Lipid Biomarkers in Depression: Does Antidepressant Therapy Have an Impact?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Psychosocial Measurement
2.4. Anthropometric Measurement
2.5. Blood Sampling
2.6. Statistical Analysis
3. Results
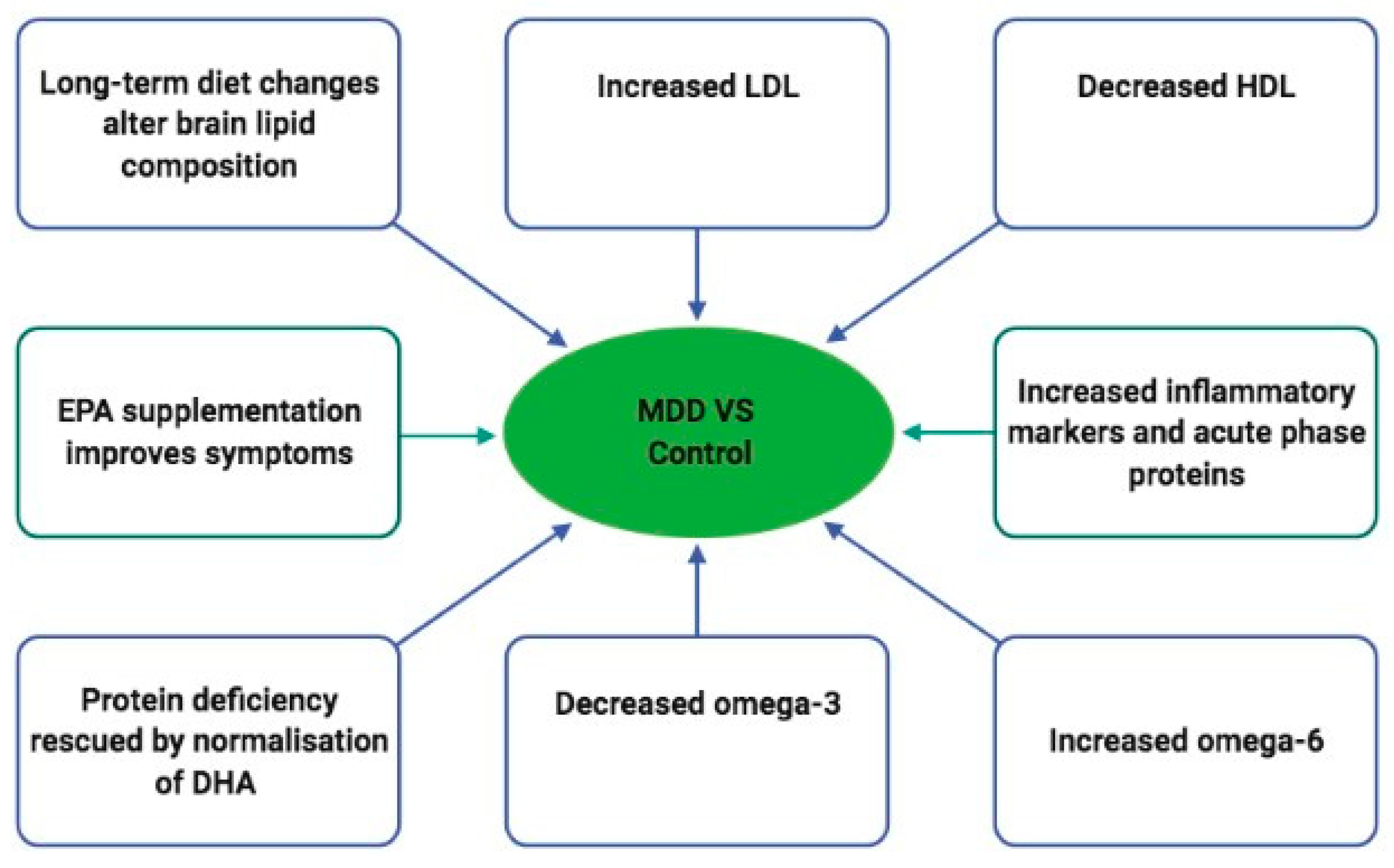
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Depression. 2020. Available online: https://www.who.int/news-room/fact-sheets/de-tail/depression (accessed on 10 September 2021).
- Maske, U.E.; Buttery, A.K.; Beesdo-Baum, K.; Riedel-Heller, S.; Hapke, U.; Busch, M.A. Prevalence and correlates of DSM-IV-TR major depressive disorder, self-reported diagnosed depression and current depressive symptoms among adults in Germany. J. Affect. Disord. 2016, 190, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, H.; Rommel, A.; Thom, J.; Schmidt, C.; König, H.-H.; Brettschneider, C.; Konnopka, A. The excess costs of depression and the influence of sociodemographic and socioeconomic factors: Results from the German Health Interview and Examination Survey for Adults (DEGS). Pharmacoeconomics 2021, 39, 667–680. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Soczynska, J.K.; Konarski, J.Z.; Kennedy, S.H. The effect of antidepressants on lipid homeostasis: A cardiac safety concern? Expert Opin. Drug Saf. 2006, 5, 523–537. [Google Scholar] [CrossRef]
- Dhar, A.K.; Barton, D.A. Depression and the link with cardiovascular disease. Front. Psychiatry 2016, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- Kopf, D.; Westphal, S.; Luley, C.W.; Ritter, S.; Gilles, M.; Weber-Hamann, B.; Lederbogen, F.; Lehnert, H.; Henn, F.A.; Heuser, I.; et al. Lipid metabolism and insulin resistance in depressed patients: Significance of weight, hypercortisolism, and antidepressant treatment. J. Clin. Psychopharmacol. 2004, 24, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Sarchiapone, M.; Camardese, G.; Roy, A.; Della Casa, S.; Satta, M.; Gonzalez, B.; Berman, J.; De Risio, S. Cholesterol and serotonin indices in depressed and suicidal patients. J. Affect. Disord. 2001, 62, 217–219. [Google Scholar] [CrossRef]
- Wu, S.; Ding, Y.; Wu, F.; Xie, G.; Hou, J.; Mao, P. Serum lipid levels and suicidality: A meta-analysis of 65 epidemiological studies. J. Psychiatry Neurosci. 2016, 41, 56–69. [Google Scholar] [CrossRef] [Green Version]
- Bilici, M.; Efe, H.; Köroğlu, M.A.; Uydu, H.A.; Bekaroğlu, M.; Değer, O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J. Affect. Disord. 2001, 64, 43–51. [Google Scholar] [CrossRef]
- Barthel, A.; Benker, G.; Berens, K.; Diederich, S.; Manfras, B.; Gruber, M.; Kanczkowski, W.; Kline, G.; Kamvissi-Lorenz, V.; Hahner, S.; et al. An update on Addison’s disease. Exp. Clin. Endocrinol. Diabetes 2019, 127, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Parekh, A.; Smeeth, D.; Milner, Y.; Thuret, S. The role of lipid biomarkers in major depression. Healthcare 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hryhorczuk, C.; Sharma, S.; Fulton, S.E. Metabolic disturbances connecting obesity and depression. Front. Neurosci. 2013, 7, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bschor, T.; Adli, M. Certified advanced training (cme)—Therapy of depressive disorders. Therapie. Dtsch. Arztebl. 2008, 105, 782. [Google Scholar] [CrossRef]
- Schwabe, U.; Paffrath, D.; Ludwig, W.-D.; Klauber, J. Arzneiverordnungs—Report 2017; Springer: Heidelberg, Germany, 2018. [Google Scholar]
- Chi, K.F.; Korgaonkar, M.; Grieve, S.M. Imaging predictors of remission to anti-depressant medications in major depressive disorder. J. Affect. Disord. 2015, 186, 134–144. [Google Scholar] [CrossRef]
- Johanna, G.; Lea-Teresa, N.; Tom, B. Guideline-Based Pharmacy. Pharmacotherapy of Depression According to Guidelines. 2018. Available online: https://www.akdae.de/Arzneimitteltherapie/AVP/Artikel/201803/141h/index.php (accessed on 20 December 2021).
- Depression Guideline Panel. Depression in Primary Care: Treatment of Major Depression. In Clinical Practice Guideline; US Dept of Health and Human Services, Public Health Service, and Agency for Health Care Policy and Research: Rockville, MD, USA, 1993; Volume 2. [Google Scholar]
- Gelenberg, A.J.; Freeman, M.P.; Markowitz, J.C.; Rosenbaum, J.; Thase, M.; Trivedi, M.; Van Rhoads, R.S. Work group on major depressive disorder. In Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd ed.; American Psychiatric Association: Arlington, VA, USA, 2010. [Google Scholar]
- Hersey, M. The Role of Acute and Chronic Neuroinflammation in Depression: Uncovering the Relationship between Histamine and Serotonin Transmission. Ph.D. Thesis, University of South Carolina, Columbia, SC, USA, 2020. [Google Scholar]
- Vogelzangs, N.; Beekman, A.T.F.; Dortland, A.K.V.R.; Schoevers, R.A.; Giltay, E.J.; de Jonge, P.; Penninx, B.W.J.H. Inflammatory and metabolic dysregulation and the 2-year course of depressive disorders in antidepressant users. Neuropsychopharmacology 2014, 39, 1624–1634. [Google Scholar] [CrossRef] [Green Version]
- Hazell, P.; Mirzaie, M. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst. Rev. 2013, 6, CD002317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelton, R.C.; Osuntokun, O.; Heinloth, A.N.; Corya, S.A. Therapeutic options for treatment-resistant depression. CNS Drugs 2010, 24, 131–161. [Google Scholar] [CrossRef]
- DGPPN, BÄK, KBV, AWMF (Eds.) for the Guideline Group on Unipolar Depression*. S3-Guideline/National Care Guideline Unipolar Depression-Long Version. 2015. Available online: https://www.leitlinien.de/themen/depression (accessed on 20 December 2021). [CrossRef]
- Uher, R.; Mors, O.; Rietschel, M.; Rajewska-Rager, A.; Petrović, A.; Zobel, A.; Henigsberg, N.; Mendlewicz, J.; Aitchison, K.J.; Farmer, A.; et al. Early and delayed onset of response to antidepressants in individual trajectories of change during treatment of major depression: A secondary analysis of data from the Genome-Based Therapeutic Drugs for Depression (GENDEP) study. J. Clin. Psychiatry 2011, 72, 5274. [Google Scholar] [CrossRef]
- Szegedi, A.A.; Jansen, W.T.; Van Willigenburg, A.P.P.; Van Der Meulen, E.; Stassen, H.H.; Thase, M.E. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: A meta-analysis including 6562 patients. J. Clin. Psychiatry 2009, 70, 5290. [Google Scholar] [CrossRef]
- Roohi, E.; Jaafari, N.; Hashemian, F. On inflammatory hypothesis of depression: What is the role of IL-6 in the middle of the chaos? J. Neuroinflamm. 2021, 18, 45. [Google Scholar] [CrossRef]
- Wang, H.; Li, P.; Zhang, Y.; Zhang, C.; Li, K.; Song, C. Cytokine changes in different types of depression: Specific or general? Neurol. Psychiatry Brain Res. 2020, 36, 39–51. [Google Scholar] [CrossRef]
- Diller, G.-P.; Bräutigam, A.; Kempny, A.; Uebing, A.; Alonso-Gonzalez, R.; Swan, L.; Babu-Narayan, S.V.; Baumgartner, H.; Dimopoulos, K.; Gatzoulis, M.A. Depression requiring anti-depressant drug therapy in adult congenital heart disease: Prevalence, risk factors, and prognostic value. Eur. Heart J. 2016, 37, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Maj, M.; Stein, D.J.; Parker, G.; Zimmerman, M.; Fava, G.A.; De Hert, M.; Demyttenaere, K.; McIntyre, R.S.; Widiger, T.; Wittchen, H.-U. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry 2020, 19, 269–293. [Google Scholar] [CrossRef]
- Koponen, H.; Saari, K.; Savolainen, M.; Isohanni, M. Weight gain and glucose and lipid metabolism disturbances during antipsychotic medication. Eur. Arch. Psychiatry Clin. Neurosci. 2002, 252, 294–298. [Google Scholar] [CrossRef]
- Wippert, P.-M.; Block, A.; Mansuy, I.M.; Peters, E.M.J.; Rose, M.; Rapp, M.A.; Huppertz, A.; Wuertz-Kozak, K. Alterations in bone homeostasis and microstructure related to depression and allostatic load. Psychother. Psychosom. 2019, 88, 383–385. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory (BDI-II); Pearson: New York, NY, USA, 1996; Volume 10. [Google Scholar]
- Kühner, C.; Bürger, C.; Keller, F.; Hautzinger, M. Reliability and validity of the revised Beck Depression Inventory (BDI-II). Results from German samples. Nervenarzt 2007, 78, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Hennings, J.M.; Heel, S.; Lechner, K.; Uhr, M.; Dose, T.; Schaaf, L.; Holsboer, F.; Lucae, S.; Fulda, S.; Kloiber, S. Effect of mirtazapine on metabolism and energy substrate partitioning in healthy men. JCI Insight 2019, 4, e123786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roessner, V.; Demling, J.; Bleich, S. Doxepin increases serum cholesterol levels. Can. J. Psychiatry 2004, 49, 74–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deisenhammer, E.A.; Kramer-Reinstadler, K.; Liensberger, D.; Kemmler, G.; Hinterhuber, H.; Fleischhacker, W.W. No evidence for an association between serum cholesterol and the course of depression and suicidality. Psychiatry Res. 2004, 121, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Arain, A.A.; Memon, A.R.; Kazi, H.; Mashori, B.A. Reduction of serum lipid profile by escitalopram in depressive patients: A cardio protective aspect of SSRI use. J. Cardiol. Cardiovasc. Ther. 2017, 4, 555642. [Google Scholar]
- Eker, Ö.O.; Özsoy, S.; Eker, B.; Doğan, H. Metabolic effects of antidepressant treatment. Arch. Neuropsychiatry 2017, 54, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laimer, M.; Kramer-Reinstadler, K.; Rauchenzauner, M.; Lechner-Schoner, T.; Strauss, R.; Engl, J.; Deisenhammer, E.A.; Hinterhuber, H.; Patsch, J.R.; Ebenbichler, C.F. Effect of mirtazapine treatment on body composition and metabolism. J. Clin. Psychiatry 2006, 67, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, J.W. Antipsychotic medications: Metabolic and cardiovascular risk. J. Clin. Psychiatry 2007, 64, 8–13. [Google Scholar]
- van Reedt Dortland, A.K.B.; Giltay, E.J.; Van Veen, T.; Zitman, F.G.; Penninx, B.W.J.H. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr. Scand. 2010, 122, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.; Duivis, H.E.; Beekman, A.T.F.; Kluft, C.; Neuteboom, J.; Hoogendijk, W.; Smit, J.H.; de Jonge, P.; Penninx, B.W.J.H. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl. Psychiatry 2012, 2, e79. [Google Scholar] [CrossRef] [Green Version]
- Licht, C.M.M.; de Geus, E.J.C.; Seldenrijk, A.; van Hout, H.P.J.; Zitman, F.G.; Van Dyck, R.; Penninx, B.W.J.H. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension 2009, 53, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Kessing, L.V.; Vradi, E.; McIntyre, R.S.; Andersen, P.K. Causes of decreased life expectancy over the life span in bipolar disorder. J. Affect. Disord. 2015, 180, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-J.; Zhou, Y.-J.; Wang, D.-F.; Li, Y.; Wang, D.-M.; Liu, T.-Q.; Zhang, X.-Y. Association of lipid profile and suicide attempts in a large sample of first episode drug-naive patients with major depressive disorder. Front. Psychiatry 2020, 11, 543632. [Google Scholar] [CrossRef]
- Bot, M.; Milaneschi, Y.; Al-Shehri, T.; Amin, N.; Garmaeva, S.; Onderwater, G.L.J.; Pool, R.; Thesing, C.S.; Vijfhuizen, L.S.; Vogelzangs, N.; et al. Metabolomics profile in depression: A pooled analysis of 230 metabolic markers in 5283 cases with depression and 10,145 controls. Biol. Psychiatry 2020, 87, 409–418. [Google Scholar] [CrossRef]
- Goldston, K.; Baillie, A.J. Depression and coronary heart disease: A review of the epidemiological evidence, explanatory mechanisms and management approaches. Clin. Psychol. Rev. 2008, 28, 288–306. [Google Scholar] [CrossRef]
- González, A.; Fazzino, F.; Castillo, M.; Mata, S.; Lima, L. Serotonin, 5-HT1A serotonin receptors and proliferation of lymphocytes in major depression patients. Neuroimmunomodulation 2007, 14, 8–15. [Google Scholar] [CrossRef]
- Schiepers, O.J.G.; Wichers, M.C.; Maes, M. Cytokines and major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 201–217. [Google Scholar] [CrossRef]
- Joseph, S.B.; Castrillo, A.; Laffitte, B.A.; Mangelsdorf, D.J.; Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003, 9, 213–219. [Google Scholar] [CrossRef]
- Deighton, S.; Neville, A.; Pusch, D.; Dobson, K. Biomarkers of adverse childhood experiences: A scoping review. Psychiatry Res. 2018, 269, 719–732. [Google Scholar] [CrossRef]
- Vega, C.; Becker, R.V.; Mucha, L.; Lorenz, B.H.; Eaddy, M.T.; Ogbonnaya, A.O. Impact of adherence to antidepressants on healthcare outcomes and costs among patients with type 2 diabetes and comorbid major depressive disorder. Curr. Med Res. Opin. 2017, 33, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Lamers, F.; Berk, M.; Penninx, B.W.J.H. Depression heterogeneity and its biological underpinnings: Toward immunometabolic depression. Biol. Psychiatry 2020, 88, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Fava, M. Weight gain and antidepressants. J. Clin. Psychiatry 2000, 61, 37–41. [Google Scholar] [PubMed]
- Shimizu, S.; Akiyama, T.; Kawada, T.; Shishido, T.; Mizuno, M.; Kamiya, A.; Yamazaki, T.; Sano, S.; Sugimachi, M. In vivo direct monitoring of interstitial norepinephrine levels at the sinoatrial node. Auton. Neurosci. 2010, 152, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Dickens, C.M.; McGowan, L.; Percival, C.; Tomenson, B.; Cotter, L.; Heagerty, A.; Creed, F.H. Contribution of depression and anxiety to impaired health-related quality of life following first myocardial infarction. Br. J. Psychiatry 2006, 189, 367–372. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, C.-Y.; Deng, W.-M. The role of pro-inflammatory cytokines in lipid metabolism of metabolic diseases. Int. Rev. Immunol. 2019, 38, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, J.A.; Berbée, J.F.P.; Havekes, L.M.; Rensen, P.C.N. Interactions between inflammation and lipid metabolism: Relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis 2013, 228, 306–315. [Google Scholar] [CrossRef] [PubMed]
| Variables | M/N | (SD)/% |
|---|---|---|
| Age (years) | 49.45 | 7.42 |
| Gender | ||
| Female | 13 | 86.67 |
| Male | 2 | 13.33 |
| Marital Status | ||
| Married/in relationship | 10 | 66.67 |
| Single/divorced/widowed | 5 | 33.33 |
| Educational level | ||
| <10 years | 7 | 46.67 |
| =10 years | 2 | 13.33 |
| 10 years | 6 | 40.00 |
| Depression Severity 1 | ||
| None | 0 | 0.00 |
| Mild | 1 | 6.67 |
| Moderate | 6 | 40.00 |
| Severe | 6 | 40.00 |
| No information | 2 | 13.33 |
| Height (cm) | 179.36 | 6.13 |
| Weight (kg) | 80.58 | 24.06 |
| BMI (kg/m2) | 27.83 | 7.06 |
| Smoking | ||
| Regularly | 10 | 66.67 |
| Not smoking | 5 | 33.33 |
| Drinking | ||
| Regularly | 9 | 60.00 |
| Not drinking | 6 | 40.00 |
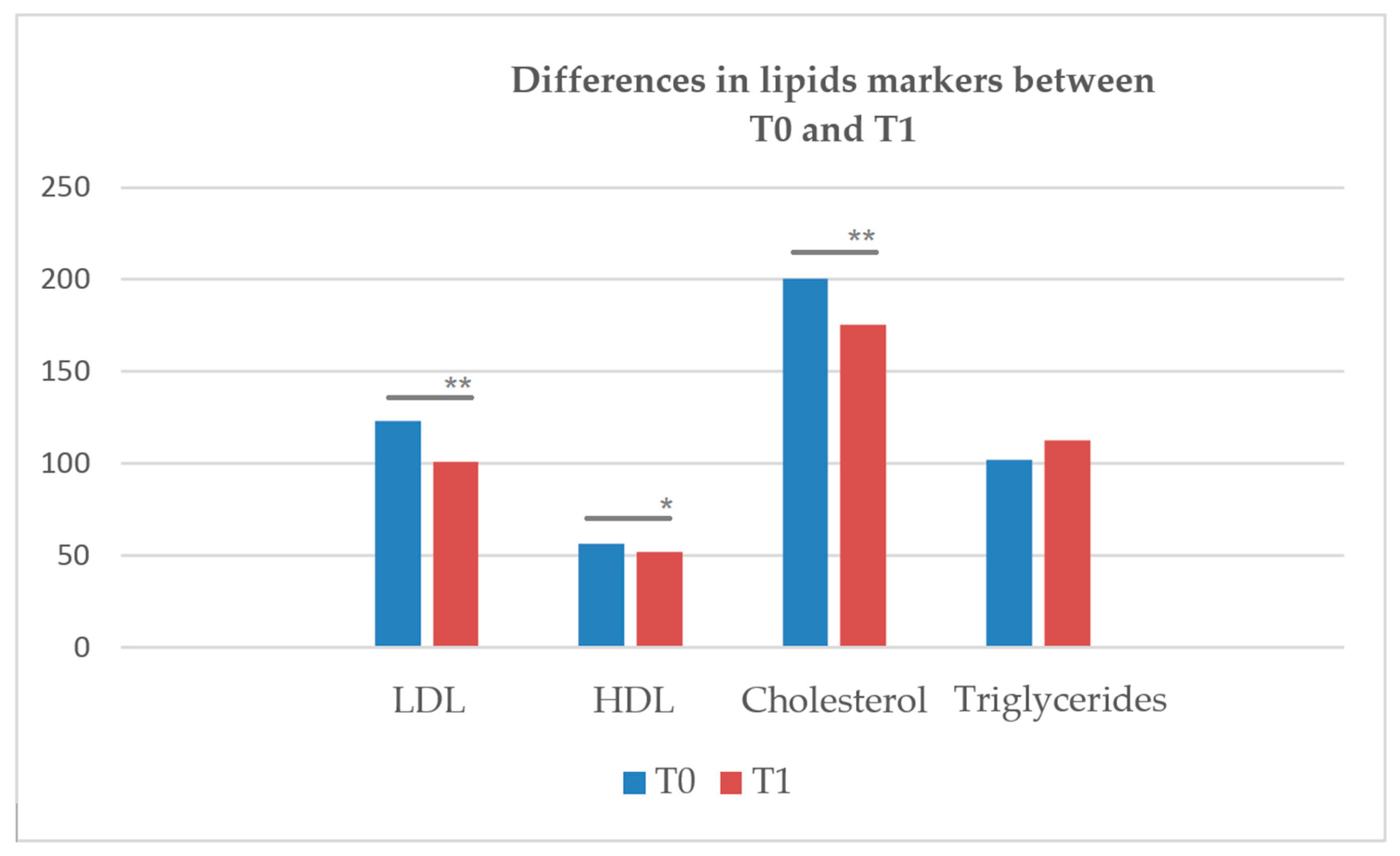
| Variables | T0 | T1 | Z | p-Value | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| LDL (mg/dL) | 31.60 | 44.39 | 107.33 | 36.10 | −3.297 | <0.001 ** |
| HDL (mg/dL) | 55.58 | 16.00 | 51.84 | 14.58 | −2.104 | 0.035 * |
| Cholesterol (mg/dL) | 203.28 | 45.53 | 183.13 | 42.55 | −3.233 | <0.001 ** |
| Triglyceride (mg/dL) | 107.74 | 51.20 | 115.34 | 46.00 | −0.910 | 0.363 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuchtey, F.C.; Block, A.; Osei, F.; Wippert, P.-M. Lipid Biomarkers in Depression: Does Antidepressant Therapy Have an Impact? Healthcare 2022, 10, 333. https://doi.org/10.3390/healthcare10020333
Stuchtey FC, Block A, Osei F, Wippert P-M. Lipid Biomarkers in Depression: Does Antidepressant Therapy Have an Impact? Healthcare. 2022; 10(2):333. https://doi.org/10.3390/healthcare10020333
Chicago/Turabian StyleStuchtey, Fidelis Christin, Andrea Block, Francis Osei, and Pia-Maria Wippert. 2022. "Lipid Biomarkers in Depression: Does Antidepressant Therapy Have an Impact?" Healthcare 10, no. 2: 333. https://doi.org/10.3390/healthcare10020333
APA StyleStuchtey, F. C., Block, A., Osei, F., & Wippert, P.-M. (2022). Lipid Biomarkers in Depression: Does Antidepressant Therapy Have an Impact? Healthcare, 10(2), 333. https://doi.org/10.3390/healthcare10020333







