The Influence of Social Determinants of Health on the Provision of Postpartum Contraceptives in Medicaid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
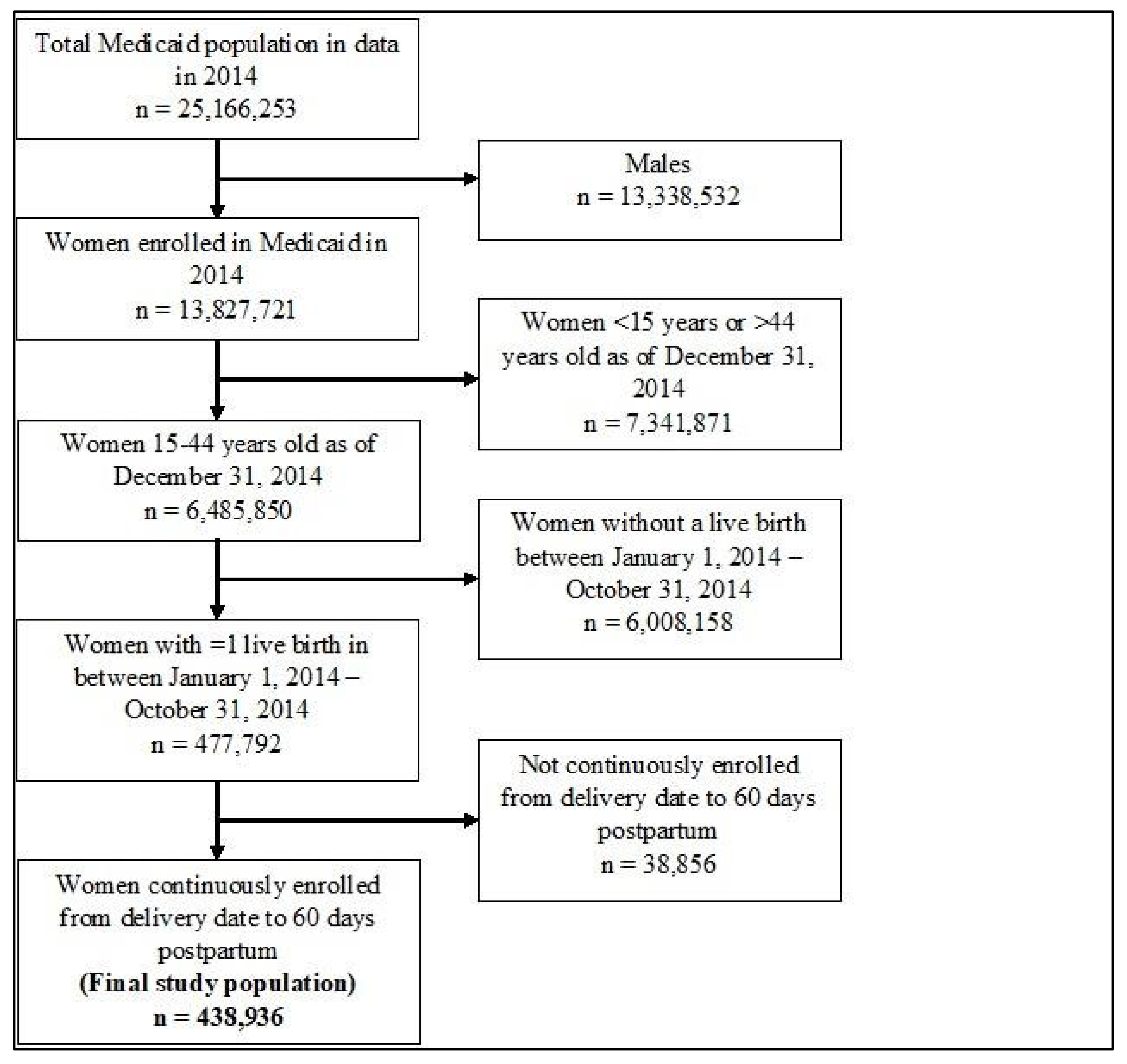
2.2. Study Population
2.3. Postpartum Contraception Provision
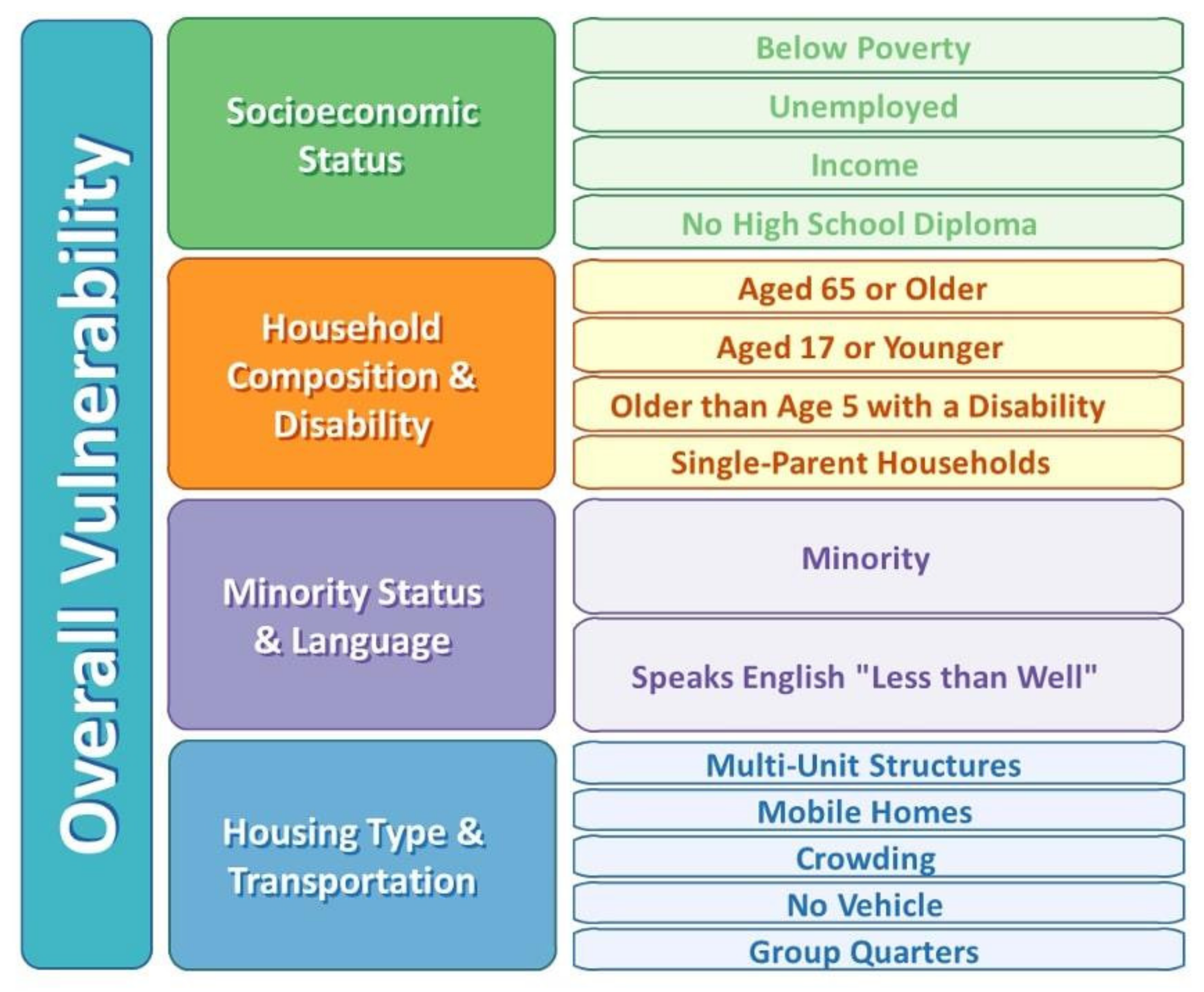
2.4. Theoretical Framework
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Implications for Policy and/or Practice
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Differences in Maternal Mortality among Black and White Women—United States, 1990. MMWR 1995, 44, 6–7. [Google Scholar]
- Hoyert, D.L. Maternal Mortality Rates in The United States, 2019; NCHS Health E-Stats; Center for Disease Control & Prevention: Atlanta, GA, USA, 2021. [Google Scholar] [CrossRef]
- Bingham, D.; Suplee, P.D.; Morris, M.H.; McBride, M. Healthcare Strategies for Reducing Pregnancy-Related Morbidity and Mortality in the Postpartum Period. J. Perinat. Neonat. Nurs. 2018, 32, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.E.; Davis, N.L.; Goodman, D.; Cox, S.; Mayes, N.; Johnston, E.; Syverson, C.; Seed, K.; Shapiro-Mendoza, C.K.; Calaghan, W.M.; et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortney, J.; Smith, J. The Base of the Iceberg: Prevalence and Perceptions of Maternal Morbidity in Four Developing Countries; Family Health International Maternal and Neonatal Health Center: Research Triangle Park, NC, USA, 1997. [Google Scholar]
- Chen, J.; Cox, S.; Kuklina, E.V.; Ferre, C.; Barfield, W.; Li, R. Assessment of Incidence and Factors Associated With Severe Maternal Morbidity After Delivery Discharge Among Women in the US. JAMA Netw. Open 2021, 4, e2036148. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, W.M.; Creanga, A.A.; Kuklina, E.V. Severe Maternal Morbidity Among Delivery and Postpartum Hospitalizations in the United States. Obstet. Gynecol. 2012, 120, 1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creanga, A.A.; Berg, C.J.; Ko, J.Y.; Farr, S.L.; Tong, V.T.; Bruce, F.C.; Callaghan, W.M. Maternal Mortality and Morbidity in the United States: Where Are We Now? J. Women’s Health 2014, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Severe Maternal Morbidity in the United States. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html (accessed on 25 October 2021).
- Garg, B.; Darney, B.; Pilliod, R.A.; Caughey, A.B. Long and Short Interpregnancy Intervals Increase Severe Maternal Morbidity. Am. J. Obstet. Gynecol. 2021, 225, 331.e1–331.e8. [Google Scholar] [CrossRef]
- Hutcheon, J.A.; Nelson, H.D.; Stidd, R.; Moskosky, S.; Ahrens, K.A. Short Interpregnancy Intervals and Adverse Maternal Outcomes in High-Resource Settings: An Updated Systematic Review. Paediatr. Perinat. Epidemiol. 2019, 33, O48–O59. [Google Scholar] [CrossRef] [Green Version]
- McKinney, D.; House, M.; Chen, A.; Muglia, L.; DeFranco, E. The Influence of Interpregnancy Interval on Infant Mortality. Am. J. Obstet. Gynecol. 2017, 216, 316.e1–316.e9. [Google Scholar] [CrossRef] [Green Version]
- Schummers, L.; Hutcheon, J.A.; Hernandez-Diaz, S.; Williams, P.L.; Hacker, M.R.; VanderWeele, T.J.; Norman, W.V. Association of Short Interpregnancy Interval With Pregnancy Outcomes According to Maternal Age. JAMA Intern. Med. 2018, 178, 1661–1670. [Google Scholar] [CrossRef]
- Appareddy, S.; Pryor, J.; Bailey, B. Inter-Pregnancy Interval and Adverse Outcomes: Evidence for an Additional Risk in Health Disparate Populations. J. Matern. Fetal Neonatal Med. 2017, 30, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- American College of Nurse-Midwives and the National Association of Nurse Practitioners in Women’s Health; American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine; Louis, J.M.; Bryant, A.; Ramos, D.; Stuebe, A.; Blackwell, S.C. Interpregnancy Care. Am. J. Obstet. Gynecol. 2019, 220, B2–B18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washington, C.I.; Jamshidi, R.; Thung, S.F.; Nayeri, U.A.; Caughey, A.B.; Werner, E.F. Timing of Postpartum Intrauterine Device Placement: A Cost-Effectiveness Analysis. Fertil. Steril. 2015, 103, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.I.; Caughey, A.B.; Edelman, A.; Darney, P.D.; Foster, D.G. Cost–Benefit Analysis of State- and Hospital-Funded Postpartum Intrauterine Contraception at a University Hospital for Recent Immigrants to the United States. Contraception 2010, 81, 304–308. [Google Scholar] [CrossRef]
- Zapata, L.B.; Murtaza, S.; Whiteman, M.K.; Jamieson, D.J.; Robbins, C.L.; Marchbanks, P.A.; D’Angelo, D.V.; Curtis, K.M. Contraceptive Counseling and Postpartum Contraceptive Use. Am. J. Obstet. Gynecol. 2015, 212, 171.e1–171.e8. [Google Scholar] [CrossRef] [Green Version]
- Oduyebo, T.; Zapata, L.B.; Boutot, M.E.; Tepper, N.K.; Curtis, K.M.; D’Angelo, D.V.; Marchbanks, P.A.; Whiteman, M.K. Factors Associated with Postpartum Use of Long-Acting Reversible Contraception. Am. J. Obstet. Gynecol. 2019, 221, 43.e1–43.e11. [Google Scholar] [CrossRef]
- Loewenberg Weisband, Y.; Keder, L.M.; Keim, S.A.; Gallo, M.F. Postpartum Intentions on Contraception Use and Method Choice among Breastfeeding Women Attending a University Hospital in Ohio: A Cross-Sectional Study. Reprod. Health 2017, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Gatwood, J.; Shuvo, S.; Hohmeier, K.C.; Hagemann, T.; Chiu, C.-Y.; Tong, R.; Ramachandran, S. Pneumococcal Vaccination in Older Adults: An Initial Analysis of Social Determinants of Health and Vaccine Uptake. Vaccine 2020, 38, 5607–5617. [Google Scholar] [CrossRef]
- Centers for Medicare and Medicaid Services. June 2021 Medicaid & CHIP Enrollment Data Highlights. Available online: https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/report-highlights/index.html (accessed on 25 October 2021).
- Centers for Disease Control and Prevention (CDC). CDC/ATSDR Social Vulnerability Index (SVI). Available online: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html (accessed on 19 December 2020).
- Azap, R.A.; Paredes, A.Z.; Diaz, A.; Hyer, J.M.; Pawlik, T.M. The Association of Neighborhood Social Vulnerability with Surgical Textbook Outcomes among Patients Undergoing Hepatopancreatic Surgery. Surgery 2020, 168, 868–875. [Google Scholar] [CrossRef]
- Diaz, A.; Hyer, J.M.; Barmash, E.; Azap, R.; Paredes, A.Z.; Pawlik, T.M. County-Level Social Vulnerability Is Associated with Worse Surgical Outcomes Especially Among Minority Patients. Ann. Surg. 2020, 274, 881–891. [Google Scholar] [CrossRef]
- An, R.; Xiang, X. Social Vulnerability and Obesity among U.S. Adults. Int J. Health Sci. 2015, 3, 7–21. [Google Scholar] [CrossRef] [Green Version]
- An, R.; Xiang, X. Social Vulnerability and Leisure-Time Physical Inactivity among US Adults. Am. J. Health Behav. 2015, 39, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Thakore, N.; Khazanchi, R.; Orav, E.J.; Ganguli, I. Association of Social Vulnerability, COVID-19 Vaccine Site Density, and Vaccination Rates in the United States. Health 2021, 9, 100583. [Google Scholar] [CrossRef]
- Karaye, I.M.; Horney, J.A. The Impact of Social Vulnerability on COVID-19 in the U.S.: An Analysis of Spatially Varying Relationships. Am. J. Prev. Med. 2020, 59, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Khazanchi, R.; Beiter, E.R.; Gondi, S.; Beckman, A.L.; Bilinski, A.; Ganguli, I. County-Level Association of Social Vulnerability with COVID-19 Cases and Deaths in the USA. J. Gen. Intern. Med. 2020, 35, 2784–2787. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Office of Population Affairs. Contraceptive Care Measures. Available online: https://opa.hhs.gov/evaluation-research/title-x-services-research/contraceptive-care-measures (accessed on 13 February 2021).
- Healthy People 2020. Social Determinants of Health. Available online: https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health (accessed on 19 December 2020).
- Centers for Medicare and Medicaid Services (CMS). Adult Health Care Quality Measures. Available online: https://www.medicaid.gov/medicaid/quality-of-care/performance-measurement/adult-and-child-health-care-quality-measures/adult-health-care-quality-measures/index.html (accessed on 19 December 2020).
- Centers for Medicare and Medicaid Services (CMS). Maternal & Infant Health Care Quality. Available online: https://www.medicaid.gov/medicaid/quality-of-care/improvement-initiatives/maternal-infant-health-care-quality/index.html (accessed on 19 December 2020).
- Healthy People 2020: MICH-16.6 Increase the Proportion of Women Delivering a Live Birth Who Used a Most Effective or Moderately Effective Contraception Method Postpartum. Available online: https://www.healthypeople.gov/node/4850/data_details (accessed on 5 August 2021).
- The American College of Obstetricians and Gynecologists. Medicaid Reimbursement for Postpartum LARC. Available online: https://www.acog.org/en/Programs/Long-ActingReversibleContraceptionLARC/ActivitiesInitiatives/MedicaidReimbursementforPostpartumLARC (accessed on 19 December 2020).
- Dunlop, A.L.; Joski, P.; Strahan, A.E.; Sierra, E.; Adams, E.K. Postpartum Medicaid Coverage and Contraceptive Use Before and After Ohio’s Medicaid Expansion Under the Affordable Care Act. Women’s Health Issues 2020, 30, 426–435. [Google Scholar] [CrossRef]
- Robbins, C.; Boulet, S.L.; Morgan, I.; D’Angelo, D.V.; Zapata, L.B.; Morrow, B.; Sharma, A.; Kroelinger, C.D. Disparities in Preconception Health Indicators—Behavioral Risk Factor Surveillance System, 2013–2015, and Pregnancy Risk Assessment Monitoring System, 2013–2014. MMWR Surveill. Summ. 2018, 67, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Thiel de Bocanegra, H.; Braughton, M.; Bradsberry, M.; Howell, M.; Logan, J.; Schwarz, E.B. Racial and Ethnic Disparities in Postpartum Care and Contraception in California’s Medicaid Program. Am. J. Obstet. Gynecol. 2017, 217, 47.e1–47.e7. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin No. 186: Long-Acting Reversible Contraception: Implants and Intrauterine Devices. Obstet Gynecol. 2017, 130, e251–e269. [Google Scholar] [CrossRef]
| Characteristic | n | % |
|---|---|---|
| Age group | ||
| 15–20 years | 61,751 | 14.07% |
| 21–44 years | 377,186 | 85.93% |
| Race/ethnicity | ||
| White | 193,594 | 44.11% |
| Black | 109,319 | 24.91% |
| Asian | 11,808 | 2.69% |
| Hispanic | 83,490 | 19.02% |
| Other/Unknown | 40,725 | 9.28% |
| State | ||
| CA | 129,625 | 29.53% |
| GA | 50,115 | 11.42% |
| IA | 11,610 | 2.65% |
| ID | 7249 | 1.65% |
| LA | 27,668 | 6.3% |
| MI | 37,850 | 8.62% |
| MN | 18,271 | 4.16% |
| MO | 21,594 | 4.92% |
| MS | 18,325 | 4.17% |
| NJ | 19,276 | 4.39% |
| PA | 42,972 | 9.79% |
| SD | 3167 | 0.72% |
| TN | 30,667 | 6.99% |
| UT | 9426 | 2.15% |
| VT | 2181 | 0.5% |
| WV | 6787 | 1.55% |
| WY | 2153 | 0.49% |
| Characteristic | 3-Day LARC Provision Rate | p-Value | 60-Day MMEC Provision Rate | p-Value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Overall | 787 | 0.18% | 160,615 | 36.31% | ||
| Age group | ||||||
| 15–20 years | 141 | 0.23% | 0.002 | 21,343 | 34.29% | <0.001 |
| 21–44 years | 646 | 0.17% | 139,272 | 36.64% | ||
| Race/ethnicity | ||||||
| White | 220 | 0.11% | <0.001 | 78,926 | 40.48% | <0.001 |
| Black | 382 | 0.35% | 42,299 | 38.41% | ||
| Asian | 20 | 0.17% | 3225 | 27.05% | ||
| Hispanic | 95 | 0.11% | 23,780 | 28.25% | ||
| Other/Unknown | 70 | 0.17% | 12,385 | 30.07% | ||
| State | ||||||
| CA | 172 | 0.13% | <0.001 | 32,467 | 24.84% | <0.001 |
| GA | 29 | 0.06% | 21,580 | 42.94% | ||
| IA | 85 | 0.73% | 4685 | 40.15% | ||
| ID | 1 | 0.01% | 3089 | 42.15% | ||
| LA | 59 | 0.21% | 13,400 | 48.18% | ||
| MI | 15 | 0.04% | 14,578 | 38.31% | ||
| MN | 49 | 0.27% | 7058 | 38.34% | ||
| MO | 51 | 0.23% | 9757 | 44.80% | ||
| MS | 8 | 0.04% | 8982 | 48.52% | ||
| NJ | 8 | 0.04% | 5910 | 30.54% | ||
| PA | 233 | 0.54% | 16,075 | 37.13% | ||
| SD | 4 | 0.12% | 1325 | 41.01% | ||
| TN | 43 | 0.14% | 14,973 | 48.14% | ||
| UT | 18 | 0.19% | 4262 | 44.21% | ||
| VT | 11 | 0.50% | 1013 | 46.15% | ||
| WV | 0 | 0.00% | 488 | 7.10% | ||
| WY | 1 | 0.05% | 973 | 44.23% | ||
| Characteristic | 3-Day LARC Provision | 60-Day MMEC Provision | ||
|---|---|---|---|---|
| aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | |
| Age Group (Ref = 21–44 years) | ||||
| 15–20 years | 1.37 (1.14, 1.64) | 0.001 | 0.87 (0.86,0.89) | <0.001 |
| Race/ethnicity (Ref = White) | ||||
| Black | 2.91 (2.42, 3.49) | <0.001 | 1.00 (0.98,1.01) | 0.756 |
| Asian | 1.19 (0.74, 1.91) | 0.486 | 0.69 (0.66,0.72) | <0.001 |
| Hispanic | 0.76 (0.58, 0.99) | 0.039 | 0.73 (0.72,0.75) | <0.001 |
| Other/Unknown | 1.29 (0.98, 1.70) | 0.072 | 0.73 (0.72,0.75) | <0.001 |
| Medicaid Eligibility >60 days postpartum (Ref = Discontinued) | ||||
| Continued | 1.32 (1.11, 1.58) | 0.002 | 0.97 (0.95,0.98) | <0.001 |
| Social Vulnerability Index | ||||
| Socioeconomic Theme (Ref = Least vulnerable–Bottom Quartile) | ||||
| Moderately vulnerable (Q2/Q3) | 0.41 (0.34, 0.51) | <0.001 | 1.00 (0.98,1.02) | 0.932 |
| Most vulnerable (Top Quartile) | 0.76 (0.58, 0.99) | 0.043 | 1.07 (1.04,1.09) | <0.001 |
| Household Composition and Disability (Ref = Least vulnerable–Bottom Quartile) | ||||
| Moderately vulnerable (Q2/Q3) | 0.81 (0.68, 0.96) | 0.014 | 1.27 (1.25,1.29) | <0.001 |
| Most vulnerable (Top Quartile) | 0.27 (0.20, 0.38) | <0.001 | 1.37 (1.34,1.41) | <0.001 |
| Minority status and Language (Ref = Least vulnerable–Bottom Quartile) | ||||
| Moderately vulnerable (Q2/Q3) | 0.63 (0.45, 0.87) | 0.005 | 1.18 (1.15,1.21) | <0.001 |
| Most vulnerable (Top Quartile) | 0.77 (0.55, 1.08) | 0.126 | 0.86 (0.84,0.89) | <0.001 |
| Housing and Transportation (Ref = Least vulnerable–Bottom Quartile) | ||||
| Moderately vulnerable (Q2/Q3) | 2.01 (1.45, 2.78) | <0.001 | 0.98 (0.96,1.00) | 0.070 |
| Most vulnerable (Top Quartile) | 2.44 (1.72, 3.45) | <0.001 | 0.92 (0.90,0.94) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nsiah, I.; Mali, N.V.; Barnard, M.; Goswami, S.; Lyle, C.; Ramachandran, S. The Influence of Social Determinants of Health on the Provision of Postpartum Contraceptives in Medicaid. Healthcare 2022, 10, 298. https://doi.org/10.3390/healthcare10020298
Nsiah I, Mali NV, Barnard M, Goswami S, Lyle C, Ramachandran S. The Influence of Social Determinants of Health on the Provision of Postpartum Contraceptives in Medicaid. Healthcare. 2022; 10(2):298. https://doi.org/10.3390/healthcare10020298
Chicago/Turabian StyleNsiah, Irene, Nidhi Vij Mali, Marie Barnard, Swarnali Goswami, Christy Lyle, and Sujith Ramachandran. 2022. "The Influence of Social Determinants of Health on the Provision of Postpartum Contraceptives in Medicaid" Healthcare 10, no. 2: 298. https://doi.org/10.3390/healthcare10020298
APA StyleNsiah, I., Mali, N. V., Barnard, M., Goswami, S., Lyle, C., & Ramachandran, S. (2022). The Influence of Social Determinants of Health on the Provision of Postpartum Contraceptives in Medicaid. Healthcare, 10(2), 298. https://doi.org/10.3390/healthcare10020298






