerning interesting topics. | MMSE VF ADAS-Cog | T0 = Baseline T1 = 4 months T2 = 7 months | There were no differences from baseline between groups at 4 months (−0.27, 95% CI −1.4 to 0.87, p = 0.644) or at 7 months in MMSE (−1.15, 95% CI −2.32 to 0.03, p = 0.056) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-Campo, Y.; García-Garro, P.A.; Aibar-Almazán, A.; Martínez-Amat, A.; Vega-Ávila, G.C.; Afanador-Restrepo, D.F.; León-Morillas, F.; Hita-Contreras, F. The Effects of High-Intensity Functional Training on Cognition in Older Adults with Cognitive Impairment: A Systematic Review. Healthcare 2022, 10, 670. https://doi.org/10.3390/healthcare10040670
Rivas-Campo Y, García-Garro PA, Aibar-Almazán A, Martínez-Amat A, Vega-Ávila GC, Afanador-Restrepo DF, León-Morillas F, Hita-Contreras F. The Effects of High-Intensity Functional Training on Cognition in Older Adults with Cognitive Impairment: A Systematic Review. Healthcare. 2022; 10(4):670. https://doi.org/10.3390/healthcare10040670
Chicago/Turabian StyleRivas-Campo, Yulieth, Patricia Alexandra García-Garro, Agustín Aibar-Almazán, Antonio Martínez-Amat, Gloria Cecilia Vega-Ávila, Diego Fernando Afanador-Restrepo, Felipe León-Morillas, and Fidel Hita-Contreras. 2022. "The Effects of High-Intensity Functional Training on Cognition in Older Adults with Cognitive Impairment: A Systematic Review" Healthcare 10, no. 4: 670. https://doi.org/10.3390/healthcare10040670
APA StyleRivas-Campo, Y., García-Garro, P. A., Aibar-Almazán, A., Martínez-Amat, A., Vega-Ávila, G. C., Afanador-Restrepo, D. F., León-Morillas, F., & Hita-Contreras, F. (2022). The Effects of High-Intensity Functional Training on Cognition in Older Adults with Cognitive Impairment: A Systematic Review. Healthcare, 10(4), 670. https://doi.org/10.3390/healthcare10040670