Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases
Abstract
:1. Introduction
2. Data Source
3. Coenzyme Q10
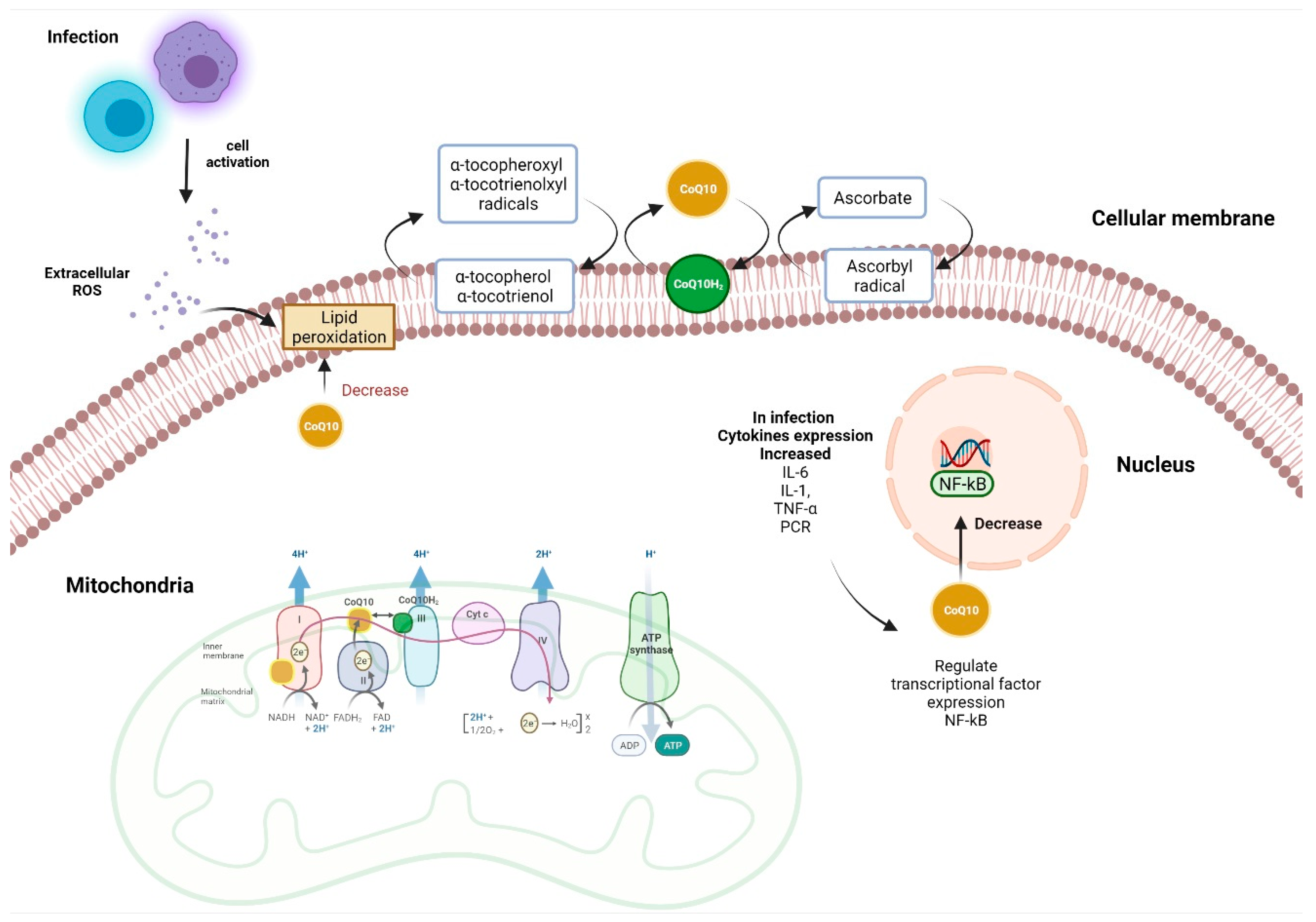
4. Mechanism of Coenzyme Q10 Action Associated Antioxidant and Anti-Inflammatory Activity
4.1. Antioxidant Functions
4.2. Anti-Inflammatory and Immune Functions
5. CoQ10 as a Possible Adjuvant in Infectious Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Further Research
References
- Quinzii, C.M.; Lopez, L.C.; Von-Moltke, J.; Naini, A.; Krishna, S.; Schuelke, M.; Salviati, L.; Navas, P.; DiMauro, S.; Hirano, M. Respiratory chain dysfunction and oxidative stress correlate with severity of primary COQ10 deficiency. FASEB J. 2008, 22, 1874–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Fonarow, G.C.; Butler, J.; Ezekowitz, J.A.; Felker, G.M. Coenzyme Q10 and heart failure: A state-of-the-art review. Circ. Heart Fail. 2016, 9, e002639. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Arenas-de Larriva, A.P.; Limia-Perez, L.; Romero-Cabrera, J.L.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2020, 21, 7870. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Kaminski, V.L.; Chies, J. Emerging infectious disease prevention: Where should we invest our resources and efforts? J. Infect. Public Health 2019, 12, 313–316. [Google Scholar] [CrossRef]
- Nii-Trebi, N.I. Emerging and Neglected Infectious Diseases: Insights, Advances, and Challenges. BioMed. Res. Int. 2017, 2017, 5245021. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline, Including Tuberculosis; WHO: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/258965 (accessed on 20 February 2022).
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients 2020, 12, 2358. [Google Scholar] [CrossRef]
- Szkopińska, A. Ubiquinone. Biosynthesis of quinone ring and its isoprenoid side chain. Intracellular localization. Acta Biochim. Pol. 2000, 47, 469–480. [Google Scholar] [CrossRef] [Green Version]
- MBentinger, M.; Tekle, G. Dallner Coenzyme Q–biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010, 396, 74–79. [Google Scholar] [CrossRef]
- Pallotti, F.; Bergamini, C.; Lamperti, C.; Fato, R. The Roles of Coenzyme Q in Disease: Direct and Indirect Involvement in Cellular Functions. Int. J. Mol. Sci. 2021, 23, 128. [Google Scholar] [CrossRef]
- Pravst, I.; Zmitek, K.; Zmitek, J. Coenzyme Q10 contents in foods and fortification strategies. Crit. Rev. Food Sci. Nutr. 2020, 50, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Dybring, A. Bioavailability of Coenzyme Q10: An Overview of the Absorption Process and Subsequent Metabolism. Antioxidants 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Raizner, A.E. Coenzyme Q10. Methodist DeBakey Cardiovasc. J. 2019, 15, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and Immune Function: An Overview. Antioxidants 2021, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Xu, H.; Luo, X.; Yu, J.; Liu, J.; Chang, R.C. Neuroprotection of Coenzyme Q10 in Neurodegenerative Diseases. Curr. Top. Med. Chem. 2016, 16, 858–866. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Yang, K.L.; Zeng, L.T.; Wu, X.H.; Huang, H.Y. Effectiveness of Coenzyme Q10 Supplementation for Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2018, 2018, 6484839. [Google Scholar] [CrossRef]
- Hargreaves, I.P.; Mantle, D. Coenzyme Q10 Supplementation in Fibrosis and Aging. Adv. Exp. Med. Biol. 2019, 1178, 103–112. [Google Scholar]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef]
- Farough, S.; Karaa, A.; Walker, M.; Slate, N.; Dasu, T.; Verbsky, J.; Fusunyan, R.; Canapari, C.; Kinane, T.; Van Cleave, J.; et al. Coenzyme Q10 and immunity: A case report and new implications for treatment of recurrent infections in metabolic diseases. Clin. Immunol. 2014, 155, 209–212. [Google Scholar] [CrossRef]
- Hidaka, T.; Fujii, K.; Funahashi, I.; Fukutomi, N.; Hosoe, K. Safety assessment of coenzyme Q10 (CoQ10). BioFactors 2008, 32, 199–208. [Google Scholar] [CrossRef]
- Deramaudt, T.B.; Dill, C.; Bonay, M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Med. Mal. Infect. 2013, 43, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hekimi, S. Understanding Ubiquinone. Trends Cell Biol. 2016, 26, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fabra, M.; Navas, P.; Brea-Calvo, G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta 2016, 1857, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Letts, J.A.; Fiedorczuk, K.; Degliesposti, G.; Skehel, M.; Sazanov, L.A. Structures of respiratory supercomplex I+ III2 reveal functional and conformational crosstalk. Mol. Cell 2019, 75, 1131–1146.e6. [Google Scholar] [CrossRef] [Green Version]
- Sohal, R.S. Coenzyme Q and vitamin E interactions. Methods Enzymol. 2004, 378, 146–151. [Google Scholar]
- Castaneda, O.A.; Lee, S.C.; Ho, C.T.; Huang, T.C. Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J. Food Drug Anal. 2017, 25, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Torres, M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002, 166, S4–S8. [Google Scholar] [CrossRef]
- Mazidi, M.; Kengne, A.P.; Banach, M.; Lipid and Blood Pressure Meta-analysis Collaboration Group. Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2018, 128, 130–136. [Google Scholar] [CrossRef]
- Heidari, A.; Hamidi, G.; Soleimani, A.; Aghadavod, E.; Asemi, Z. Effects of Coenzyme Q10 Supplementation on Gene Expressions Related to Insulin, Lipid, and Inflammation Pathways in Patients with Diabetic Nephropathy. Iran. J. Kidney Dis. 2018, 12, 14–21. [Google Scholar]
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Döring, F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors 2008, 32, 179–183. [Google Scholar] [CrossRef]
- Farsi, F.; Mohammadshahi, M.; Alavinejad, P.; Rezazadeh, A.; Zarei, M.; Engali, K.A. Functions of Coenzyme Q10 Supplementation on Liver Enzymes, Markers of Systemic Inflammation, and Adipokines in Patients Affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J. Am. Coll. Nutr. 2016, 35, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Lindahl, T.L.; Aaseth, J.; Svensson, E.; Johansson, P. Levels of sP-selectin and hs-CRP Decrease with Dietary Intervention with Selenium and Coenzyme Q10 Combined: A Secondary Analysis of a Randomized Clinical Trial. PLoS ONE 2015, 10, e0137680. [Google Scholar]
- Alehagen, U.; Alexander, J.; Aaseth, J.; Larsson, A. Decrease in inflammatory biomarker concentration by intervention with selenium and coenzyme Q10: A subanalysis of osteopontin, osteoprotergerin, TNFr1, TNFr2 and TWEAK. J. Inflamm. 2019, 16, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkers, K.; Brown, R.; Judy, W.V.; Morita, M. Survival of cancer patients on therapy with coenzyme Q10. Biochem. Biophys. Res. Commun. 1993, 192, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, K.; Moesgaard, S.; Folkers, K. Partial and complete regression of breast cancer in patients in relation to dosage of coenzyme Q10. Biochem. Biophys. Res. Commun. 1994, 199, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-T.; Huang, Y.-C.; Cheng, S.-B.; Huang, Y.-T.; Lin, P.-T. Effects of coenzyme Q10 supplementation on antioxidant capacity and inflammation in hepatocellular carcinoma patients after surgery: A randomized, placebo-controlled trial. Nutr. J. 2015, 15, 85. [Google Scholar] [CrossRef] [Green Version]
- Dahri, M.; Tarighat-Esfanjani, A.; Asghari-Jafarabadi, M.; Hashemilar, M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr. Neurosci. 2019, 22, 607–615. [Google Scholar] [CrossRef]
- Shastri, M.D.; Shukla, S.D.; Chong, W.C.; Dua, K.; Peterson, G.M.; Patel, R.P.; Hansbro, P.M.; Eri, R.; O’Toole, R.F. Role of Oxidative Stress in the Pathology and Management of Human Tuberculosis. Oxid. Med. Cell Longev. 2018, 2018, 7695364. [Google Scholar] [CrossRef] [Green Version]
- Chase, M.; Cocchi, M.N.; Liu, X.; Andersen, L.W.; Holmberg, M.J.; Donnino, M.W. Coenzyme Q10 in acute influenza. Influenza Other Respir. Viruses 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Sumbalova, Z.; Kucharska, J.; Palacka, P.; Rausova, Z.; Langsjoen, P.H.; Langsjoen, A.M.; Gvozdjakova, A. Platelet mitochondrial function and endogenous coenzyme Q10 levels are reduced in patients after COVID-19. Bratisl. Lek. Listy 2022, 123, 9–15. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Pedersen, J.Z.; Incerpi, S. Computational studies reveal mechanism by which quinone derivatives can inhibit SARS-CoV-2. Study of embelin and two therapeutic compounds of interest, methyl prednisolone and dexamethasone. J. Infect. Public Health 2020, 13, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K.; Rehman, T.; Alam, P.; Al-Dosari, M.S.; Alqasoumi, S.I.; Mohammed, F.; Alajmi, M.F. Plant-derived antiviral drugs as novel hepatitis B virus inhibitors: Cell culture and molecular docking study. Saudi Pharm. J. 2019, 27, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Alikiaie, B.; Shafiee, F.; Amiri, H.; Mousavi, S. Coenzyme Q10 improves the survival and reduces inflammatory markers in septic patients. Bratisl. Lek. Listy 2020, 121, 154–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnino, M.W.; Mortensen, S.J.; Andersen, L.W.; Chase, M.; Berg, K.M.; Balkema, J.; Radhakrishnan, J.; Gazmuri, R.J.; Liu, X.; Cocchi, M.N. Ubiquinol (reduced Coenzyme Q10) in patients with severe sepsis or septic shock: A randomized, double-blind, placebo-controlled, pilot trial. Crit. Care 2015, 19, 275. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, F.; Roozbeh, F. The effect of coenzyme Q10 in comparison with placebo on CD4 in HIV-infected patients. Clin. Epidemiol. Glob. Health 2019, 7, 306–308. [Google Scholar] [CrossRef] [Green Version]
- De Luca, C.; Kharaeva, Z.; Raskovic, D.; Pastore, P.; Luci, A.; Korkina, L. Coenzyme Q(10), vitamin E, selenium, and methionine in the treatment of chronic recurrent viral mucocutaneous infections. Nutrition 2012, 28, 509–514. [Google Scholar] [CrossRef]
- Shao, L.; Ma, A.; Figtree, G.; Zhang, P. Combination Therapy with Coenzyme Q10 and Trimetazidine in Patients With Acute Viral Myocarditis. J. Cardiovasc. Pharmacol. 2016, 68, 150–154. [Google Scholar] [CrossRef]
- Rangel, G.R.; Camara, C.R.; Garcia, A.G.; Rodriguez, A.C.; Galarza, D.A. Could coenzyme Q10 supplementation have a role in the treatment of anti-NMDA receptor encephalitis? Acta Neurol. Belg. 2015, 115, 85–86. [Google Scholar] [CrossRef]
| Authors | Study Design | Sample Size | Objective | Results |
|---|---|---|---|---|
| Soltani et al. [44] | Randomized, controlled trial. Follow-up: 7 days-oral administration of 200 mg daily of CoQ10 |
| Evaluate the effects of CoQ10 supplementation in patients with early sepsis to determine its effects on markers of inflammation and oxidative stress, as well as the clinical impact |
|
| Dominno et al. [45] | Randomized, double-blind, pilot trial | 38 patients with severe sepsis | To evaluate the effect of parenteral administration of ubiquinol in patients with severe sepsis on markers of inflammation and markers of vascular endothelial injury. |
|
| Yousefi et al. [46] | Randomized, double blind, placebo controlled, parallel group clinical trial. Follow-up: 3 months-oral administration of 200 mg daily of CoQ10 | 73 patients with HIV infected | To determine the effect on the effects of CoQ10 on CD4 count in HIV infected patients. | In both study groups the mean TCD4+ cell count increased significantly at the end of treatment. there were no changes in markers of liver and kidney function |
| De Luca et al. [47] | Two clinical trials were carried out:
|
| To assess the effect of nutritional therapies with coenzyme Q10, RRR-a-tocopherol, selenium aspartate, and L-methionine associated with established therapies in patients with chronic mucocutaneous infections. |
|
| Shao et al. [48] | Randomized, double blind. Three study groups: CQ10 group, trimetazidine group and CQ10 + trimetazidine group Follow-up: two weeks in patients with acute viral myocarditis | CQ10 group (n = 42), trimetazidine group (n = 39), and CQ10 + trimetazidine group (n = 43) | To evaluate the effect of CQ10 and trimetazidine as monotherapy and in combination for the treatment of acute viral myocarditis. | Inflammation and oxidative markers and myocardial enzymes decreased in all group, but the combination group showed the most powerful effect |
| Rangel-Guerra et al. [49] | Case report | Case report (1) | Report the case of a patient with anti-NMDAR encephalitis than after poor response to standard immunotherapy, and demonstrate improvement after starting coenzyme Q10 (CoQ10) supplementation | A significant improvement is observed in the patient after the administration of CoQ10, however the results are not conclusive, the effects could be due to the late response to immunotherapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sifuentes-Franco, S.; Sánchez-Macías, D.C.; Carrillo-Ibarra, S.; Rivera-Valdés, J.J.; Zuñiga, L.Y.; Sánchez-López, V.A. Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases. Healthcare 2022, 10, 487. https://doi.org/10.3390/healthcare10030487
Sifuentes-Franco S, Sánchez-Macías DC, Carrillo-Ibarra S, Rivera-Valdés JJ, Zuñiga LY, Sánchez-López VA. Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases. Healthcare. 2022; 10(3):487. https://doi.org/10.3390/healthcare10030487
Chicago/Turabian StyleSifuentes-Franco, Sonia, Dellaneira Carolina Sánchez-Macías, Sandra Carrillo-Ibarra, Juan José Rivera-Valdés, Laura Y. Zuñiga, and Virginia Aleyda Sánchez-López. 2022. "Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases" Healthcare 10, no. 3: 487. https://doi.org/10.3390/healthcare10030487
APA StyleSifuentes-Franco, S., Sánchez-Macías, D. C., Carrillo-Ibarra, S., Rivera-Valdés, J. J., Zuñiga, L. Y., & Sánchez-López, V. A. (2022). Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases. Healthcare, 10(3), 487. https://doi.org/10.3390/healthcare10030487







