Intrasession Reliability Analysis for Oscillometric Blood Pressure Method Using a Digital Blood Pressure Monitor in Peruvian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Ethical Considerations
2.4. Instruments and Measurements
2.5. Statistical Analysis
3. Results
3.1. Systolic Blood Pressure Reliability
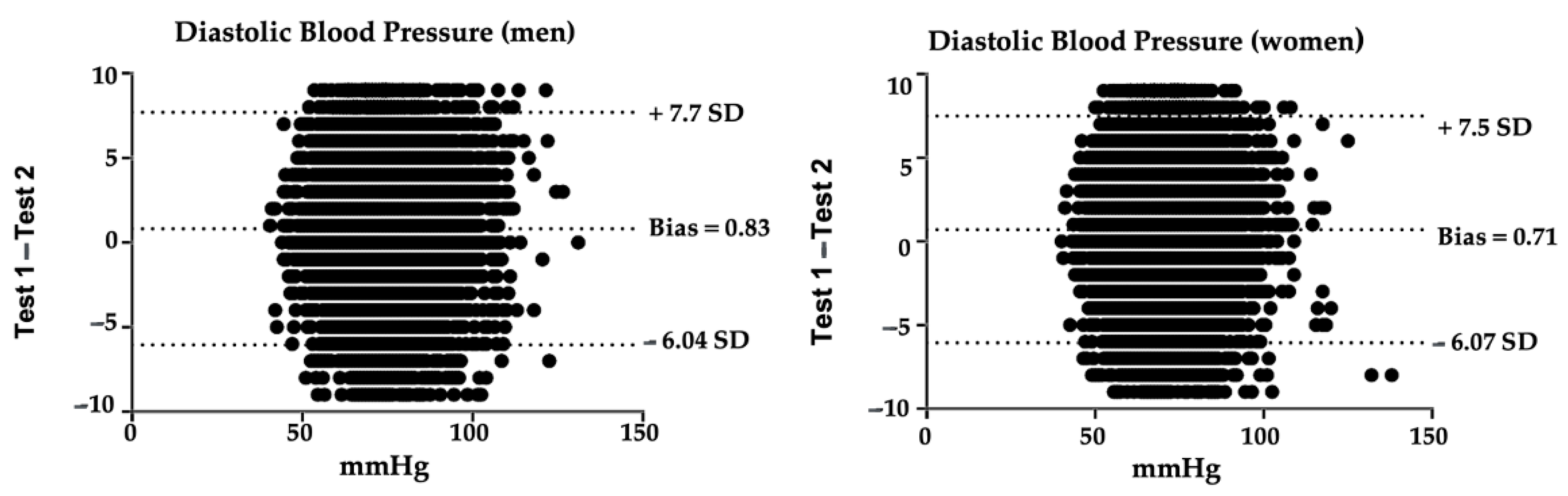
3.2. Diastolic Blood Pressure Reliability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Hypertension. Available online: https://www.who.int/health-topics/hypertension (accessed on 5 May 2021).
- Magder, S. The Meaning of Blood Pressure. Crit. Care 2018, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, W.A. Blood Pressure. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterwoths: Boston, MA, USA, 1990. [Google Scholar]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Hastings, J.A.; Boyd, K.; Krainski, F.; Perhonen, M.A.; Scheer, F.A.J.L.; Levine, B.D. Day/Night Variability in Blood Pressure: Influence of Posture and Physical Activity. Am. J. Hypertens. 2013, 26, 822–828. [Google Scholar] [CrossRef]
- Eşer, İ.; Khorshid, L.; Yapucu Güneş, Ü.; Demir, Y. The Effect of Different Body Positions on Blood Pressure. J. Clin. Nurs. 2007, 16, 137–140. [Google Scholar] [CrossRef]
- Sala, C.; Santin, E.; Rescaldani, M.; Magrini, F. How Long Shall the Patient Rest before Clinic Blood Pressure Measurement? Am. J. Hypertens. 2006, 19, 713–717. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; Palatini, P.; Parati, G.; O’Brien, E.; Januszewicz, A.; Lurbe, E.; Persu, A.; Mancia, G.; Kreutz, R. 2021 European Society of Hypertension Practice Guidelines for Office and out-of-Office Blood Pressure Measurement. J. Hypertens. 2021, 39, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- On behalf of the HOPE Asia Network; Park, S.; Buranakitjaroen, P.; Chen, C.-H.; Chia, Y.-C.; Divinagracia, R.; Hoshide, S.; Shin, J.; Siddique, S.; Sison, J.; et al. Expert Panel Consensus Recommendations for Home Blood Pressure Monitoring in Asia: The Hope Asia Network. J. Hum. Hypertens. 2018, 32, 249–258. [Google Scholar] [CrossRef]
- Meidert, A.S.; Saugel, B. Techniques for Non-Invasive Monitoring of Arterial Blood Pressure. Front. Med. 2018, 4, 231. [Google Scholar] [CrossRef]
- Gijón-Conde, T.; Gorostidi, M.; Camafort, M.; Abad-Cardiel, M.; Martín-Rioboo, E.; Morales-Olivas, F.; Vinyoles, E.; Armario, P.; Banegas, J.R.; Coca, A.; et al. Documento de la Sociedad Española de Hipertensión-Liga Española para la Lucha contra la Hipertensión Arterial (SEH-LELHA) sobre las guías ACC/AHA 2017 de hipertensión arterial. Hipertens. Riesgo Vasc. 2018, 35, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Read, S.H.; Wild, S.H. Prevention of Premature Cardiovascular Death Worldwide. Lancet 2020, 395, 758–760. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Ren, M.; Wang, R.; Zhao, F.; Liu, T.; Zhang, Y.; Guo, Z.; Cong, H. Distribution of Risk Factors of Hypertension Patients in Different Age Groups in Tianjin. BMC Public Health 2021, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Deere, B.P.; Ferdinand, K.C. Hypertension and Race/Ethnicity. Curr. Opin. Cardiol. 2020, 35, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; McNaughton, S.A. Diet Quality Is Associated with Obesity and Hypertension in Australian Adults: A Cross Sectional Study. BMC Public Health 2016, 16, 1037. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Mukamal, K.J. Alcohol Consumption and Risk of Hypertension: Does the Type of Beverage or Drinking Pattern Matter? Rev. Esp. Cardiol. 2009, 62, 603–605. [Google Scholar] [CrossRef]
- Virdis, A.; Giannarelli, C.; Fritsch Neves, M.; Taddei, S.; Ghiadoni, L. Cigarette Smoking and Hypertension. Curr. Pharm. Des. 2010, 16, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.S. Association of Obesity with Hypertension. Ann. Transl. Med. 2017, 5, 350. [Google Scholar] [CrossRef]
- Ibekwe, R. Modifiable Risk Factors of Hypertension and Socio-Demographic Profile in Oghara, Delta State; Prevalence and Correlates. Ann. Med. Health Sci. Res. 2015, 5, 71. [Google Scholar] [CrossRef]
- Egan, B.M. Physical Activity and Hypertension: Knowing Is Not Enough; We Must Apply. Willing Is Not Enough; We Must Do—von Goethe. Hypertension 2017, 69, 404–406. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Yin, S.; Fan, H.; Feng, F.; Yuan, J. Effects of Psychological Stress on Hypertension in Middle-Aged Chinese: A Cross-Sectional Study. PLoS ONE 2015, 10, e0129163. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Prevent High Blood Pressure. Available online: https://www.cdc.gov/bloodpressure/prevent.htm (accessed on 10 May 2021).
- Imai, Y.; Hosaka, M.; Elnagar, N.; Satoh, M. Clinical Significance of Home Blood Pressure Measurements for the Prevention and Management of High Blood Pressure. Clin. Exp. Pharmacol. Physiol. 2014, 41, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujita, T.; Ito, S.; Naritomi, H.; Ogihara, T.; Shimamoto, K.; Tanaka, H.; Yoshiike, N. The Importance of Home Blood Pressure Measurement for Preventing Stroke and Cardiovascular Disease in Hypertensive Patients: A Sub-Analysis of the Japan Hypertension Evaluation with Angiotensin II Antagonist Losartan Therapy (J-HEALTH) Study, a Prospective Nationwide Observational Study. Hypertens. Res. 2008, 31, 1903–1911. [Google Scholar] [CrossRef][Green Version]
- Centers for Disease Control and Prevention. Measure Your Blood Pressure. Available online: https://www.cdc.gov/bloodpressure/measure.htm (accessed on 10 May 2021).
- Musini, V.M.; Wright, J.M. Factors Affecting Blood Pressure Variability: Lessons Learned from Two Systematic Reviews of Randomized Controlled Trials. PLoS ONE 2009, 4, e5673. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud. Especificaciones Técnicas de La OMS Para Dispositivos Automáticos de Medición de La Presión Arterial No Invasivos y Con Brazalete; Organización Panamericana de la Salud: Washington, DC, USA, 2020. [Google Scholar]
- Lombardi, C.; Ordunez, P. Technical Resources Relevant to the Accuracy of Blood Pressure Measurement; Organizacion Panamericana de la Salud: Washington, DC, USA, 2020. [Google Scholar]
- World Health Organization (Ed.) Affordable Technology: Blood Pressure Measuring Devices for Low Resource Settings; World Health Organization: Geneva, Switzerland, 2005; ISBN 978-92-4-159264-2.
- Nerenberg, K.A.; Zarnke, K.B.; Leung, A.A.; Dasgupta, K.; Butalia, S.; McBrien, K.; Harris, K.C.; Nakhla, M.; Cloutier, L.; Gelfer, M.; et al. Hypertension Canada’s 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can. J. Cardiol. 2018, 34, 506–525. [Google Scholar] [CrossRef] [PubMed]
- Grupo de Trabajo de la Sociedad Europea de Cardiología (ESC) y la European Society of Hypertension (ESH) sobre el diagnóstico y tratamiento de la hipertensión arteria. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Rev. Esp. Cardiol. 2019, 72, 160.e1–160.e78. [Google Scholar] [CrossRef]
- Padwal, R.; Campbell, N.R.C.; Schutte, A.E.; Olsen, M.H.; Delles, C.; Etyang, A.; Cruickshank, J.K.; Stergiou, G.; Rakotz, M.K.; Wozniak, G.; et al. Optimizing Observer Performance of Clinic Blood Pressure Measurement: A Position Statement from the Lancet Commission on Hypertension Group. J. Hypertens. 2019, 37, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Vilagut, G. Test-Retest Reliability. In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 6622–6625. ISBN 978-94-007-0752-8. [Google Scholar]
- Salkind, N.J. (Ed.) Encyclopedia of Research Design; SAGE Publications: Thousand Oaks, CA, USA, 2010; ISBN 978-1-4129-6127-1. [Google Scholar]
- Daly, L.E.; Bourke, G.J.; Bourke, G.J. Interpretation and Uses of Medical Statistics, 5th ed.; Blackwell Science: Oxford, UK; Malden, MA, USA, 2000; ISBN 978-0-632-04763-5. [Google Scholar]
- Instituto Nacional de Estadística e Informática. Encuesta Demográfica y de Salud Familiar 2019; Instituto Nacional de Estadística e Informática: Lima, Perú, 2020.
- Instituto Nacional de Estadística e Informática. Ficha Técnica de La Encuesta Demográfica y de Salud Familiar 2019; Instituto Nacional de Estadística e Informática: Lima, Perú, 2020.
- Dirección Técnica de Demografía e Indicadores Sociales. Manual de La Entrevistadora; Instituto Nacional de Estadística e Informática: Lima, Perú, 2015.
- Plichta, S.B.; Kelvin, E.A.; Munro, B.H. Munro’s Statistical Methods for Health Care Research, 6th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; ISBN 978-1-4511-1561-1. [Google Scholar]
- Weir, J.P. Quantifying Test-Retest Reliability Using the Intraclass Correlation Coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Alpert, B.S.; Quinn, D.; Gallick, D. Oscillometric Blood Pressure: A Review for Clinicians. J. Am. Soc. Hypertens. 2014, 8, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Duncombe, S.L.; Voss, C.; Harris, K.C. Oscillometric and Auscultatory Blood Pressure Measurement Methods in Children: A Systematic Review and Meta-Analysis. J. Hypertens. 2017, 35, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Maselli, M.; Giantin, V.; Corrado, D.; Franchin, A.; Attanasio, F.; Pengo, V.; Tramontano, A.; De Toni, P.; Perissinotto, E.; Manzato, E. Reliability of Oscillometric Blood Pressure Monitoring in Atrial Fibrillation Patients Admitted for Electric Cardioversion. J. Clin. Hypertens. 2015, 17, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Choi, Y.-K. Measurement Reliability of Automated Oscillometric Blood Pressure Monitor in the Elderly with Atrial Fibrillation: A Systematic Review and Meta-Analysis. Blood Press. Monit. 2020, 25, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Stoner, L.; Bonner, C.; Credeur, D.; Lambrick, D.; Faulkner, J.; Wadsworth, D.; Williams, M.A. Reliability of Oscillometric Central Hemodynamic Responses to an Orthostatic Challenge. Atherosclerosis 2015, 241, 761–765. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitchelmore, A.; Stoner, L.; Lambrick, D.; Jobson, S.; Faulkner, J. Reliability of Oscillometric Central Blood Pressure and Central Systolic Loading in Individuals over 50 Years: Effects of Posture and Fasting. Atherosclerosis 2018, 269, 79–85. [Google Scholar] [CrossRef]
- Fryer, S.; Stone, K.; Dickson, T.; Faulkner, J.; Lambrick, D.; Corres, P.; Jerred, L.; Stoner, L. Reliability of Oscillometric Central Blood Pressure Responses to Lower Limb Resistance Exercise. Atherosclerosis 2018, 268, 157–162. [Google Scholar] [CrossRef]
- Lim, W.; Faulkner, J.; Lambrick, D.; Stoner, L. Reliability of Oscillometric Central Blood Pressure Responses to Submaximal Exercise. J. Hypertens. 2016, 34, 1084–1090. [Google Scholar] [CrossRef]
- Fonseca-Reyes, S.; Romero-Velarde, E.; Torres-Gudiño, E.; Illescas-Zarate, D.; Forsyth-MacQuarrie, A.M. Comparison of Auscultatory and Oscillometric BP Measurements in Children with Obesity and Their Effect on the Diagnosis of Arterial Hypertension. Arch. Cardiol. México 2018, 88, 16–24. [Google Scholar] [CrossRef]
- Šelmytė-Besusparė, A.; Barysienė, J.; Petrikonytė, D.; Aidietis, A.; Marinskis, G.; Laucevičius, A. Auscultatory versus Oscillometric Blood Pressure Measurement in Patients with Atrial Fibrillation and Arterial Hypertension. BMC Cardiovasc. Disord. 2017, 17, 87. [Google Scholar] [CrossRef][Green Version]
- Pioli, M.R.; Ritter, A.M.V.; de Faria, A.P.; Modolo, R. White Coat Syndrome and Its Variations: Differences and Clinical Impact. Integr. Blood Press. Control 2018, 11, 73–79. [Google Scholar] [CrossRef]
- Ramli, A.; Halmey, N.; Teng, C. White Coat Effect and White Coat Hypertension: One and the Same? Malays. Fam. Physician 2008, 3, 158–161. [Google Scholar]
- Haskard-Zolnierek, K.; Cobos, B.; Howard, K. White Coat Hypertension: Improving the Patients; Health Care Practitioner Relationship. Psychol. Res. Behav. Manag. 2015, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, E.; Tikhonoff, V.; Albertini, F.; Palatini, P. Poor Reliability of Wrist Blood Pressure Self-Measurement at Home: A Population-Based Study. Hypertension 2016, 68, 896–903. [Google Scholar] [CrossRef]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2007, 25, 1105–1187. [Google Scholar] [CrossRef] [PubMed]
- Climie, R.E.D.; Schultz, M.G.; Nikolic, S.B.; Ahuja, K.D.K.; Fell, J.W.; Sharman, J.E. Validity and Reliability of Central Blood Pressure Estimated by Upper Arm Oscillometric Cuff Pressure. Am. J. Hypertens. 2012, 25, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Takci, S.; Yigit, S.; Korkmaz, A.; Yurdakök, M. Comparison between Oscillometric and Invasive Blood Pressure Measurements in Critically Ill Premature Infants: Reliability of Noninvasive Blood Pressure Measurements. Acta Paediatr. 2012, 101, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Bulut, U.; Gunvar, T.; Guldal, A.D. Efficacy of Oscillometric Method for Screening Periferic Arterial Disease in Primary Care. Niger. J. Clin. Pract. 2020, 23, 668–674. [Google Scholar] [CrossRef] [PubMed]
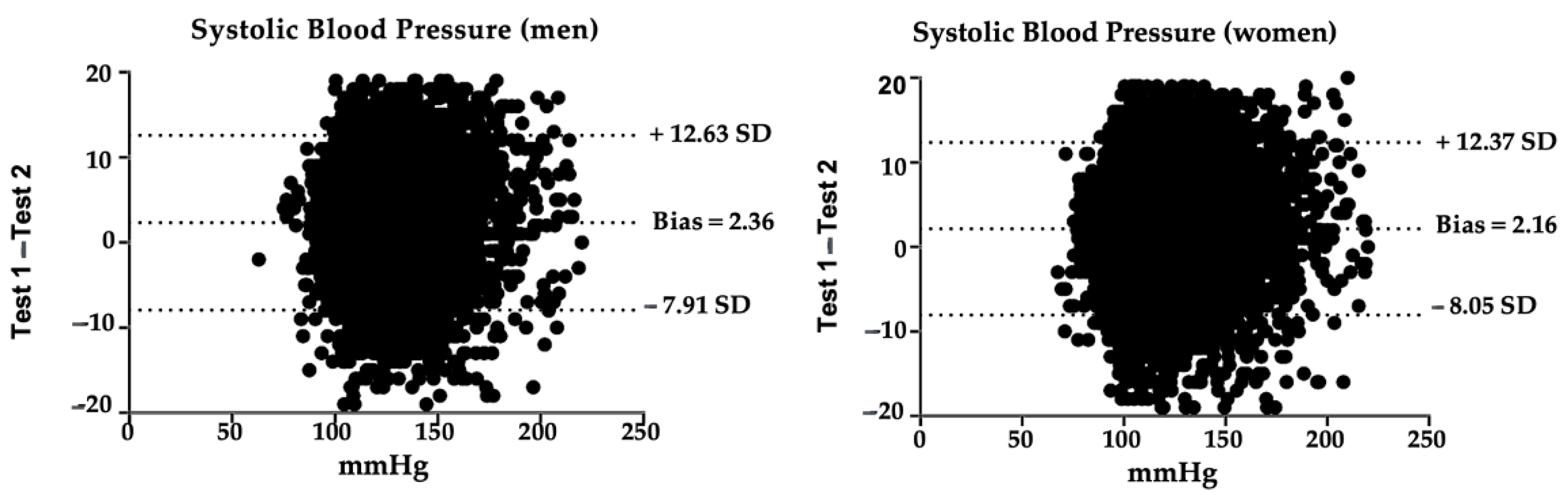
| Measurement 1 ± TD | Measurement 2 ± TD | p * | ||
|---|---|---|---|---|
| All (n = 33,522) | Systolic blood pressure (mmHg) | 120.31 ± 15.56 | 118.07 ± 17.17 | <0.001 |
| ICC (95% CI) | SEM (mmHg) | %SEM | SRD (mmHg) | %SRD |
|---|---|---|---|---|
| 0.95 (0.94–0.95) | 3.99 | 3.35 | 11.07 | 9.28 |
| Measurement 1 ± TD | Measurement 2 ± TD | p * | ||
|---|---|---|---|---|
| Men with Hypertension diagnosis (n = 1106) | ||||
| 15–17 (n = 6) | Systolic blood pressure (mmHg) | 119.83 ± 5.88 | 119.33 ± 4.97 | 0.768 |
| 18–29 (n = 48) | Systolic blood pressure (mmHg) | 127.06 ± 15.48 | 125.27 ± 15.72 | 0.021 |
| 30–39 (n = 107) | Systolic blood pressure (mmHg) | 131.10 ± 15.42 | 127.66 ± 14.53 | <0.001 |
| 40–49 (n = 181) | Systolic blood pressure (mmHg) | 132.82 ± 20.24 | 130.77 ± 18.87 | <0.001 |
| 50–59 (n = 199) | Systolic blood pressure (mmHg) | 140.98 ± 22.71 | 138.65 ± 21.73 | <0.001 |
| 60–69 (n = 220) | Systolic blood pressure (mmHg) | 143.34 ± 23.36 | 140.35 ± 23.78 | <0.001 |
| 70–79 (n = 220) | Systolic blood pressure (mmHg) | 147.50 ± 25.16 | 144.90 ± 24.44 | <0.001 |
| 80–89 (n = 114) | Systolic blood pressure (mmHg) | 151.02 ± 26.50 | 147.63 ± 26.80 | <0.001 |
| >90 (n = 11) | Systolic blood pressure (mmHg) | 142.91 ± 15.37 | 142.64 ± 17.88 | 0.885 |
| Men without Hypertension diagnosis (n = 13,133) | ||||
| 15–17 (n = 876) | Systolic blood pressure (mmHg) | 118.76 ± 12.97 | 116.19 ± 11.81 | <0.001 |
| 18–29 (n = 3210) | Systolic blood pressure (mmHg) | 123.09 ± 11.81 | 120.74 ± 11.37 | <0.001 |
| 30–39 (n = 3535) | Systolic blood pressure (mmHg) | 123.67 ± 11.99 | 121.41 ± 11.70 | <0.001 |
| 40–49 (n = 2208) | Systolic blood pressure (mmHg) | 125.37 ± 14.17 | 123.34 ± 13.82 | <0.001 |
| 50–59 (n = 1479) | Systolic blood pressure (mmHg) | 127.86 ± 16.28 | 125.21 ± 15.67 | <0.001 |
| 60–69 (n = 1012) | Systolic blood pressure (mmHg) | 131.23 ± 19.44 | 128.79 ± 18.91 | <0.001 |
| 70–79 (n = 541) | Systolic blood pressure (mmHg) | 134.59 ± 21.08 | 132.32 ± 20.73 | <.001 |
| 80–89 (n = 240) | Systolic blood pressure (mmHg) | 132.76 ± 21.59 | 130.06 ± 21.13 | <0.001 |
| >90 (n = 32) | Systolic blood pressure (mmHg) | 137.06 ± 26.83 | 132.56 ± 28.69 | <0.001 |
| Age Intervals (years) | ICC (95% CI) | SEM (mmHg) | %SEM | SRD (mmHg) | %SRD |
|---|---|---|---|---|---|
| Men with Hypertension diagnosis (n = 1106) | |||||
| 15–17 (n = 6) | 0.77 (0.13–0.96) | 2.58 | 2.16 | 7.716 | 5.99 |
| 18–29 (n = 48) | 0.94 (0.89–0.96) | 3.85 | 3.05 | 10.68 | 8.47 |
| 30–39 (n = 107) | 0.91 (0.89–0.94) | 4.37 | 3.38 | 12.10 | 9.36 |
| 40–49 (n = 181) | 0.95 (0.94–0.96) | 4.24 | 3.22 | 12 | 8.92 |
| 50–59 (n = 199) | 0.96 (0.95–0.97) | 4.50 | 3.22 | 12.47 | 8.92 |
| 60–69 (n = 220) | 0.96 (0.95–0.97) | 4.66 | 3.28 | 12.90 | 9.10 |
| 70–79 (n = 220) | 0.95 (0.94–0.96) | 5.26 | 3.60 | 14.58 | 9.97 |
| 80–89 (n = 114) | 0.96 (0.95–0.97) | 5.14 | 3.45 | 14.29 | 9.55 |
| >90 (n = 11) | 0.94 (0.80–0.98) | 4.11 | 2.88 | 11.38 | 7.97 |
| Men without Hypertension diagnosis (n = 13,133) | |||||
| 15–17 (n = 876) | 0.89 (0.86–0.89) | 4.17 | 3.55 | 11.56 | 9.84 |
| 18–29 (n = 3210) | 0.89 (0.88–0.90) | 3.81 | 3.12 | 10.56 | 8.66 |
| 30–39 (n = 3535) | 0.90 (0.89–0.90) | 3.76 | 3.07 | 10.43 | 8.52 |
| 40–49 (n = 2208) | 0.93 (0.92–0.93) | 3.81 | 3.06 | 10.55 | 8.49 |
| 50–59 (n = 1479) | 0.93 (0.92–0.94) | 4.20 | 3.32 | 11.63 | 9.19 |
| 60–69 (n = 1012) | 0.94 (0.94–0.95) | 4.58 | 3.52 | 12.69 | 9.76 |
| 70–79 (n = 541) | 0.95 (0.94–0.96) | 4.77 | 3.57 | 13.21 | 9.9 |
| 80–89 (n = 240) | 0.95 (0.94–0.96) | 4.63 | 3.52 | 12.84 | 9.77 |
| <90 (n = 32) | 0.96 (0.93–0.98) | 5.19 | 3.85 | 14.39 | 10.68 |
| Measurement 1 ± TD | Measurement 2 ± TD | p * | ||
|---|---|---|---|---|
| Women with Hypertension diagnosis (n = 1106) | ||||
| 15–17 (n = 12) | Systolic blood pressure (mmHg) | 113.42 ± 9.76 | 111.25 ± 8.69 | 0.031 |
| 18–29 (n = 126) | Systolic blood pressure (mmHg) | 113.00 ± 13.05 | 110.66 ± 13.07 | <0.001 |
| 30–39 (n = 194) | Systolic blood pressure (mmHg) | 117.20 ± 16.73 | 114.67 ± 16.28 | <0.001 |
| 40–49 (n = 261) | Systolic blood pressure (mmHg) | 127.98 ± 21.65 | 126.00 ± 22.40 | <0.001 |
| 50–59 (n = 343) | Systolic blood pressure (mmHg) | 132.25 ± 21.66 | 129.85 ± 21.47 | <0.001 |
| 60–69 (n = 428) | Systolic blood pressure (mmHg) | 138.13 ± 23.01 | 135.24 ± 22.63 | <0.001 |
| 70–79 (n = 287) | Systolic blood pressure (mmHg) | 147.44 ± 26.00 | 144.64 ± 25.60 | <0.001 |
| 80–89 (n = 151) | Systolic blood pressure (mmHg) | 152.54 ± 25.10 | 150.00 ± 25.05 | <0.001 |
| >90 (n = 18) | Systolic blood pressure (mmHg) | 147.06 ± 31.90 | 144.22 ± 32.67 | 0.055 |
| Women without Hypertension diagnosis (n = 17,414) | ||||
| 15–17 (n = 989) | Systolic blood pressure (mmHg) | 108.23 ± 10.72 | 105.77 ± 10.34 | <0.001 |
| 18–29 (n = 5451) | Systolic blood pressure (mmHg) | 108.81 ± 10.44 | 106.90 ± 10.15 | <0.001 |
| 30–39 (n = 4903) | Systolic blood pressure (mmHg) | 111.30 ± 11.54 | 109.29 ± 11.25 | <0.001 |
| 40–49 (n = 2555) | Systolic blood pressure (mmHg) | 115.90 ± 14.19 | 113.96 ± 14.03 | <0.001 |
| 50–59 (n = 1586) | Systolic blood pressure (mmHg) | 120.36 ± 16.11 | 117.83 ± 16.10 | <0.001 |
| 60–69 (n = 1053) | Systolic blood pressure (mmHg) | 126.95 ± 18.53 | 124.34 ± 18.35 | <.001 |
| 70–79 (n = 598) | Systolic blood pressure (mmHg) | 130.87 ± 20.02 | 127.88 ± 19.80 | <0.001 |
| 80–89 (n = 238) | Systolic blood pressure (mmHg) | 136.67 ± 23.28 | 134.00 ± 23.39 | <0.001 |
| >90 (n = 41) | Systolic blood pressure (mmHg) | 132.27 ± 26.33 | 129.88 ± 27.04 | 0.009 |
| Age Intervals (years) | ICC (95% CI) | SEM (mmHg) | %SEM | SRD (mmHg) | %SRD |
|---|---|---|---|---|---|
| Women with Hypertension diagnosis (n = 1820) | |||||
| 15–17 (n = 12) | 0.92 (0.77–0.98) | 2.54 | 2.26 | 7.05 | 6.27 |
| 18–29 (n = 126) | 0.92 (0.89–0.94) | 3.65 | 3.26 | 10.11 | 9.04 |
| 30–39 (n = 194) | 0.94 (0.93–0.96) | 3.91 | 3.37 | 10.83 | 9.34 |
| 40–49 (n = 261) | 0.96 (0.95–0.97) | 4.41 | 3.47 | 12.21 | 9.62 |
| 50–59 (n = 343) | 0.95 (0.94–0.96) | 4.82 | 3.68 | 13.37 | 10.20 |
| 60–69 (n = 428) | 0.94 (0.93–0.95) | 5.4 | 3.95 | 14.96 | 10.95 |
| 70–79 (n = 287) | 0.96 (0.95–0.97) | 5.22 | 3.58 | 14.48 | 9.92 |
| 80–89 (n = 151) | 0.96 (0.95–0.97) | 4.69 | 3.10 | 13 | 8.6 |
| <90 (n = 18) | 0.98 (0.95–0.99) | 4.45 | 3.06 | 12.34 | 8.47 |
| Women without Hypertension diagnosis (n = 17,414) | |||||
| 15–17 (n = 989) | 0.87 (0.86–0.89) | 3.75 | 3.51 | 10.4 | 9.72 |
| 18–29 (n = 5451) | 0.88 (0.88–0.89) | 3.54 | 3.73 | 9.8 | 9.09 |
| 30–39 (n = 4903) | 0.89 (0.89–0.90) | 3.74 | 3.4 | 10.38 | 9.41 |
| 40–49 (n = 2555) | 0.92 (0.91–0.93) | 3.99 | 3.47 | 11.06 | 9.62 |
| 50–59 (n = 1586) | 0.93 (0.92–0.94) | 4.29 | 3.6 | 11.9 | 9.99 |
| 60–69 (n = 1053) | 0.94 (0.93–0.95) | 4.48 | 3.57 | 12.42 | 9.88 |
| 70–79 (n = 598) | 0.94 (0.93–0.95) | 4.92 | 3.8 | 13.63 | 10.35 |
| 80–89 (n = 238) | 0.96 (0.95–0.97) | 4.73 | 3.49 | 13.1 | 9.68 |
| <90 (n = 41) | 0.97 (0.95–0.99) | 4.22 | 3.22 | 11.7 | 8.92 |
| Measurement 1 ± TD | Measurement 2 ± TD | p * | ||
|---|---|---|---|---|
| All (n = 33,522) | Diastolic blood pressure (mmHg) | 71.97 ± 10.07 | 71.21 ± 10.05 | <0.001 |
| ICC (95% CI) | SEM (mmHg) | %SEM | SRD (mmHg) | %SRD |
|---|---|---|---|---|
| 0.94 (0.94–0.94) | 2.53 | 3.53 | 7 | 9.78 |
| Measurement 1 ± TD | Measurement 2 ± TD | p * | ||
|---|---|---|---|---|
| Men with Hypertension diagnosis (n = 1106) | ||||
| 15–17 (n = 6) | Diastolic blood pressure (mmHg) | 68.83 ± 5.98 | 65.83 ± 4.87 | 0.068 |
| 18–29 (n = 48) | Diastolic blood pressure (mmHg) | 74.92 ± 12.87 | 74.42 ± 11.40 | 0.371 |
| 30–39 (n = 107) | Diastolic blood pressure (mmHg) | 81.09 ± 10.86 | 79.88 ± 10.37 | 0.002 |
| 40–49 (n = 181) | Diastolic blood pressure (mmHg) | 82.03 ± 12.99 | 81.07 ± 12.62 | <0.001 |
| 50–59 (n = 199) | Diastolic blood pressure (mmHg) | 83.24 ± 12.68 | 82.31 ± 12.47 | <0.001 |
| 60–69 (n = 220) | Diastolic blood pressure (mmHg) | 79.45 ± 11.83 | 78.46 ± 11.22 | <0.001 |
| 70–79 (n = 220) | Diastolic blood pressure (mmHg) | 76.93 ± 12.50 | 76.51 ± 12.39 | 0.100 |
| 80–89 (n = 114) | Diastolic blood pressure (mmHg) | 73.23 ± 12.88 | 72.92 ± 12.38 | 0.380 |
| >90 (n = 11) | Diastolic blood pressure (mmHg) | 80.55 ± 9.97 | 78.00 ± 9.85 | 0.034 |
| Men without Hypertension diagnosis (n = 13,133) | ||||
| 15–17 (n = 876) | Diastolic blood pressure (mmHg) | 67.93 ± 8.00 | 66.42 ± 8.13 | <0.001 |
| 18–29 (n = 3210) | Diastolic blood pressure (mmHg) | 72.04 ± 9.23 | 70.82 ± 9.18 | <0.001 |
| 30–39 (n = 3535) | Diastolic blood pressure (mmHg) | 74.91 ± 9.10 | 74.20 ± 9.19 | <0.001 |
| 40–49 (n = 2208) | Diastolic blood pressure (mmHg) | 76.80 ± 10.03 | 76.33 ± 9.96 | <0.001 |
| 50–59 (n = 1479) | Diastolic blood pressure (mmHg) | 76.57 ± 9.80 | 75.96 ± 9.82 | <0.001 |
| 60–69 (n = 1012) | Diastolic blood pressure (mmHg) | 75.56 ± 10.34 | 74.87 ± 10.24 | <0.001 |
| 70–79 (n = 541) | Diastolic blood pressure (mmHg) | 73.28 ± 10.68 | 72.70 ± 10.82 | <0.001 |
| 80–89 (n = 240) | Diastolic blood pressure (mmHg) | 69.55 ± 10.68 | 68.78 ± 11.00 | 0.001 |
| >90 (n = 32) | Diastolic blood pressure (mmHg) | 68.28 ± 10.54 | 67.50 ± 11.11 | 0.212 |
| Age Intervals (years) | ICC (95% CI) | SEM (mmHg) | %SEM | SRD (mmHg) | %SRD |
|---|---|---|---|---|---|
| Men with Hypertension diagnosis (n = 1106) | |||||
| 15–17 (n = 6) | 0.73 (0.02–0,95) | 2.24 | 4.22 | 7.88 | 11.7 |
| 18–29 (n = 48) | 0.95 (0.91–0.97) | 2.69 | 3.6 | 7.45 | 9.97 |
| 30–39 (n = 107) | 0.93 (0.90–0.95) | 2.85 | 3.5 | 7.9 | 9.81 |
| 40–49 (n = 181) | 0.96 (0.95–0.97) | 2.53 | 3.10 | 7.01 | 8.6 |
| 50–59 (n = 199) | 0.96 (0.94–0.97) | 2.61 | 3.15 | 7.23 | 8.73 |
| 60–69 (n = 220) | 0.95 (0.93–0.96) | 2.63 | 3.33 | 7.29 | 9.23 |
| 70–79 (n = 220) | 0.95 (0.94–0.96) | 2.64 | 3.44 | 7.32 | 9.54 |
| 80–89 (n = 114) | 0.96 (0.94–0.97) | 2.62 | 3.58 | 7.26 | 9.93 |
| <90 (n = 11) | 0.91 (0.72–0.98) | 2.92 | 3.69 | 8.10 | 10.22 |
| Men without Hypertension diagnosis (n = 13,133) | |||||
| 15–17 (n = 876) | 0.87 (0.85–0.88) | 2.92 | 4.35 | 8.09 | 12.05 |
| 18–29 (n = 3210) | 0.91 (0.91–0.92) | 2.7 | 3.78 | 7.48 | 10.49 |
| 30–39 (n = 3535) | 0.93 (0.92–0.93) | 2.47 | 3.31 | 6.85 | 9.18 |
| 40–49 (n = 2208) | 0.94 (0.94–0.95) | 2.43 | 3.17 | 6.73 | 8.79 |
| 50–59 (n = 1479) | 0.94 (0.93–0.95) | 2.38 | 3.13 | 6.61 | 8.66 |
| 60–69 (n = 1012) | 0.95 (0.94–0.95) | 2.35 | 3.12 | 6.50 | 8.65 |
| 70–79 (n = 541) | 0.95 (0.94–0.95) | 2.5 | 3.42 | 6.92 | 9.49 |
| 80–89 (n = 240) | 0.95 (0.93–0.96) | 2.47 | 3.57 | 6.85 | 9.9 |
| <90 (n = 32) | 0.95 (0.90–0.97) | 2.44 | 3.6 | 6.78 | 9.98 |
| Measurement 1 ± TD | Measurement 2 ± TD | p * | ||
|---|---|---|---|---|
| Women with Hypertension diagnosis (n = 1106) | ||||
| 15–17 (n = 12) | Diastolic blood pressure (mmHg) | 71.67 ± 6.72 | 72.75 ± 7.46 | 0.218 |
| 18–29 (n = 126) | Diastolic blood pressure (mmHg) | 71.32 ± 9.47 | 70.21 ± 9.46 | <0.001 |
| 30–39 (n = 194) | Diastolic blood pressure (mmHg) | 74.48 ± 11.24 | 73.69 ± 11.62 | 0.001 |
| 40–49 (n = 261) | Diastolic blood pressure (mmHg) | 79.64 ± 13.16 | 79.24 ± 12.90 | 0.073 |
| 50–59 (n = 343) | Diastolic blood pressure (mmHg) | 77.06 ± 10.76 | 76.23 ± 10.52 | <0.001 |
| 60–69 (n = 428) | Diastolic blood pressure (mmHg) | 74.54 ± 11.03 | 73.79 ± 10.98 | <0.001 |
| 70–79 (n = 287) | Diastolic blood pressure (mmHg) | 73.26 ± 12.00 | 72.33 ± 12.34 | <0.001 |
| 80–89 (n = 151) | Diastolic blood pressure (mmHg) | 71.09 ± 10.96 | 70.15 ± 10.73 | 0.004 |
| >90 (n = 18) | Diastolic blood pressure (mmHg) | 69.33 ± 11.79 | 68.56 ± 11.37 | 0.353 |
| Women without Hypertension diagnosis (n = 17,414) | ||||
| 15–17 (n = 989) | Diastolic blood pressure (mmHg) | 66.49 ± 8.49 | 65.24 ± 8.53 | <0.001 |
| 18–29 (n = 5451) | Diastolic blood pressure (mmHg) | 67.60 ± 8.36 | 66.83 ± 8.23 | <0.001 |
| 30–39 (n = 4903) | Diastolic blood pressure (mmHg) | 70.08 ± 8.95 | 69.48 ± 8.88 | <0.001 |
| 40–49 (n = 2555) | Diastolic blood pressure (mmHg) | 71.88 ± 9.41 | 71.37 ± 9.41 | <0.001 |
| 50–59 (n = 1586) | Diastolic blood pressure (mmHg) | 71.93 ± 9.59 | 71.22 ± 9.45 | <0.001 |
| 60–69 (n = 1053) | Diastolic blood pressure (mmHg) | 71.32 ± 9.36 | 70.65 ± 9.35 | <0.001 |
| 70–79 (n = 598) | Diastolic blood pressure (mmHg) | 69.82 ± 10.29 | 68.90 ± 10.22 | <0.001 |
| 80–89 (n = 238) | Diastolic blood pressure (mmHg) | 68.41 ± 10.59 | 67.67 ± 10.46 | 0.001 |
| >90 (n = 41) | Diastolic blood pressure (mmHg) | 68.02 ± 10.23 | 67.56 ± 10.85 | 0.318 |
| Age Intervals (years) | ICC (95% CI) | SEM (mmHg) | %SEM | SRD (mmHg) | %SRD |
|---|---|---|---|---|---|
| Women with Hypertension diagnosis (n = 1820) | |||||
| 15–17 (n = 12) | 0.91 (0.74–0.97) | 2.09 | 2.9 | 5.8 | 8.03 |
| 18–29 (n = 126) | 0.93 (0.90–0.95) | 2.47 | 3.49 | 6.84 | 9.67 |
| 30–39 (n = 194) | 0.96 (0.94–0.97) | 2.37 | 3.2 | 6.57 | 8.87 |
| 40–49 (n = 261) | 0.96 (0.95–0.97) | 2.57 | 3.24 | 7.13 | 8.98 |
| 50–59 (n = 343) | 0.95 (0.94–0.96) | 2.4 | 3.13 | 6.66 | 8.69 |
| 60–69 (n = 428) | 0.95 (0.94–0.96) | 2.53 | 3.42 | 7.02 | 9.47 |
| 70–79 (n = 287) | 0.96 (0.94–0.96) | 2.55 | 3.51 | 7.08 | 9.72 |
| 80–89 (n = 151) | 0.93 (0.90–0.95) | 2.87 | 4.06 | 7.96 | 11.27 |
| <90 (n = 18) | 0.96 (0.89–0.98) | 2.43 | 3.52 | 6.73 | 9.77 |
| Women without Hypertension diagnosis (n = 17,414) | |||||
| 15–17 (n = 989) | 0.89 (0.88–0.91) | 2.76 | 4.19 | 7.65 | 11.61 |
| 18–29 (n = 5451) | 0.90 (0.90–0.91) | 2.56 | 3.81 | 7.09 | 10.55 |
| 30–39 (n = 4903) | 0.92 (0.92–0.93) | 2.46 | 3.52 | 6.81 | 9.76 |
| 40–49 (n = 2555) | 0.93 (0.93–0.94) | 2.4 | 3.35 | 6.65 | 9.29 |
| 50–59 (n = 1586) | 0.94 (0.93–0.94) | 2.39 | 3.34 | 6.63 | 9.26 |
| 60–69 (n = 1053) | 0.93 (0.92–0.94) | 2.48 | 3.49 | 6.86 | 9.67 |
| 70–79 (n = 598) | 0.94 (0.93–0.95) | 2.47 | 3.56 | 6.85 | 9.87 |
| 80–89 (n = 238) | 0.95 (0.93–0.96) | 2.38 | 3.49 | 6.59 | 9.68 |
| <90 (n = 41) | 0.96 (0.93–0.98) | 2.08 | 3.07 | 5.77 | 8.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrios-Fernandez, S.; Sosa-Sánchez, E.M.; Carlos-Vivas, J.; Muñoz-Bermejo, L.; Morenas-Martín, J.; Apolo-Arenas, M.D.; Adsuar, J.C.; Domínguez-Muñoz, F.J. Intrasession Reliability Analysis for Oscillometric Blood Pressure Method Using a Digital Blood Pressure Monitor in Peruvian Population. Healthcare 2022, 10, 209. https://doi.org/10.3390/healthcare10020209
Barrios-Fernandez S, Sosa-Sánchez EM, Carlos-Vivas J, Muñoz-Bermejo L, Morenas-Martín J, Apolo-Arenas MD, Adsuar JC, Domínguez-Muñoz FJ. Intrasession Reliability Analysis for Oscillometric Blood Pressure Method Using a Digital Blood Pressure Monitor in Peruvian Population. Healthcare. 2022; 10(2):209. https://doi.org/10.3390/healthcare10020209
Chicago/Turabian StyleBarrios-Fernandez, Sabina, Eduardo Manuel Sosa-Sánchez, Jorge Carlos-Vivas, Laura Muñoz-Bermejo, Jesús Morenas-Martín, María Dolores Apolo-Arenas, Jose Carmelo Adsuar, and Francisco Javier Domínguez-Muñoz. 2022. "Intrasession Reliability Analysis for Oscillometric Blood Pressure Method Using a Digital Blood Pressure Monitor in Peruvian Population" Healthcare 10, no. 2: 209. https://doi.org/10.3390/healthcare10020209
APA StyleBarrios-Fernandez, S., Sosa-Sánchez, E. M., Carlos-Vivas, J., Muñoz-Bermejo, L., Morenas-Martín, J., Apolo-Arenas, M. D., Adsuar, J. C., & Domínguez-Muñoz, F. J. (2022). Intrasession Reliability Analysis for Oscillometric Blood Pressure Method Using a Digital Blood Pressure Monitor in Peruvian Population. Healthcare, 10(2), 209. https://doi.org/10.3390/healthcare10020209












