Value of Adding Bioelectrical Impedance Analysis to Anthropometric Indices in the Diagnosis of Metabolic Syndrome in 10–16 Years Old Schoolgirls
Abstract
:1. Introduction
2. Materials and Methods
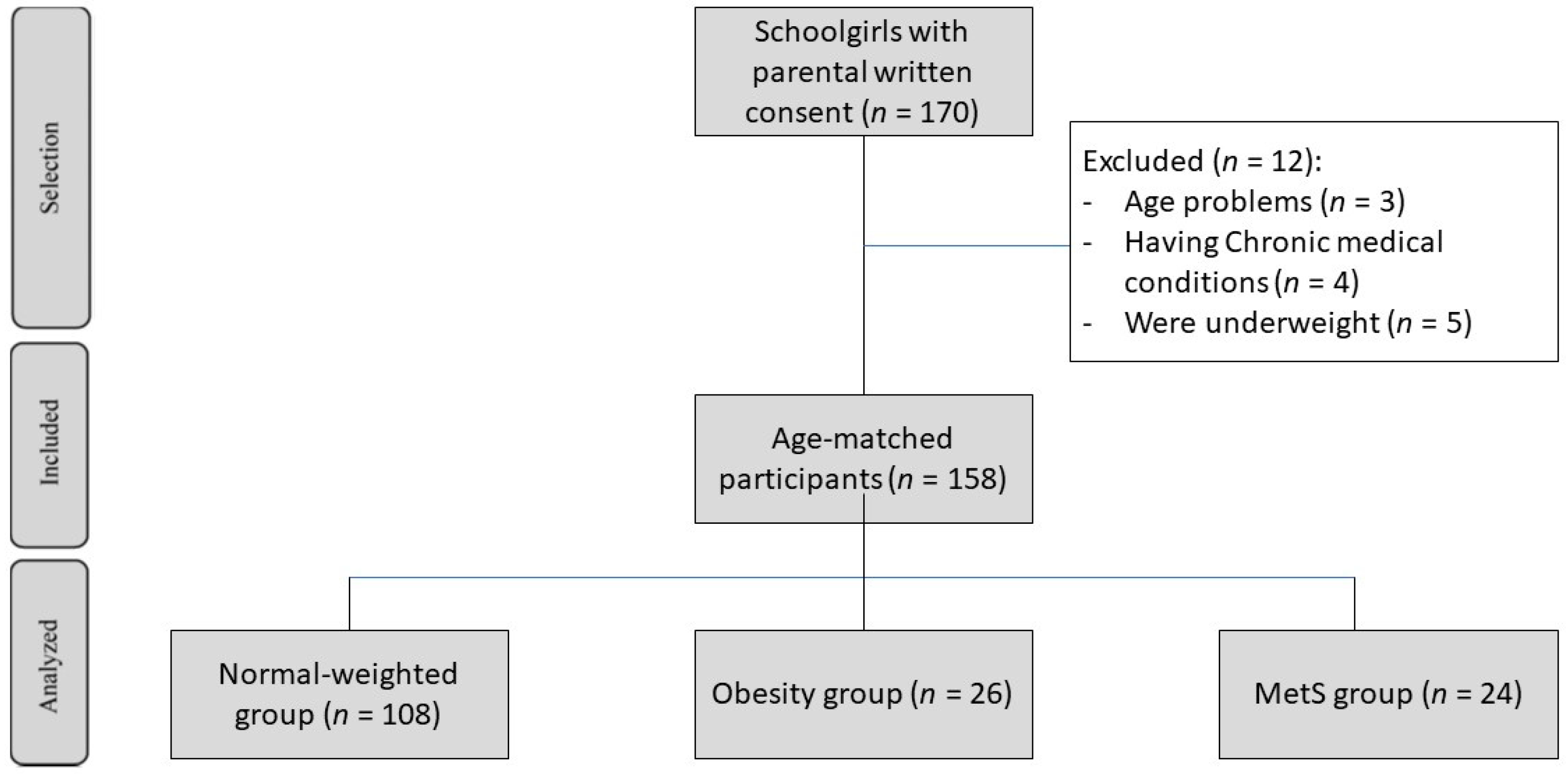
2.1. Study Design
2.2. Anthropometric Measurements
2.3. Blood Pressure and Biochemical Measures
2.4. Bioelectrical Impedance Analysis
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Correlations of Anthropometric Measurements and BIA with the Components of MetS
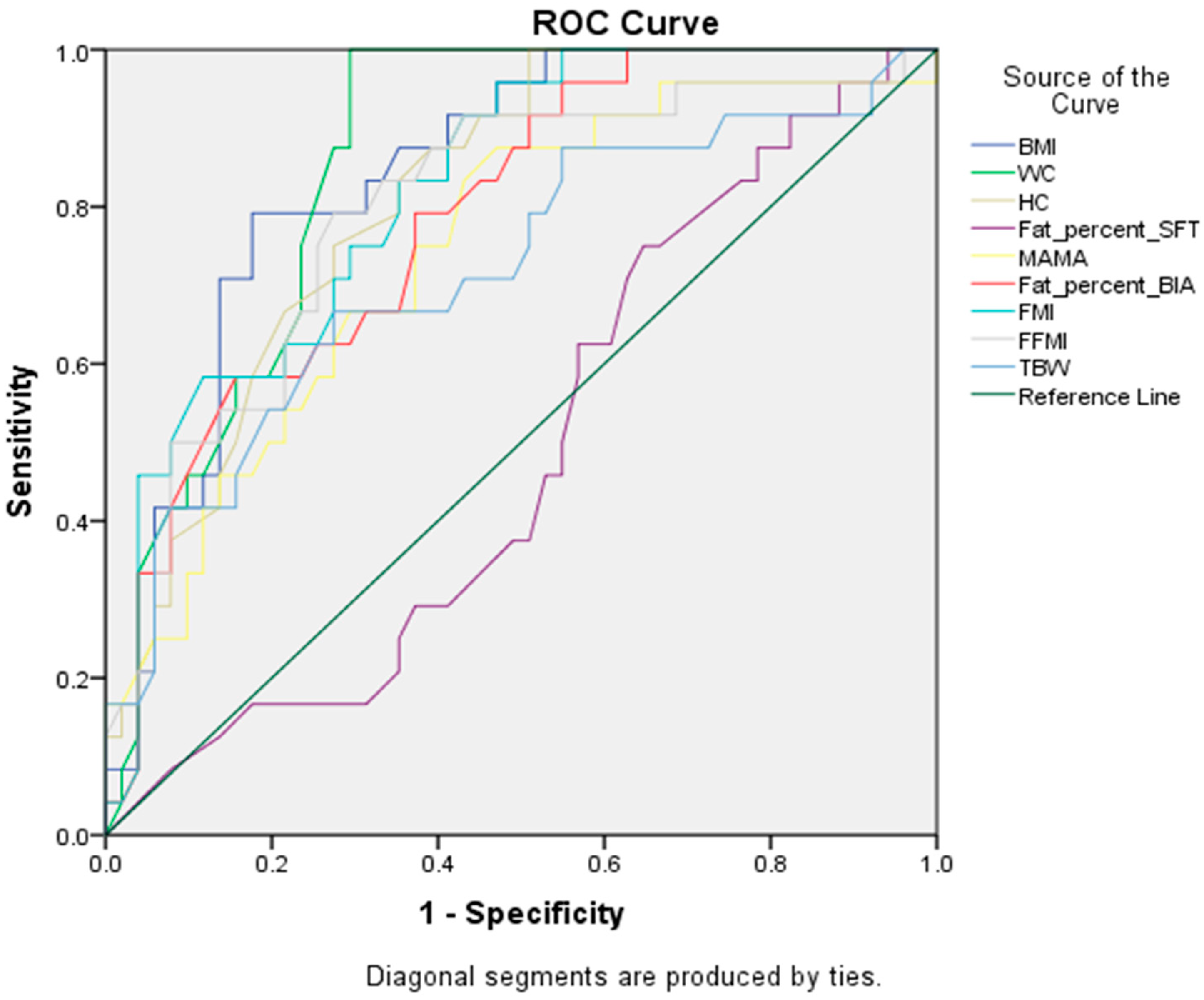
3.3. Sensitivity and Specificity of Anthropometric Measures and BIA Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Adult Obesity Causes & Consequences|Overweight & Obesity|CDC. Centers for Disease Control and Prevention. 2020. Available online: https://www.cdc.gov/obesity/adult/causes.html (accessed on 17 March 2021).
- Ritchie, S.A.; Connell, J.M. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef] [PubMed]
- van Vliet-Ostaptchouk, J.V.; Nuotio, M.L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalina, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef] [Green Version]
- Agudelo, G.M.; Bedoya, G.; Estrada, A.; Patiño, F.A.; Muñoz, A.M.; Velásquez, C.M. Variations in the prevalence of metabolic syndrome in adolescents according to different criteria used for diagnosis: Which definition should be chosen for this age group? Metab. Syndr. Relat. Disord. 2014, 12, 202–209. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016633371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friend, A.; Craig, L.; Turner, S. The prevalence of metabolic syndrome in children: A systematic review of the literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.R.; Leonard, M.B.; Shults, J.; Zemel, B.S. A comparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. J. Clin. Endocrinol. Metab. 2014, 99, 3208–3216. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, M.; Leto, G.; Petrone, A.; Zampetti, S.; Papa, R.E.; Osimani, M.; Spoletini, M.; Lenzi, A.; Osborn, J.; Mastantuono, M.; et al. Wrist circumference is a clinical marker of insulin resistance in overweight and obese children and adolescents. Circulation 2011, 123, 1757–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logue, J.; Thompson, L.; Romanes, F.; Wilson, D.C.; Thompson, J.; Sattar, N. Management of obesity: Summary of SIGN guideline. BMJ 2010, 340, c154. [Google Scholar] [CrossRef] [PubMed]
- Flemming, C.; Matthias, G.; Bussler, S.; Körner, A.; Kiess, W. Definition and early diagnosis of metabolic syndrome in children. J. Pediatr. Endocrinol. Metab. 2020, 33, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Fernandes, R.A.; Rosa, C.S.; Buonani, C.; Oliveira, A.R.; Freitas Júnior, I.F. The use of bioelectrical impedance to detect excess visceral and subcutaneous fat. J. Pediatr. 2007, 83, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Kyle, U.G.; Earthman, C.P.; Pichard, C.; Coss-Bu, J.A. Body composition during growth in children: Limitations and perspectives of bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2015, 69, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Spolidoro, J.V.; Pitrez Filho, M.L.; Vargas, L.T.; Santana, J.C.; Pitrez, E.; Hauschild, J.A.; Bruscato, N.M.; Moriguchi, E.H.; Medeiros, A.K.; Piva, J.P. Waist circumference in children and adolescents correlate with metabolic syndrome and fat deposits in young adults. Clin. Nutr. 2013, 32, 93–97. [Google Scholar] [CrossRef]
- El-Mouzan, M.; Al-Herbish, A.; Al-Salloum, A.; Qurachi, M.; Al-Omar, A. Growth charts for Saudi children and adolescents. Saudi Med. J. 2007, 28, 1555–1568. [Google Scholar] [PubMed]
- Marrodán, M.D.; Pèrez, B.; Morales, E.; Santos-Beneit, G.; Cabañas, M.D. Contrast and agreement among body composition equations in pediatric age: Application in Spanish and Venezuelan population. Nutr. Clin. Diet. Hosp. 2009, 29, 4–11. [Google Scholar]
- Nasreddine, L.; Naja, F.; Hills, A.P.; Youssef, S.K.; Chahine, J.; Hwalla, N. Validity of predictive equations developed to estimate body fat from anthropometry and bioelectrical impedance analysis in 8–10 year-old children. Clin. Nutr. 2012, 31, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Schutz, Y.; Kyle, U.U.G.; Pichard, C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int. J. Obes. 2002, 26, 953–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Wang, Y.; Shan, X.; Cheng, H.; Hou, D.; Zhao, X.; Wang, T.; Zhao, D.; Mi, J. Association between childhood obesity and metabolic syndrome: Evidence from a large sample of Chinese children and adolescents. PLoS ONE 2012, 7, e47380. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, J.L.; Bernstein, L. Epidemiology and prevention of breast cancer. Ann. Rev. Public Health 1996, 17, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Namwongprom, S.; Rerkasem, K.; Wongthanee, A.; Pruenglampoo, S.; Mangklabruks, A. Relationship between body composition parameters and metabolic syndrome in young Thai adults. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 227–232. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Rodriguez-Colon, S.; Fernandez-Mendoza, J.; Vgontzas, A.N.; Bixler, E.O.; Berg, A.; Kawasawa, Y.I.; Sawyed, M.D.; Liao, D. Abdominal obesity and metabolic syndrome burden in adolescents—Penn State children cohort study. J. Clin. Densitom 2015, 18, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafiu, O.O.; Burke, C.; Lee, J.; Voepel-Lewis, T.; Malviya, S.; Tremper, K.K. Neck circumference as a screening measure for identifying children with high body mass index. Pediatrics 2010, 126, e306–e310. [Google Scholar] [CrossRef]
- Kelishadi, R.; Heidari-Beni, M.; Qorbani, M.; Motamed-Gorji, N.; Motlagh, M.E.; Ziaodini, H.; Taheri, M.; Ahadi, Z.; Aminaee, T.; Heshmat, R. Association between neck and wrist circumferences and cardiometabolic risk in children and adolescents: The CASPIAN-V study. Nutrition 2017, 43–44, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, S.S.d.B.S.; Mastroeni, M.F.; Ekwaru, J.P.; Setayeshgar, S.; Veugelers, P.J.; Gonçalves, M.d.C.; de Carvalho Rondó, P.H. Anthropometric measurements as a potential non-invasive alternative for the diagnosis of metabolic syndrome in adolescents. Arch. Endocrinol. Metab. 2019, 63, 30–39. [Google Scholar] [CrossRef]
- Wicklow, B.A.; Becker, A.; Chateau, D.; Palmer, K.; Kozyrskij, A.; Sellers, E.A. Comparison of anthropometric measurements in children to predict metabolic syndrome in adolescence: Analysis of prospective cohort data. Int. J. Obes. 2015, 39, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Correa-Bautista, J.E.; Sanders-Tordecilla, A.; Ojeda-Pardo, M.L.; Cobo-Mejía, E.A.; Castellanos-Vega, R.D.P.; García-Hermoso, A.; González-Jiménez, E.; Schmidt-RioValle, J.; González-Ruíz, K. Percentage of body fat and fat mass index as a screening tool for metabolic syndrome prediction in Colombian University students. Nutrients 2017, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Lamb, M.J.; Byrne, C.D.; Wilson, J.F.; Wild, S.H. Evaluation of bioelectrical impedance analysis for identifying overweight individuals at increased cardiometabolic risk: A cross-sectional study. PLoS ONE 2014, 9, e106134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Daghri, N.M.; Al-Attas, O.S.; Wani, K.; Alnaami, A.M.; Sabico, S.; Al-Ajlan, A.; Chrousos, G.P.; Alokail, M.S. Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovasc. Diabetol. 2015, 14, 101. [Google Scholar] [CrossRef]
- Oliveira, R.G.; Guedes, D.P. Performance of anthropometric indicators as predictors of metabolic syndrome in Brazilian adolescents. BMC Pediatr. 2018, 18, 33. [Google Scholar] [CrossRef] [Green Version]
- El Mouzan, M.I.; Foster, P.J.; Al Herbish, A.S.; Al Salloum, A.A.; Al Omer, A.A.; Qurachi, M.M.; Kecojevic, T. Prevalence of overweight and obesity in Saudi children and adolescents. Ann. Saudi Med. 2010, 30, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Normal Weight (n = 108) | None-MetS (n = 26) | MetS (n = 24) |
|---|---|---|---|
| Wt | 48.60 ± 8.92 | 68.95 ± 11.70 p < 0.01 * | 76.31 ± 15.17 p < 0.01 * p < 0.01 ** |
| Ht | 154.2 ± 7.63 | 157.46 ± 9.13 p = 0.17 * | 157.87 ± 8.26 p = 0.13 * p = 0.86 ** |
| BMI | 20.28 ± 2.76 | 27.74 ± 3.54 p < 0.01 * | 30.40 ± 4.37 p < 0.01 * p < 0.05 ** |
| WC | 65.2 ± 6.68 | 84.53 ± 9.86 p < 0.01 * | 91.58 ± 9.52 p < 0.01 * p < 0.01 ** |
| BFP-SFT | 28.84 ± 2.72 | 30.26 ± 1.74 p < 0.05 * | 29.84 ± 1.73 p = 0.10 * p = 0.48 ** |
| BFP | 27.11 ± 4.94 | 37.31 ± 4.90 p < 0.01 * | 39.70 ± 6.30 p < 0.01 * p = 0.122 ** |
| FM | 13.47 ± 4.49 | 25.68 ± 6.15 p < 0.01 * | 30.50 ± 8.72 p < 0.01 * p < 0.05 ** |
| FFM | 35.16 ± 5.29 | 42.70 ± 6.22 p < 0.01 * | 45.51 ± 8.70 p < 0.01 * p = 0.152 ** |
| TBW | 25.74 ± 3.87 | 31.27 ± 4.55 p < 0.01 * | 33.31 ± 6.37 p < 0.01 * p = 0.155 ** |
| FBG | 91.44 ± 6.75 | 94.15 ± 12.20 p = 0.362 * | 103.04 ± 11.80 p < 0.01 * p < 0.05 ** |
| HDL | 46.48 ± 7.51 | 44.26 ± 12.59 p = 0.389 * | 34.79 ± 5.35 p < 0.01 * p < 0.01 ** |
| TG | 67.80 ± 16.13 | 84.76 ± 20.33 p = 0.161 * | 123.66 ± 70.70 p < 0.01 * p < 0.01 ** |
| SBP | 118 ± 10.00 | 123.26 ± 12.76 p = 0.116 * | 133.50 ± 12.45 p < 0.01 * p < 0.01 ** |
| DBP | 69.64 ± 8.99 | 70.92 ± 9.26 p = 0.649 * | 78.91 ± 11.70 p < 0.01 * p < 0.01 ** |
| MetS Components | Anthropometric Measurements | BIA Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | HC | WHR | MAC | MAMA | BFP-SFT | BFP | FMI | FFM | TBW | ||
| None-MetS group | |||||||||||
| WC | r | 0.47 * | 0.65 ** | 0.58 ** | 0.47 * | 0.34 | −0.24 | 0.09 | 0.22 | 0.49 * | 0.57 ** |
| FBG | r | 0.11 | 0.36 | 0.05 | 0.27 | 0.37 | −0.09 | 0.12 | −0.03 | −0.29 | 0.11 |
| HDL | r | −0.25 | 0.07 | −0.14 | −0.29 | −0.25 | 0.18 | 0.11 | −0.11 | −0.44 * | −0.03 |
| TG | r | 0.09 | 0.14 | 0.15 | 0.11 | 0.18 | −0.27 | −0.00 | 0.14 | 0.36 | 0.25 |
| SBP | r | −0.13 | −0.04 | −0.34 | 0.05 | −0.10 | 0.22 | −0.06 | −0.02 | 0.11 | 0.07 |
| DBP | r | −0.08 | −0.05 | 0.12 | 0.31 | 0.18 | −0.11 | 0.07 | −0.06 | −0.20 | 0.05 |
| MetS group | |||||||||||
| WC | r | 0.54 ** | 0.67 ** | 0.45 * | 0.05 | −0.08 | −0.57 ** | 0.14 | 0.35 | 0.50 * | 0.38 |
| FBG | r | 0.07 | 0.26 | 0.28 | −0.03 | −0.20 | −0.43 * | 0.01 | 0.15 | 0.32 | 0.22 |
| HDL | r | 0.15 | −0.09 | 0.21 | 0.09 | 0.11 | −0.18 | 0.11 | 0.15 | 0.08 | −0.10 |
| TG | r | 0.23 | 0.32 | 0.01 | −0.19 | −0.29 | −0.32 | 0.04 | 0.16 | 0.24 | 0.31 |
| SBP | r | 0.64 ** | −0.09 | −0.46 * | 0.20 | 0.22 | 0.50 * | 0.32 | 0.16 | −0.37 | −0.27 |
| DBP | r | 0.48 * | 0.01 | −0.10 | 0.06 | 0.16 | 0.38 | 0.35 | 0.29 | −0.14 | −0.12 |
| Measures | Sensitivity | Specificity | AUC (95% CI) | Cut-Off Point |
|---|---|---|---|---|
| BMI (kg/m2) | 79% | 80.4% | 0.84 (0.75–0.93) | 26.9 |
| WC (cm) | 87.5% | 72.5% | 0.86 (0.77–0.94) | 81.5 |
| HC (cm) | 75% | 72.5% | 0.81 (0.72–0.91) | 100.0 |
| BFP-SFT(%) | 62.5% | 43.1% | 0.49 (0.35–0.62) | 29.9 |
| MAMA (cm2) | 75% | 60.8% | 0.74 (0.62–0.86) | 26.0 |
| BFP | 79.2% | 60.8% | 0.79 (0.68–0.89) | 34.7 |
| FMI | 83.3% | 60.8% | 0.82 (0.73–0.92) | 8.8 |
| FFMI | 79.2% | 72.5% | 0.80 (0.69–0.91) | 16.6 |
| TBW | 70.8% | 56.9% | 0.72 (0.59–0.85) | 29.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhanna, R.G.; Aljuraiban, G.S.; Almadani, N.K.; Alquraishi, M.; El-Sharkawy, M.S.; Abulmeaty, M.M.A. Value of Adding Bioelectrical Impedance Analysis to Anthropometric Indices in the Diagnosis of Metabolic Syndrome in 10–16 Years Old Schoolgirls. Healthcare 2022, 10, 419. https://doi.org/10.3390/healthcare10030419
Muhanna RG, Aljuraiban GS, Almadani NK, Alquraishi M, El-Sharkawy MS, Abulmeaty MMA. Value of Adding Bioelectrical Impedance Analysis to Anthropometric Indices in the Diagnosis of Metabolic Syndrome in 10–16 Years Old Schoolgirls. Healthcare. 2022; 10(3):419. https://doi.org/10.3390/healthcare10030419
Chicago/Turabian StyleMuhanna, Rawan G., Ghadeer S. Aljuraiban, Najwa K. Almadani, Mohammed Alquraishi, Mohamed S. El-Sharkawy, and Mahmoud M. A. Abulmeaty. 2022. "Value of Adding Bioelectrical Impedance Analysis to Anthropometric Indices in the Diagnosis of Metabolic Syndrome in 10–16 Years Old Schoolgirls" Healthcare 10, no. 3: 419. https://doi.org/10.3390/healthcare10030419
APA StyleMuhanna, R. G., Aljuraiban, G. S., Almadani, N. K., Alquraishi, M., El-Sharkawy, M. S., & Abulmeaty, M. M. A. (2022). Value of Adding Bioelectrical Impedance Analysis to Anthropometric Indices in the Diagnosis of Metabolic Syndrome in 10–16 Years Old Schoolgirls. Healthcare, 10(3), 419. https://doi.org/10.3390/healthcare10030419







