Cost-Effectiveness of Prostate Cancer Detection in Biopsy-Naïve Men: Ultrasound Shear Wave Elastography vs. Multiparametric Diagnostic Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
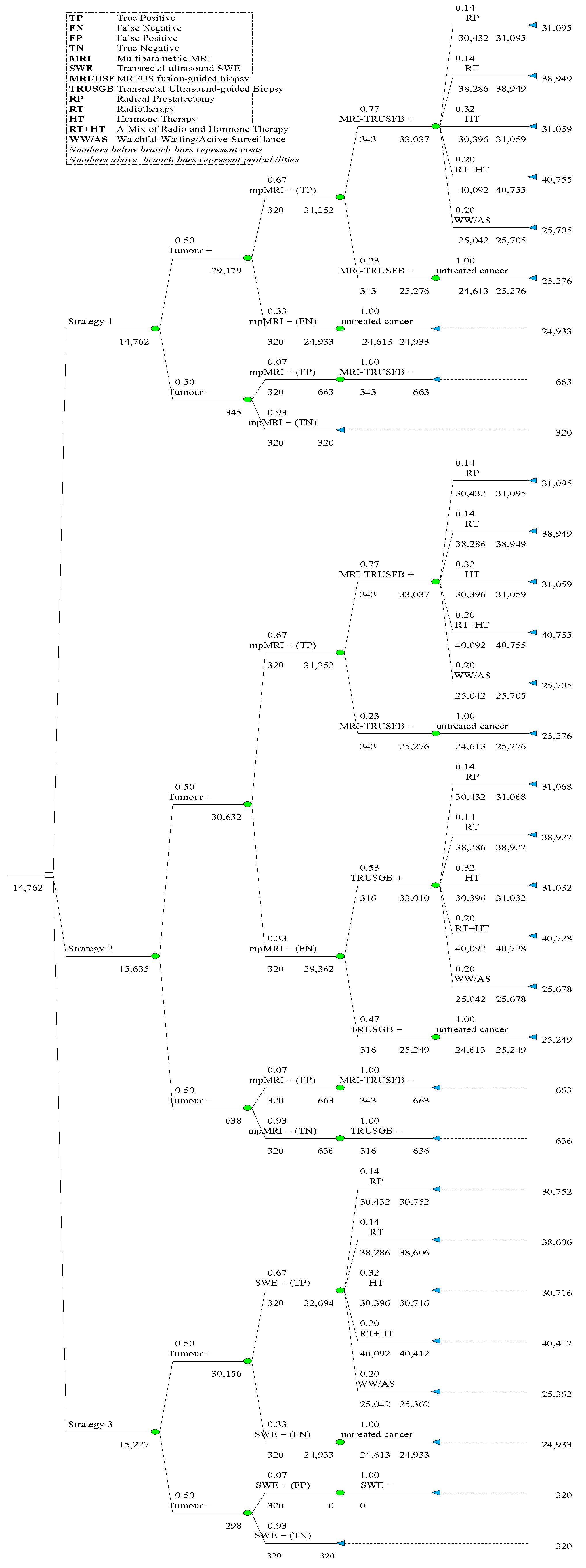
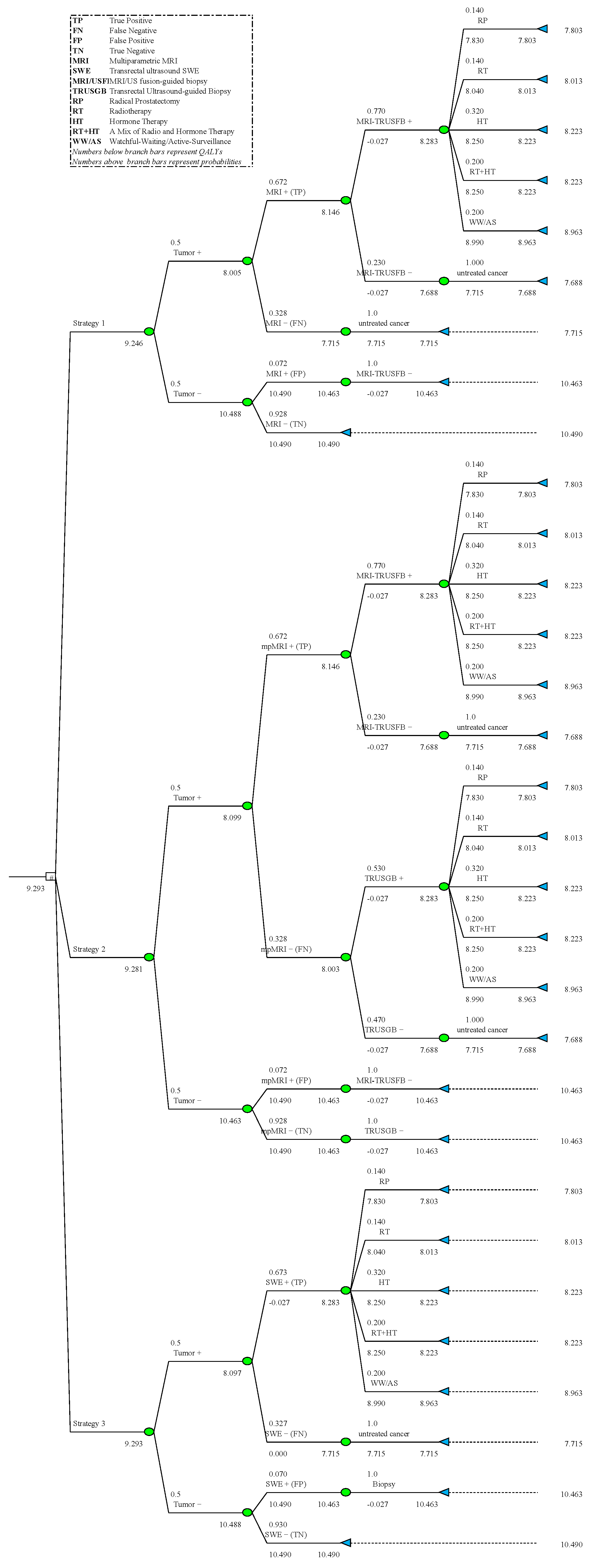
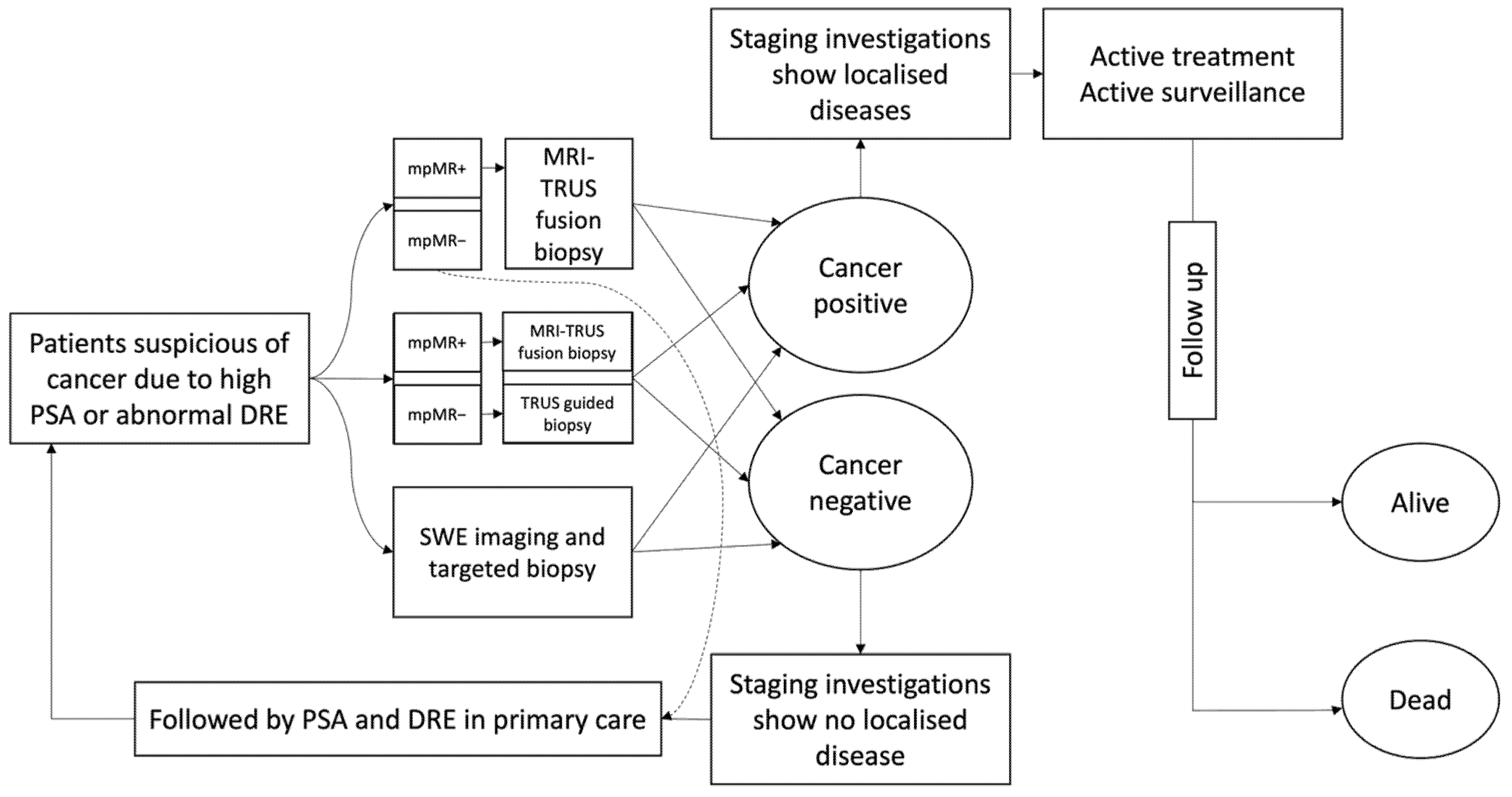
2.1. Model Development
2.2. Costs, Probabilities, and QALYs
3. Results
3.1. Costs-Effectiveness Analysis
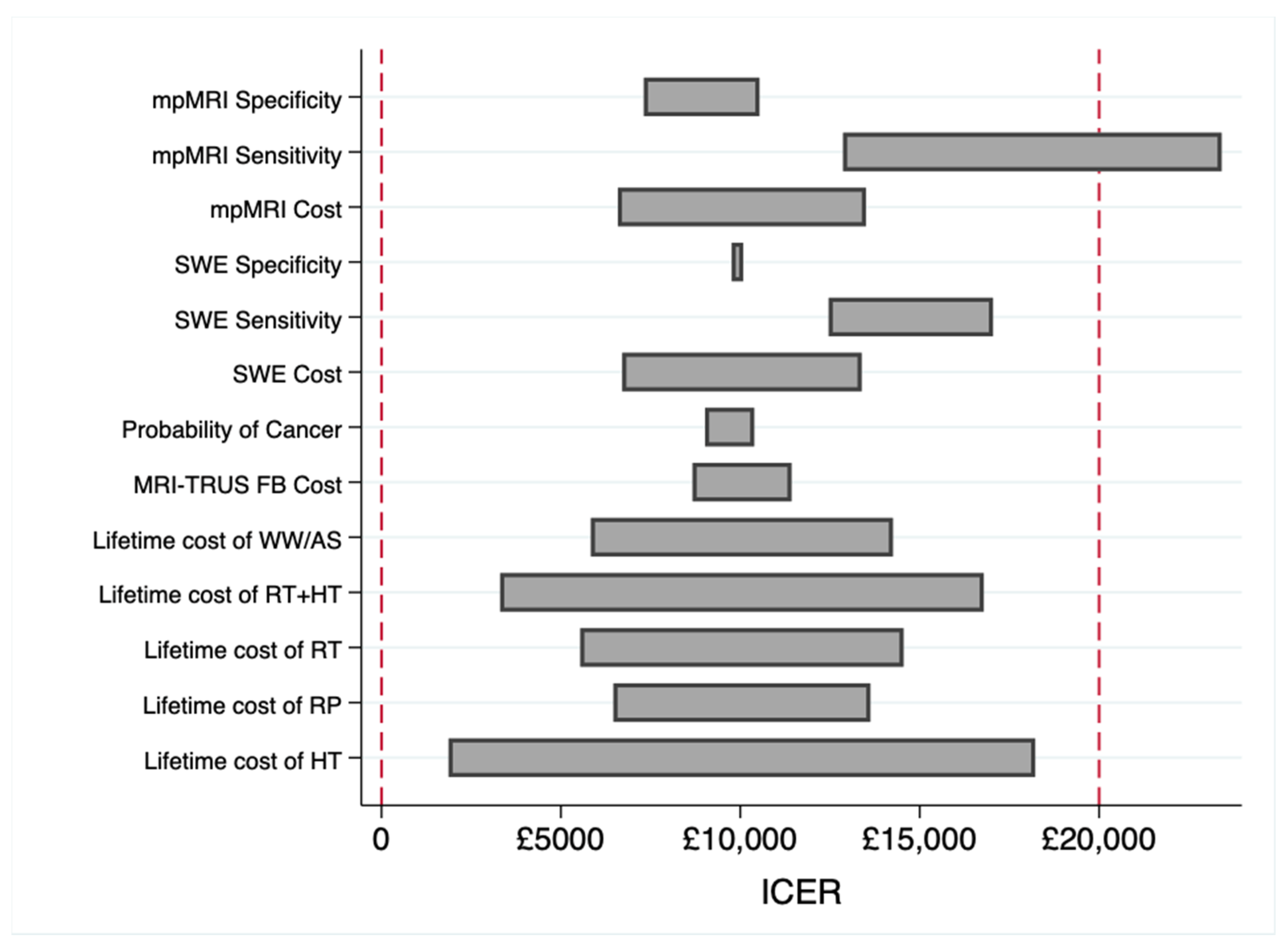
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Loeb, S.; Vellekoop, A.; Ahmed, H.U.; Catto, J.; Emberton, M.; Nam, R.; Rosario, D.J.; Scattoni, V.; Lotan, Y. Systematic review of complications of prostate biopsy. Eur. Urol. 2013, 64, 876–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, L.; Ahmed, H.U.; Allen, C.; Barentsz, J.O.; Carey, B.; Futterer, J.J.; Heijmink, S.W.; Hoskin, P.; Kirkham, A.P.; Padhani, A.R.; et al. Clinical applications of multiparametric MRI within the prostate cancer diagnostic pathway. Urol. Oncol. 2013, 31, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delongchamps, N.B.; Beuvon, F.; Eiss, D.; Flam, T.; Muradyan, N.; Zerbib, M.; Peyromaure, M.; Cornud, F. Multiparametric MRI is helpful to predict tumor focality, stage, and size in patients diagnosed with unilateral low-risk prostate cancer. Prostate Cancer Prostatic Dis. 2011, 14, 232–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NICE. Resource Impact Report: Prostate Cancer: Diagnosis and Management (Update) (NG131). 2019. Available online: https://www.nice.org.uk/guidance/ng131 (accessed on 26 January 2020).
- Wei, C.; Li, C.; Szewczyk-Bieda, M.; Upreti, D.; Lang, S.; Huang, Z.; Nabi, G. Performance characteristics of transrectal shear wave elastography imaging in the evaluation of clinically localized prostate cancer: A prospective study. J. Urol. 2018, 200, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Szewczyk-Bieda, M.; Nibblok, P.; Brown, E.; Lang, S.; Nabi, G. Quantitative transrectal shear wave elastography undergoing salvage extraperitoneal laparoscopic radical prostatectomy following failed radiotherapy. Surg. Endosc. 2018, 32, 4552–4561. [Google Scholar] [CrossRef] [Green Version]
- Sang, L.; Wang, X.M.; Xu, D.Y.; Cai, Y.F. Accuracy of shear wave elastography for the diagnosis of prostate cancer: A meta-analysis. Sci. Rep. 2017, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rosenkrantz, A.B.; Ginocchio, L.A.; Cornfeld, D.; Froemming, A.T.; Gupta, R.T.; Turkbey, B.; Westphalen, A.C.; Babb, J.S.; Margolis, D.J. Interobserver reproducibility of the PI-RADS version 2 lexicon: A multicenter study of six experienced prostate radiologists. Radiology 2016, 280, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Mowatt, G.; Scotland, G.; Boachie, C.; Cruickshank, M.; Ford, J.A.; Fraser, C.; Kurban, L.; Lam, T.B.; Padhani, A.R.; Royle, J.; et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: A systematic review and economic evaluation. Health Technol. Assess. 2013, 17, vii–xix. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.U.; Bosaily, A.E.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Faria, R.; Soares, M.O.; Spackman, E.; Ahmed, H.U.; Brown, L.C.; Kaplan, R.; Emberton, M.; Sculpher, M.J. Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: A cost-effectiveness analysis based on the Prostate MR Imaging Study (PROMIS). Eur. Urol. 2018, 73, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Ghai, S.; Trachtenberg, J. MRI-guided biopsies and minimally invasive therapy for prostate cancer. Indian J. Urol. IJU J. Urol. Soc. India 2015, 31, 209. [Google Scholar] [CrossRef]
- Marks, L.; Young, S.; Natarajan, S. MRI–ultrasound fusion for guidance of targeted prostate biopsy. Curr. Opin. Urol. 2013, 23, 43. [Google Scholar] [CrossRef] [Green Version]
- Gordon, L.G.; James, R.; Tuffaha, H.W.; Lowe, A.; Yaxley, J. Cost-effectiveness analysis of multiparametric MRI with increased active surveillance for low-risk prostate cancer in Australia. J. Magn. Reson. Imaging 2017, 45, 1304–1315. [Google Scholar] [CrossRef]
- Venderink, W.; Govers, T.M.; de Rooij, M.; Fütterer, J.J.; Sedelaar, J.M. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: Systematic transrectal ultrasound, direct in-bore MRI, and image fusion. Am. J. Roentgenol. 2017, 208, 1058–1063. [Google Scholar] [CrossRef]
- Porpiglia, F.; Manfredi, M.; Mele, F.; Cossu, M.; Bollito, E.; Veltri, A.; Cirillo, S.; Regge, D.; Faletti, R.; Passera, R.; et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: Results from a randomized prospective study in biopsy-naive patients with suspected prostate cancer. Eur. Urol. 2017, 72, 282–288. [Google Scholar] [CrossRef]
- Barnett, C.L.; Davenport, M.S.; Montgomery, J.S.; Wei, J.T.; Montie, J.E.; Denton, B.T. Cost-effectiveness of magnetic resonance imaging and targeted fusion biopsy for early detection of prostate cancer. BJU Int. 2018, 122, 50–58. [Google Scholar] [CrossRef] [Green Version]
- de Rooij, M.; Crienen, S.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M.; Grutters, J.P. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound–guided biopsy in diagnosing prostate cancer: A modelling study from a health care perspective. Eur. Urol. 2014, 66, 430–436. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: A report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health 2013, 16, 231–250. [Google Scholar]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS prostate imaging–reporting and data system: 2015, version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Pahwa, S.; Schiltz, N.K.; Ponsky, L.E.; Lu, Z.; Griswold, M.A.; Gulani, V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017, 285, 157–166. [Google Scholar] [CrossRef]
- National Cancer Intelligence Network. Treatment Routes in Prostate Cancer. NCIN. 2012. Available online: https://www.ncin.org.uk/view?rid=1260 (accessed on 6 August 2018).
- Scottish Medicine Consortium. A Guide to Quality Adjusted Life Years (QALY). 2015. Available online: https://www.scottishmedicines.org.uk/media/2839/guide-to-qalys.pdf (accessed on 7 August 2019).
- Mason, H.; Jones-Lee, M.; Donaldson, C. Modelling the monetary value of a QALY: A new approach based on UK data. Health Econ. 2009, 18, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.; Baker, R.; Donaldson, C. Willingness to pay for a QALY: Past, present and future. Expert Rev. Pharm. Outcomes Res. 2008, 8, 575–582. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Cost | Source | Probability | Source | QALY | Source |
|---|---|---|---|---|---|---|
| Diagnosis and biopsy | ||||||
| mpMRI | GBP 320 | [4] | ||||
| sensitivity | 0.67 | [6] | ||||
| specificity | 0.92 | [6] | ||||
| SWE | GBP 320 | AE i | −0.027 ii | [21] | ||
| sensitivity | 0.67 | [6] | ||||
| specificity | 0.93 | [6] | ||||
| MRI-TRUS fusion biopsy | GBP 343 iii | [4] | −0.027 ii | [21] | ||
| sensitivity | 0.77 | [15] | ||||
| specificity | 1.00 iv | [15,21] | ||||
| TRUS guided biopsy | GBP 316 | [4] | −0.027 ii | [21] | ||
| sensitivity | 0.53 | [15] | ||||
| specificity | 1.00 iv | [15,21] | ||||
| Treatment | ||||||
| Radical prostatectomy (RP) | GBP 30,432 | [21] | 0.14 | [22] | 7.83 | [21] |
| Radiotherapy (RT) | GBP 38,286 | [21] | 0.14 | [22] | 8.04 | [21] |
| Hormone therapy (HT) | GBP 30,396 | [21] | 0.32 | [22] | 8.25 | [21] |
| Radiotherapy + hormone therapy (RT + HT) | GBP 40,092 | AE v | 0.20 | [22] | 8.25 | AE vi |
| Watchful waiting/active surveillance (WW/AS) | GBP 25,042 | [21] | 0.20 | [22] | 8.99 | [21] |
| Undiagnosed, untreated, or later-diagnosed cancer | GBP 24,613 | [21] | 7.71 | [21] | ||
| No prostate cancer | 0.50 | [21] | 10.49 | [21] |
| Strategy | Total QALYs | Incremental 2 QALYs | Total Cost | Incremental 2 Cost | ICER 2 | NHB 3 (GBP 20k) | NHB 3 (GBP 30k) |
|---|---|---|---|---|---|---|---|
| 1 | 9.246 | - | GBP 14,762 | - | - | 8.5082 | 8.7542 |
| [9.09, 9.40] 4 | [14,103, 15,412] 5 | ||||||
| 2 | 9.281 | 0.0347 | GBP 15,635 | GBP 873 | GBP 25,126 | 8.4993 | 8.7599 |
| [9.04, 9.53] 4 | [−0.25, 0.107] 4 | [14,695, 16,450] 5 | [542, 1203] 5 | [14,619, 38,653] 5 | |||
| 3 | 9.293 | 0.0462 | GBP 15,227 | GBP 465 | GBP 10,048 | 8.5312 | 8.7850 |
| [8.90, 9.68] 4 | [−0.027, 0.118] 4 | [13,568, 15,509] 5 | [195, 733] 5 | [4075, 16,905] 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiva, M.; Wei, C.; Molana, H.; Nabi, G. Cost-Effectiveness of Prostate Cancer Detection in Biopsy-Naïve Men: Ultrasound Shear Wave Elastography vs. Multiparametric Diagnostic Magnetic Resonance Imaging. Healthcare 2022, 10, 254. https://doi.org/10.3390/healthcare10020254
Shiva M, Wei C, Molana H, Nabi G. Cost-Effectiveness of Prostate Cancer Detection in Biopsy-Naïve Men: Ultrasound Shear Wave Elastography vs. Multiparametric Diagnostic Magnetic Resonance Imaging. Healthcare. 2022; 10(2):254. https://doi.org/10.3390/healthcare10020254
Chicago/Turabian StyleShiva, Mehdi, Cheng Wei, Hassan Molana, and Ghulam Nabi. 2022. "Cost-Effectiveness of Prostate Cancer Detection in Biopsy-Naïve Men: Ultrasound Shear Wave Elastography vs. Multiparametric Diagnostic Magnetic Resonance Imaging" Healthcare 10, no. 2: 254. https://doi.org/10.3390/healthcare10020254
APA StyleShiva, M., Wei, C., Molana, H., & Nabi, G. (2022). Cost-Effectiveness of Prostate Cancer Detection in Biopsy-Naïve Men: Ultrasound Shear Wave Elastography vs. Multiparametric Diagnostic Magnetic Resonance Imaging. Healthcare, 10(2), 254. https://doi.org/10.3390/healthcare10020254







