Laparoscopic Resection of Synchronous Liver Metastasis Involving the Left Hepatic Vein and the Common Trunk Bifurcation: A Strategy of Parenchyma-Sparing Resection with Left Sectionectomy and 4a Subsegmentectomy by Arantius Approach
Abstract
:1. Introduction
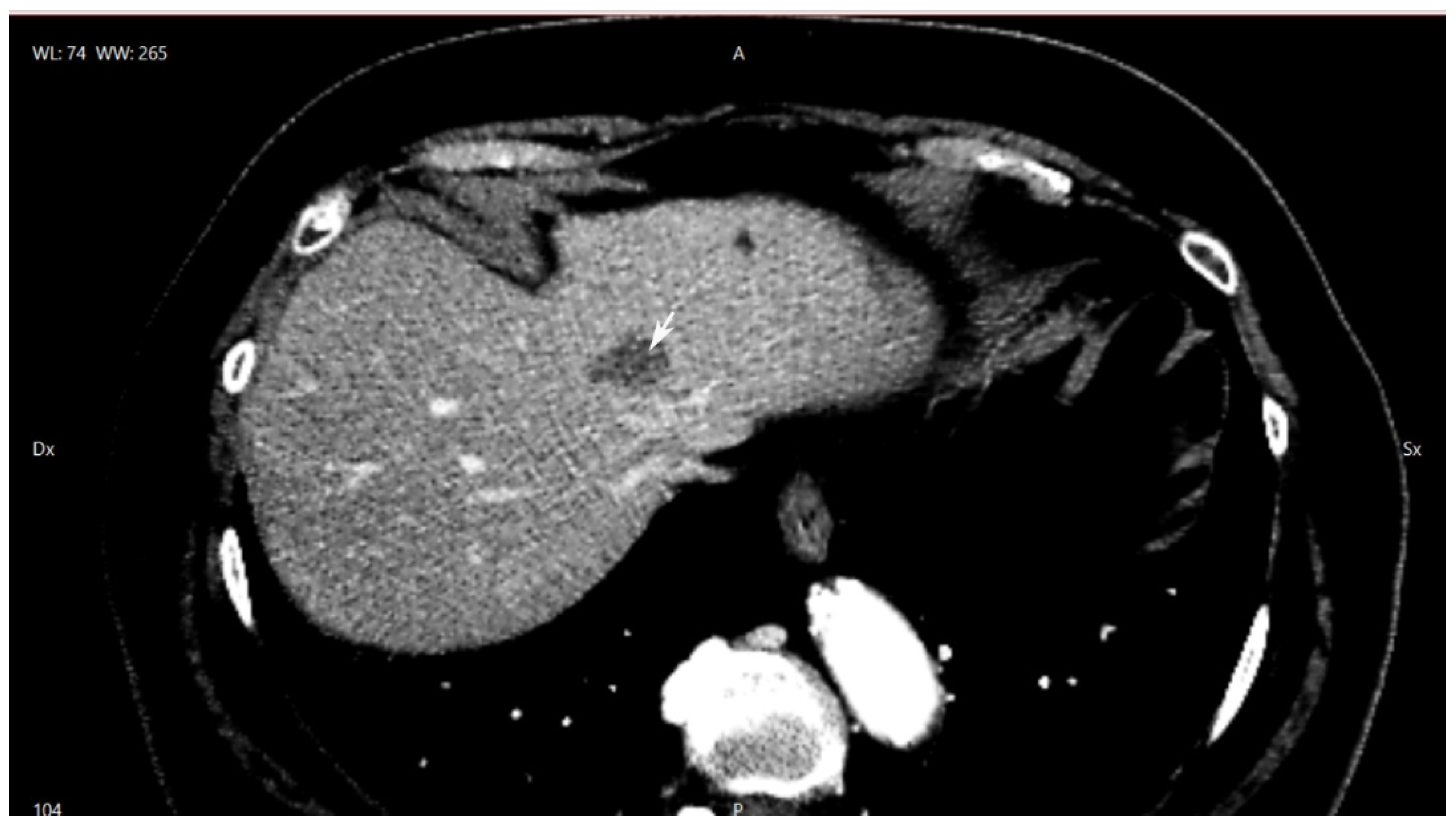
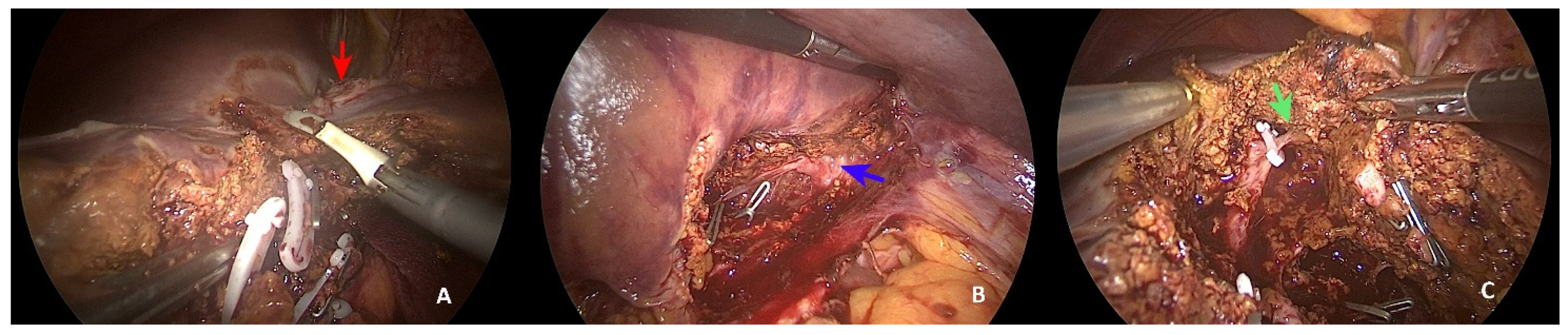
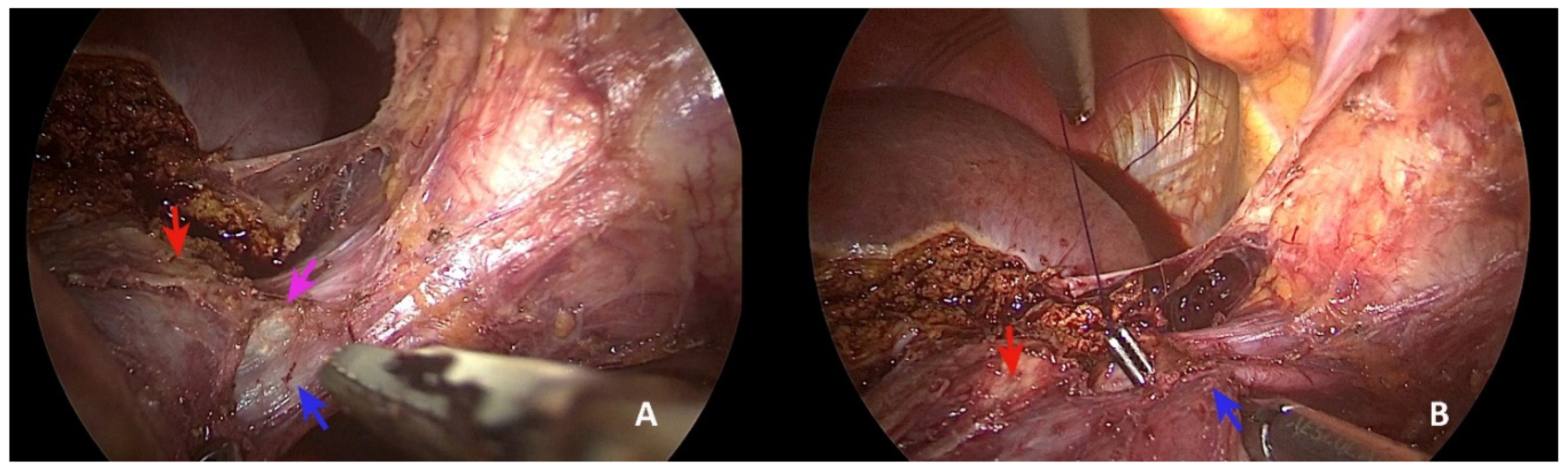
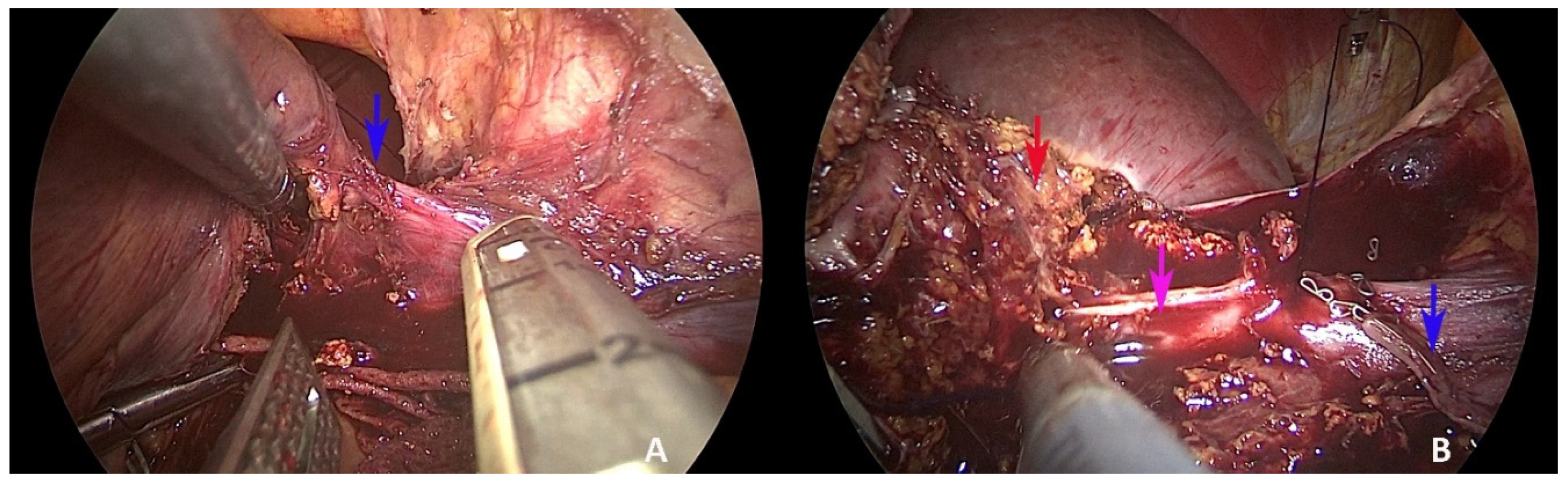
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Cutsem, E.V.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic Colorectal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2014, 25, iii1–iii9. [Google Scholar] [CrossRef] [PubMed]
- Bosma, N.A.; Keehn, A.R.; Lee-Ying, R.; Karim, S.; MacLean, A.R.; Brenner, D.R. Efficacy of Perioperative Chemotherapy in Resected Colorectal Liver Metastasis: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2021, 47, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Hackl, C.; Neumann, P.; Gerken, M.; Loss, M.; Klinkhammer-Schalke, M.; Schlitt, H.J. Treatment of Colorectal Liver Metastases in Germany: A Ten-Year Population-Based Analysis of 5772 Cases of Primary Colorectal Adenocarcinoma. BMC Cancer 2014, 14, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, R.B.; Aloia, T.A.; Loyer, E.; Pawlik, T.M.; Taouli, B.; Vauthey, J.-N.; Americas Hepato-Pancreato-Biliary Association; Society of Surgical Oncology. Society for Surgery of the Alimentary Tract Selection for Hepatic Resection of Colorectal Liver Metastases: Expert Consensus Statement. HPB 2013, 15, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Adam, R.; Laurent, A.; Azoulay, D.; Castaing, D.; Bismuth, H. Two-Stage Hepatectomy: A Planned Strategy to Treat Irresectable Liver Tumors. Ann. Surg. 2000, 232, 777–785. [Google Scholar] [CrossRef]
- De Santibañes, E.; Alvarez, F.A.; Ardiles, V. How to Avoid Postoperative Liver Failure: A Novel Method. World J. Surg. 2012, 36, 125–128. [Google Scholar] [CrossRef]
- Torzilli, G.; Cimino, M.M. Extending the Limits of Resection for Colorectal Liver Metastases ENHANCED ONE STAGE SURGERY. J. Gastrointest. Surg. 2017, 21, 187–189. [Google Scholar] [CrossRef]
- Viganò, L.; Costa, G.; Cimino, M.M.; Procopio, F.; Donadon, M.; Del Fabbro, D.; Belghiti, J.; Kokudo, N.; Makuuchi, M.; Vauthey, J.-N.; et al. R1 Resection for Colorectal Liver Metastases: A Survey Questioning Surgeons about Its Incidence, Clinical Impact, and Management. J. Gastrointest. Surg. 2018, 22, 1752–1763. [Google Scholar] [CrossRef]
- Chan, J.; Diakos, C.I.; Chan, D.L.H.; Engel, A.; Pavlakis, N.; Gill, A.J.; Clarke, S.J. Colorectal Cancer Sidedeness and Its Association with Survival and Tumor Biology in Operable Patients. JCO 2017, 35, e15110. [Google Scholar] [CrossRef]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal Liver Metastases: Current Management and Future Perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef] [PubMed]
- Dörr, N.M.; Bartels, M.; Morgul, M.H. Current Treatment of Colorectal Liver Metastasis as a Chronic Disease. Anticancer Res. 2020, 40, 1–7. [Google Scholar] [CrossRef]
- Dong, J.; Huang, Z. Precise liver resection-new concept of liver surgery in 21st century. Chin. J. Surg. 2009, 47, 1601–1605. [Google Scholar] [PubMed]
- Zhai, S.; Sun, X.; Du, L.; Chen, K.; Zhang, S.; Shi, Y.; Yuan, F. Role of Surgical Approach to Synchronous Colorectal Liver Metastases: A Retrospective Analysis. CMAR 2021, 13, 3699–3711. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.; Salminen, T.; Soveri, L.-M.; Kallio, R.; Kellokumpu, I.; Lamminmäki, A.; Halonen, P.; Ristamäki, R.; Lantto, E.; Uutela, A.; et al. Repeated Centralized Multidisciplinary Team Assessment of Resectability, Clinical Behavior, and Outcomes in 1086 Finnish Metastatic Colorectal Cancer Patients (RAXO): A Nationwide Prospective Intervention Study. Lancet Reg. Health-Eur. 2021, 3, 100049. [Google Scholar] [CrossRef]
- Mentha, G.; Majno, P.E.; Andres, A.; Rubbia-Brandt, L.; Morel, P.; Roth, A.D. Neoadjuvant Chemotherapy and Resection of Advanced Synchronous Liver Metastases before Treatment of the Colorectal Primary. Br. J. Surg. 2006, 93, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.S.; Bae, M.K.; Choi, J.K.; Hong, Y.K.; Park, I.K. Pulmonary Metastasectomy in Colorectal Cancer: A Population-Based Retrospective Cohort Study Using the Korean National Health Insurance Database. Cancer Res. Treat. 2021, 53, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, J.-M.; Wang, K.; Wang, H.-W.; Xing, B.-C. Recurrent Colorectal Liver Metastasis Patients Could Benefit from Repeat Hepatic Resection. BMC Surg. 2021, 21, 327. [Google Scholar] [CrossRef]
- Alvarez, F.A.; Sanchez Claria, R.; Oggero, S.; de Santibañes, E. Parenchymal-Sparing Liver Surgery in Patients with Colorectal Carcinoma Liver Metastases. World J. Gastrointest. Surg. 2016, 8, 407–423. [Google Scholar] [CrossRef]
- Lamade, W. The Impact of 3-Dimensional Reconstructions on Operation Planning in Liver Surgery. Arch. Surg. 2000, 135, 1256–1261. [Google Scholar] [CrossRef] [Green Version]
- Banchini, F.; Romboli, A.; Rizzi, N.; Luzietti, E.; Conti, L.; Capelli, P. Laparoscopic Dorsal Subsegmentectomy 8: Exploit the 3D Technology to Plan Liver Resection, and Predict Intraparenchymal Pedicles. A Case Report (With Video Explanation). Int. J. Surg. Case Rep. 2021, 88, 106516. [Google Scholar] [CrossRef] [PubMed]
- Banchini, F.; Luzietti, E.; Cecconi, S.; Ribolla, M.; Palmieri, G.; Capelli, P. Achieving Precision Surgery in Laparoscopic Liver Resection with the Aid of Preoperative Three-Dimensional Reconstruction: A Case Report. Int. J. Surg. Case Rep. 2021, 81, 105792. [Google Scholar] [CrossRef]
- Takasaki, K. Glissonean Pedicle Transection Method for Hepatic Resection: A New Concept of Liver Segmentation. J. Hepato-Biliary-Pancreat. Surg. 1998, 5, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, M.; Ishizawa, T.; Mise, Y.; Inoue, Y.; Ito, H.; Takahashi, Y.; Saiura, A. Applications of Fusion-Fluorescence Imaging Using Indocyanine Green in Laparoscopic Hepatectomy. Surg. Endosc. 2017, 31, 5111–5118. [Google Scholar] [CrossRef]
- Berardi, G.; Colasanti, M.; Meniconi, R.L.; Ferretti, S.; Guglielmo, N.; Mariano, G.; Burocchi, M.; Campanelli, A.; Scotti, A.; Pecoraro, A.; et al. The Applications of 3D Imaging and Indocyanine Green Dye Fluorescence in Laparoscopic Liver Surgery. Diagnostics 2021, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-M.; Xiong, J.-J.; Liu, X.-T.; Chen, H.-Y.; Iglesia-García, D.; Altaf, K.; Bharucha, S.; Huang, W.; Nunes, Q.M.; Szatmary, P.; et al. Laparoscopic Versus Open Liver Resection for Colorectal Liver Metastases: A Comprehensive Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 1012. [Google Scholar] [CrossRef] [PubMed]
- Sena, G.; Picciariello, A.; Marino, F.; Goglia, M.; Rocca, A.; Meniconi, R.L.; Gallo, G. One-Stage Total Laparoscopic Treatment for Colorectal Cancer With Synchronous Metastasis. Is It Safe and Feasible? Front. Surg. 2021, 8, 752135. [Google Scholar] [CrossRef]
- Lupinacci, R.M.; Andraus, W.; De Paiva Haddad, L.B.; Carneiro D’ Albuquerque, L.A.; Herman, P. Simultaneous Laparoscopic Resection of Primary Colorectal Cancer and Associated Liver Metastases: A Systematic Review. Tech. Coloproctol. 2014, 18, 129–135. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, X.; Luo, R.; Cai, X. The Clinical Risk Factors Associated with Postoperative Bile Leakage after Hepatectomy: A Meta-Analysis. Minerva Med. 2016, 107, 39–53. [Google Scholar]
- Agarwal, V.; Divatia, J.V. Enhanced Recovery after Surgery in Liver Resection: Current Concepts and Controversies. Korean J. Anesth. 2019, 72, 119–129. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banchini, F.; Luzietti, E.; Palmieri, G.; Bonfili, D.; Romboli, A.; Conti, L.; Capelli, P. Laparoscopic Resection of Synchronous Liver Metastasis Involving the Left Hepatic Vein and the Common Trunk Bifurcation: A Strategy of Parenchyma-Sparing Resection with Left Sectionectomy and 4a Subsegmentectomy by Arantius Approach. Healthcare 2022, 10, 517. https://doi.org/10.3390/healthcare10030517
Banchini F, Luzietti E, Palmieri G, Bonfili D, Romboli A, Conti L, Capelli P. Laparoscopic Resection of Synchronous Liver Metastasis Involving the Left Hepatic Vein and the Common Trunk Bifurcation: A Strategy of Parenchyma-Sparing Resection with Left Sectionectomy and 4a Subsegmentectomy by Arantius Approach. Healthcare. 2022; 10(3):517. https://doi.org/10.3390/healthcare10030517
Chicago/Turabian StyleBanchini, Filippo, Enrico Luzietti, Gerardo Palmieri, Deborah Bonfili, Andrea Romboli, Luigi Conti, and Patrizio Capelli. 2022. "Laparoscopic Resection of Synchronous Liver Metastasis Involving the Left Hepatic Vein and the Common Trunk Bifurcation: A Strategy of Parenchyma-Sparing Resection with Left Sectionectomy and 4a Subsegmentectomy by Arantius Approach" Healthcare 10, no. 3: 517. https://doi.org/10.3390/healthcare10030517
APA StyleBanchini, F., Luzietti, E., Palmieri, G., Bonfili, D., Romboli, A., Conti, L., & Capelli, P. (2022). Laparoscopic Resection of Synchronous Liver Metastasis Involving the Left Hepatic Vein and the Common Trunk Bifurcation: A Strategy of Parenchyma-Sparing Resection with Left Sectionectomy and 4a Subsegmentectomy by Arantius Approach. Healthcare, 10(3), 517. https://doi.org/10.3390/healthcare10030517





