Effectiveness of Constraint-Induced Movement Therapy (CIMT) on Balance and Functional Mobility in the Stroke Population: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methodology
2.1. Selection Criteria
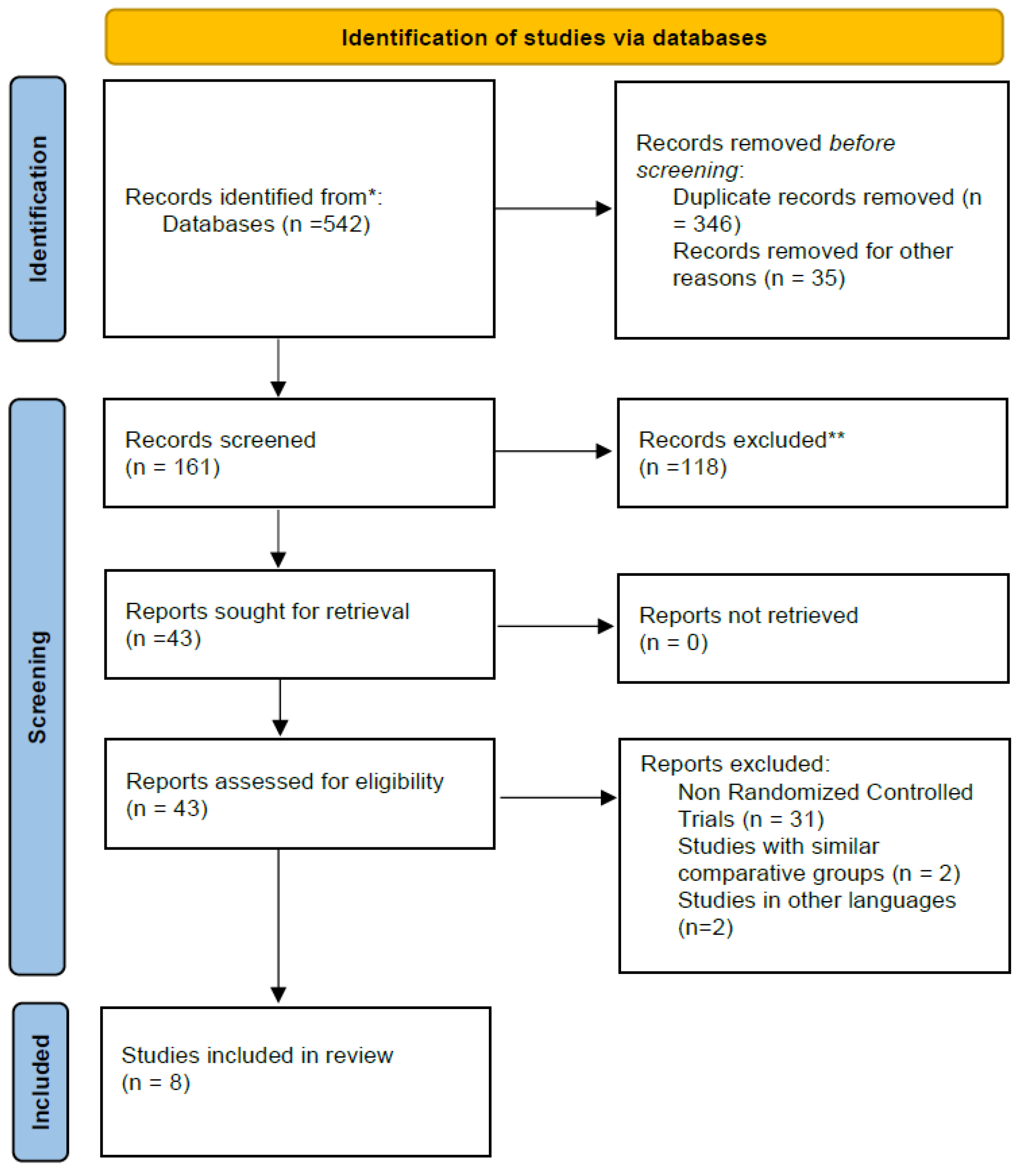
2.2. Search and Study Selection Process
2.3. Quality Evaluation of Involved RCTs
2.4. Data Analysis
3. Results
3.1. Search Results
3.2. Quality Assessment of the Involved RCTs
3.3. Descriptions of Included RCTs
3.4. Outcome Measures
3.5. Quantitative Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avan, A.; Digaleh, H.; Di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Draaisma, L.R.; Wessel, M.J.; Hummel, F.C. Non-invasive brain stimulation to enhance cognitive rehabilitation after stroke. Neurosci. Lett. 2020, 719, 133678. [Google Scholar] [CrossRef] [PubMed]
- Baniqued, P.D.E.; Stanyer, E.C.; Awais, M.; Alazmani, A.; Jackson, A.E.; Mon-Williams, M.A.; Mushtaq, F.; Holt, R.J. Brain–computer interface robotics for hand rehabilitation after stroke: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Tedla, J.S. Effectiveness of robotics in improving upper extremity functions among people with neurological dysfunction: A systematic review. Int. J. Neurosci. 2019, 129, 369–383. [Google Scholar] [CrossRef]
- Tedla, J.S.; Dixit, S.; Gular, K.; Abohashrh, M. Robotic-assisted gait training effect on function and gait speed in subacute and chronic stroke population: A systematic review and meta-analysis of randomized controlled trials. Eur. Neurol. 2019, 81, 103–111. [Google Scholar] [CrossRef]
- Kubis, N. Non-invasive brain stimulation to enhance post-stroke recovery. Front. Neural Circuits 2016, 10, 56. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Stroke 2018, 49, e160–e161. [Google Scholar] [CrossRef]
- Fathima, S.; Shankar, S.; Thajudeen, A.A. Activities of daily living rehab game play system with augmented reality based gamification therapy for automation of post stroke upper limb rehabilitation. J. Comput. Theor. Nanosci. 2018, 15, 1445–1451. [Google Scholar] [CrossRef]
- Guiu-Tula, F.X.; Cabanas-Valdés, R.; Sitjà-Rabert, M.; Urrútia, G.; Gómara-Toldrà, N. The efficacy of the proprioceptive neuromuscular facilitation (PNF) approach in stroke rehabilitation to improve basic activities of daily living and quality of life: A systematic review and meta-analysis protocol. BMJ Open 2017, 7, e016739. [Google Scholar] [CrossRef]
- Alashram, A.R.; Alghwiri, A.A.; Padua, E.; Annino, G. Efficacy of proprioceptive neuromuscular facilitation on spasticity in patients with stroke: A systematic review. Phys. Ther. Rev. 2021, 26, 168–176. [Google Scholar] [CrossRef]
- Gunning, E.; Uszynski, M.K. Effectiveness of the proprioceptive neuromuscular facilitation method on gait parameters in patients with stroke: A systematic review. Arch. Phys. Med. Rehabil. 2019, 100, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Iłżecka, J. Constraint-induced movement therapy in stroke patients. J. Educ. Health Sport 2020, 10, 50–59. [Google Scholar] [CrossRef][Green Version]
- da Silva, E.S.M.; Ocamoto, G.N.; Santos-Maia, G.L.d.; de Fatima Carreira Moreira Padovez, R.; Trevisan, C.; de Noronha, M.A.; Pereira, N.D.; Borstad, A.; Russo, T.L. The Effect of Priming on Outcomes of Task-Oriented Training for the Upper Extremity in Chronic Stroke: A Systematic Review and Meta-analysis. Neurorehabilit. Neural Repair 2020, 34, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-C.; Chang, Y.-F.; Wu, C.-Y.; Chen, Y.-A. Effects of constraint-induced therapy versus bilateral arm training on motor performance, daily functions, and quality of life in stroke survivors. Neurorehabilit. Neural Repair 2009, 23, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.C.; Conforto, A.B.; Orsini, M.; Stern, A.; Andreé, C. Similar effects of two modified constraint-induced therapy protocols on motor impairment, motor function and quality of life in patients with chronic stroke. Neurol. Int. 2015, 7, 5430. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, S.; Morris, D.; Taub, E. Constraint-induced movement therapy for lower extremity function: Describing the LE-CIMT protocol. Phys. Ther. 2020, 100, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Truijen, S.; Umar, N.A.; Useh, U.; Egwuonwu, V.A.; Van Criekinge, T.; Saeys, W. Effects of Lower Limb Constraint Induced Movement Therapy in People With Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 343. [Google Scholar] [CrossRef]
- Çoban, O. İnmeli Hastalarda Mekanik Hippoterapi Cihazı Ile Yapılan Egzersizin Postural Kontrol ve Denge Üzerine Etkisi; İstanbul Medipol Üniversitesi Sağlık Bilimleri Enstitüsü: İstanbul, Turkey, 2019. [Google Scholar]
- Bang, D.-H.; Shin, W.-S.; Choi, S.-J. The effects of modified constraint-induced movement therapy combined with trunk restraint in subacute stroke: A double-blinded randomized controlled trial. Clin. Rehabil. 2015, 29, 561–569. [Google Scholar] [CrossRef]
- Bang, D.-H.; Shin, W.-S.; Choi, H.-S. Effects of modified constraint-induced movement therapy with trunk restraint in early stroke patients: A single-blinded, randomized, controlled, pilot trial. NeuroRehabilitation 2018, 42, 29–35. [Google Scholar] [CrossRef]
- Choi, H.-S.; Shin, W.-S.; Bang, D.-H.; Choi, S.-J. Effects of game-based constraint-induced movement therapy on balance in patients with stroke: A single-blind randomized controlled trial. Am. J. Phys. Med. Rehabil. 2017, 96, 184–190. [Google Scholar] [CrossRef]
- Fuzaro, A.C.; Guerreiro, C.T.; Galetti, F.C.; Jucá, R.B.; Araujo, J.E.d. Modified constraint-induced movement therapy and modified forced-use therapy for stroke patients are both effective to promote balance and gait improvements. Braz. J. Phys. Ther. 2012, 16, 157–165. [Google Scholar] [CrossRef] [PubMed]
- e Silva, E.M.G.d.S.; Ribeiro, T.S.; da Silva, T.C.C.; Costa, M.F.P.; Cavalcanti, F.A.d.C.; Lindquist, A.R.R. Effects of constraint-induced movement therapy for lower limbs on measurements of functional mobility and postural balance in subjects with stroke: A randomized controlled trial. Top. Stroke Rehabil. 2017, 24, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, T.L.; Barr, J.O.; Moran, M.L. Geriatric Rehabilitation Manual; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Norvang, O.P.; Askim, T.; Egerton, T.; Dahl, A.E.; Thingstad, P. Associations between changes in gait parameters, balance, and walking capacity during the first 3 months after stroke: A prospective observational study. Physiother. Theory Pract. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, T.-H. The effects of balance and gait function on quality of life of stroke patients. NeuroRehabilitation 2019, 44, 37–41. [Google Scholar] [CrossRef]
- Gasq, D.; Labrunée, M.; Amarantini, D.; Dupui, P.; Montoya, R.; Marque, P. Between-day reliability of centre of pressure measures for balance assessment in hemiplegic stroke patients. J. Neuroeng. Rehabil. 2014, 11, 39. [Google Scholar] [CrossRef]
- do Carmo, A.A.; Kleiner, A.F.R.; Barros, R.M. Alteration in the center of mass trajectory of patients after stroke. Top. Stroke Rehabil. 2015, 22, 349–356. [Google Scholar] [CrossRef]
- Kamphuis, J.F.; de Kam, D.; Geurts, A.C.; Weerdesteyn, V. Is weight-bearing asymmetry associated with postural instability after stroke? A systematic review. Stroke Res. Treat. 2013, 2013, 692137. [Google Scholar] [CrossRef]
- Merchán-Baeza, J.A.; González-Sánchez, M.; Cuesta-Vargas, A. Mobile functional reach test in people who suffer stroke: A pilot study. JMIR Rehabil. Assist. Technol. 2015, 2, e4102. [Google Scholar] [CrossRef][Green Version]
- Alghadir, A.H.; Al-Eisa, E.S.; Anwer, S.; Sarkar, B. Reliability, validity, and responsiveness of three scales for measuring balance in patients with chronic stroke. BMC Neurol. 2018, 18, 141. [Google Scholar] [CrossRef]
- Aruin, A.S.; Rao, N.; Sharma, A.; Chaudhuri, G. Compelled body weight shift approach in rehabilitation of individuals with chronic stroke. Top. Stroke Rehabil. 2012, 19, 556–563. [Google Scholar] [CrossRef]
- Kim, N.-H.; Cha, Y.-J. Effect of gait training with constrained-induced movement therapy (CIMT) on the balance of stroke patients. J. Phys. Ther. Sci. 2015, 27, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, C.; Liu, Y.; Liu, J.; Jin, J.; Zhang, S.; Bai, Y.; Huang, D.; Zhu, B.; Xu, Y. Effects of modified constraint-induced movement therapy on the lower extremities in patients with stroke: A pilot study. Disabil. Rehabil. 2016, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Candan, S.; Livanelioglu, A. Effects of modified constraint-ınduced movement therapy for lower limb on motor function in stroke patients: A randomized controlled study. Int. J. Physiother 2017, 4, 269–277. [Google Scholar] [CrossRef]
- Silva, E.M.d.; Albuquerque, J.A.d. Influence of constraint induced movement therapy on functional performance in stroke patients: A randomized clinical trial. Fisioter. E Pesqui. 2017, 24, 184–190. [Google Scholar]
- Peurala, S.H.; Kantanen, M.P.; Sjögren, T.; Paltamaa, J.; Karhula, M.; Heinonen, A. Effectiveness of constraint-induced movement therapy on activity and participation after stroke: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2012, 26, 209–223. [Google Scholar] [CrossRef]
- Corbetta, D.; Sirtori, V.; Moja, L.; Gatti, R. Constraint-induced movement therapy in stroke patients: Systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2010, 46, 537–544. [Google Scholar]
- McIntyre, A.; Viana, R.; Janzen, S.; Mehta, S.; Pereira, S.; Teasell, R. Systematic review and meta-analysis of constraint-induced movement therapy in the hemiparetic upper extremity more than six months post stroke. Top. Stroke Rehabil. 2012, 19, 499–513. [Google Scholar] [CrossRef]
- Etoom, M.; Hawamdeh, M.; Hawamdeh, Z.; Alwardat, M.; Giordani, L.; Bacciu, S.; Scarpini, C.; Foti, C. Constraint-induced movement therapy as a rehabilitation intervention for upper extremity in stroke patients: Systematic review and meta-analysis. Int. J. Rehabil. Res. 2016, 39, 197–210. [Google Scholar] [CrossRef]
- Wagenaar, R.; Van Emmerik, R. Resonant frequencies of arms and legs identify different walking patterns. J. Biomech. 2000, 33, 853–861. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Anson, D.; Haller, S. Postural sway biofeedback: Its effect on reestablishing stance stability in hemiplegic patients. Arch. Phys. Med. Rehabil. 1988, 69, 395–400. [Google Scholar]
- Bennie, S.; Bruner, K.; Dizon, A.; Fritz, H.; Goodman, B.; Peterson, S. Measurements of balance: Comparison of the Timed “Up and Go” test and Functional Reach test with the Berg Balance Scale. J. Phys. Ther. Sci. 2003, 15, 93–97. [Google Scholar] [CrossRef]



| Databases (Number of Articles) | PICO Format Search with Bullion Key Words (and)(or) | |||
|---|---|---|---|---|
| Patient | Intervention | Comparison | Outcome | |
| EBSCO (95) PubMed (96) PEDro (47) Science Direct (154) Scopus (81) Web of Science (69) | Stroke OR Hemiplegia OR Hemiparesis OR Cerebrovascular accident | CIMT OR Constraint-induced movement therapy OR Restricted Limb/Extremity OR | Neuro Developmental Treatment OR NDT OR Proprioceptive Neuromuscular Facilitation OR PNF OR Conventional Physical Therapy OR CPT OR Physiotherapy OR Exercise OR Forced Arm Use Or Traditional Rehabilitation OR Standard Physical Therapy | Balance OR Functional Mobility OR Center Of Gravity OR Base of Support OR Center of Pressure |
| S.No | Author/Year | Eligibility Criteria | Random Allocation | Concealed Allocation | Baseline Comparability | Blinding of Participants | Blinding of Therapist | Blinding of Assessor | Adequate Follow up (>85%) | Intention to Treat | Between Group Comparison | Point Estimates and Variability | Pedro Score [10] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aruin AS et al., 2012 [32]. | Yes | Yes | No | Yes | No | No | No | No | No | Yes | Yes | 4 |
| 2 | Fuzaro AC et al., 2012 [22]. | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | 6 |
| 3 | Kim NH et al., 2015 [33]. | No | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 |
| 4 | Zhu Y et al., 2016 [34]. | Yes | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | 5 |
| 5 | e Silva EMG de S et al., 2017 [23]. | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 6 | Candan SA et al., 2017 [35]. | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
| 7 | Choi HS et al., 2017 [21]. | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes | Yes | 6 |
| 8 | Silva EM et al., 2017 [36]. | Yes | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | 5 |
| S.No | Author/Year | Age | Chronicity | Intervention | Duration | Outcome Measures | Inferences | |
|---|---|---|---|---|---|---|---|---|
| Experimental | Control | |||||||
| 1 | Aruin AS et al., 2012 [32]. | 57.7 ± 311.9 | 6.7 ± 3.9 Years | The management focused on accelerating muscle strength, balance, and symmetrical weight bearing by forcing body shift towards affected lower limb by shoe insert | Conventional physical therapy | 60 min per session, 1 session per week, six sessions—in total 6 h | SWB, gait speed (m/s), BBS, FM for lower limb | Post- and follow-up retention were observed for symmetrical weight bearing, gait speed, and BBS in experimental group. |
| 2 | Fuzaro AC et al., 2012 [22]. | 52.46 ± 14.29 | Experimental 19.5 ± 20.8 Months Control 30.8 ± 31.8 Months | Bimanual activities in special tasks with paretic upper limb as a main conductor with immobilization of non-paretic upper limb | Modified forced-use therapy | 50 min per single session, five sessions each week for 4 weeks | SIS, BBS, FM, T10, and TUG | Both m-CIMT and m-FUT showed equal improvements for balance, functional mobility, motor functions and quality of life at post- and follow-up sessions |
| 3 | Kim NH et al., 2015 [33]. | 54.75 ± 4.9 | Experimental 24.1 ± 10.7 Months Control 30.8 ± 11.0 Months | Overground gait training with non-paretic upper-limb constraint | Overground gait training without non-paretic upper- limb constraint | 30 min per session, three sessions each week for 4 weeks adding to central nervous system developmental treatment for 60 min per session, five times each week, over the same 4 weeks | TIS (static, dynamic and coordination) LOS TAS and LOS TUS (cm) | Experimental group showed significant improvements for TIS and LOS |
| 4 | Zhu Y et al., 2016 [34]. | 58.71 ± 6.02 | Experimental 3.90 ± 0.83 Months Control 3.72 ± 0.78 Months | Gait training consists of 2 h of sit-to-stand transfers, indoor walking, climbing up and down stairs, balance training and one-leg weight training with more repetitions in addition to this standard comprehensive rehabilitation for 45 min | Conventional physical therapy | 45 min per session, five sessions per week for four weeks | COM displacements gait speed (m/s), step width (m), step length paretic and no-paretic side (m). Paretic and non-paretic swing time (%GC) | m-CIMT gait training improved both COM displacements and spatial-temporal gait parameters |
| 5 | e Silva EMG de S et al., 2017 [23]. | 57.75 ± 3.75 | Experimental 3.5 ± 1.73 Months Control 3.7 ± 1.4 Months | Treadmill training with ankle weight on normal lower limb | Treadmill training without restraint | Nine training sessions, 30 min per session, 2 consecutive weeks | BBS, turn speed (m/s), TUG, stride time (s), stride length (s), symmetry ratio, and stride width (m) | Spatial-temporal gait parameters, balance and functional mobility improved in both groups equally. |
| 6 | Candan SA et al., 2017 [35]. | 56.4 ± 13.45 | Experimental 6.8 ± 2.7 Months Control 6.6 ± 3.1 Months | m-CIMT includes intensive practice, restraint of non-paretic lower limb and transfer package | NDT program | 120 min per session, five sessions per week, for 2 weeks | BBS, FAC, gait speed, cadence (steps/min), step length ratio and postural symmetry | Balance, functional ambulation, gait speed, and step length ratio improved significantly in m-CIMT group when compared to NDT group |
| 7 | Choi HS et al., 2017 [21]. | 61.58 ± 5.83 | Experimental 13.5 ± 5.5 Months Control 14.2 ± 4.8 Months | Game-based CIMT group undertook game-based CIMT and conventional physical therapy | Conventional physical therapy including NDT | Game-based CIMT for 30 min in a session, for three sessions a week for 4 weeks. All subjects, comprising those in the control group, underwent conventional physical therapy for 60 min a session, five sessions a week for 4 weeks | COP displacements medial-lateral and anterior-posterior (cm), sway area (cm2), sway mean velocity (cm/s), SWB, FRT (cm), m-FRT (cm), and TUG (s) | Game-based CIMT showed significant improvements in static balance, symmetrical weight bearing, and medial-lateral shift compared to control group |
| 8 | Silva EM et al., 2017 [36]. | 55.63 ± 4.93 | Experimental 13.7 ± 3.4 Months Control 29.2 ± 10.1 Months | Encouraging paretic limb to perform specific activities with constraint of non- paretic upper limb | Conventional physical therapy without constraining non-paretic upper limb | 30 min per each session, three sessions per week for 4 weeks | BBS, TUG, stairs, and gait speed | Post-intervention improvement was observed in experimental group for gait speed, BBS, TUG, and stairs up and down without any significant difference between the groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedla, J.S.; Gular, K.; Reddy, R.S.; de Sá Ferreira, A.; Rodrigues, E.C.; Kakaraparthi, V.N.; Gyer, G.; Sangadala, D.R.; Qasheesh, M.; Kovela, R.K.; et al. Effectiveness of Constraint-Induced Movement Therapy (CIMT) on Balance and Functional Mobility in the Stroke Population: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 495. https://doi.org/10.3390/healthcare10030495
Tedla JS, Gular K, Reddy RS, de Sá Ferreira A, Rodrigues EC, Kakaraparthi VN, Gyer G, Sangadala DR, Qasheesh M, Kovela RK, et al. Effectiveness of Constraint-Induced Movement Therapy (CIMT) on Balance and Functional Mobility in the Stroke Population: A Systematic Review and Meta-Analysis. Healthcare. 2022; 10(3):495. https://doi.org/10.3390/healthcare10030495
Chicago/Turabian StyleTedla, Jaya Shanker, Kumar Gular, Ravi Shankar Reddy, Arthur de Sá Ferreira, Erika Carvalho Rodrigues, Venkata Nagaraj Kakaraparthi, Giles Gyer, Devika Rani Sangadala, Mohammed Qasheesh, Rakesh Krishna Kovela, and et al. 2022. "Effectiveness of Constraint-Induced Movement Therapy (CIMT) on Balance and Functional Mobility in the Stroke Population: A Systematic Review and Meta-Analysis" Healthcare 10, no. 3: 495. https://doi.org/10.3390/healthcare10030495
APA StyleTedla, J. S., Gular, K., Reddy, R. S., de Sá Ferreira, A., Rodrigues, E. C., Kakaraparthi, V. N., Gyer, G., Sangadala, D. R., Qasheesh, M., Kovela, R. K., & Nambi, G. (2022). Effectiveness of Constraint-Induced Movement Therapy (CIMT) on Balance and Functional Mobility in the Stroke Population: A Systematic Review and Meta-Analysis. Healthcare, 10(3), 495. https://doi.org/10.3390/healthcare10030495









