Anthropometric Parameters in Patients with Fatty Acid Oxidation Disorders: A Case–Control Study, Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Case–Control Study
2.1.1. Study Population
2.1.2. Anthropometric Measurements
2.1.3. Statistical Analysis
2.2. Systemic Review and Meta-Analysis
2.2.1. Protocol and Registration
2.2.2. Search Strategy
2.2.3. Eligibility Criteria
- Study type: observational (case–control, cohort, case series) or experimental studies (any type); a study was excluded when only an abstract was available.
- Language: English.
- Study population: children (>1 month of age) and adults with confirmed diagnosis (biochemically or genetically) of fatty acid oxidation disorder (LCHADD, MCADD, VLCADD, SCADD, CPT IID or TFPD).
- Outcomes: anthropometric parameters (weight, BMI and percentiles and z-scores for these parameters).
- Study type: letters, case studies, conference abstracts, non-human studies.
- Language: papers published in a language other than English.
- Population: newborns (age < 1 month), pregnant and breastfeeding women, patients in unstable or critical clinical condition.
2.2.4. Study Selection Process
2.2.5. Data Extraction
- General information: full title of the article, list of authors, country, journal name, year of publication.
- Study characteristics: study design (experimental or observational).
- Population characteristics: number of participants in patient groups and control groups (if applicable), types of FAOD diagnosed in patients, age of the participants and sex of the participants.
- Outcomes recorded: anthropometric parameters of patients and controls (if present)—weight, BMI and percentiles and z-scores for these parameters.
2.2.6. Certainty of Evidence Assessment
2.2.7. Statistical Analysis
3. Results
3.1. Case–Control Study Results
3.2. Results from the Systematic Review and Meta-Analysis
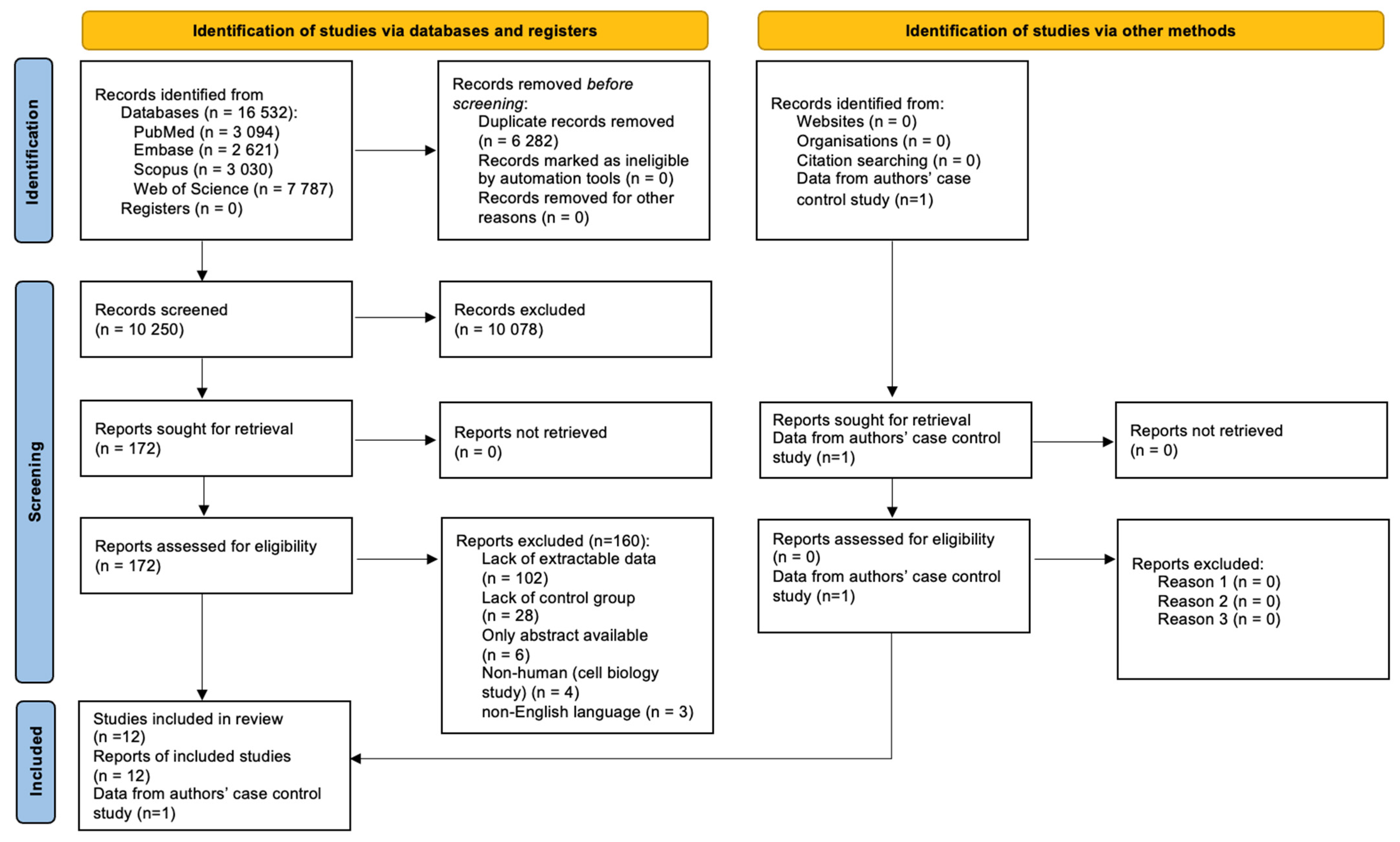
3.2.1. Search Results
3.2.2. Reported Anthropometric Parameters
3.2.3. Characteristics of the Included Studies
3.2.4. Characteristics of the Study Participants
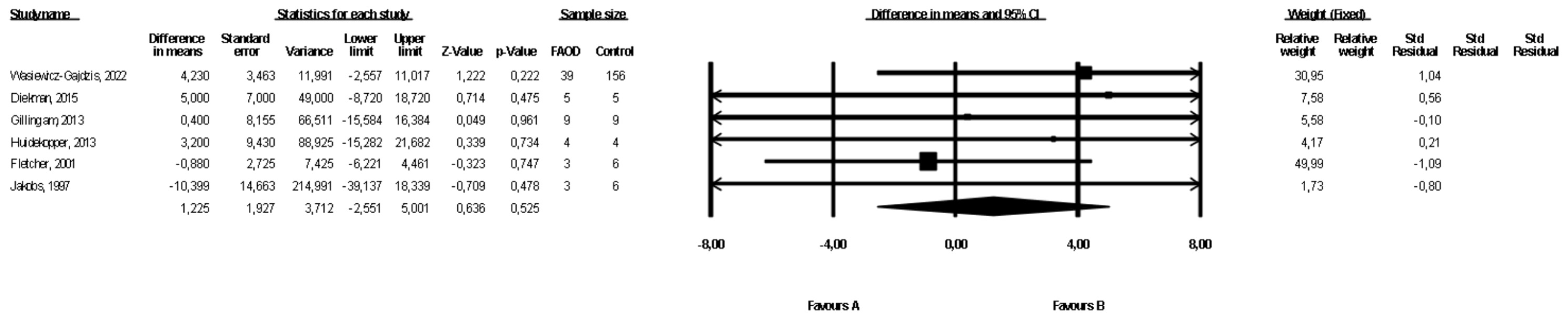
3.3. Comparison of Weight between FAOD Patients and Controls
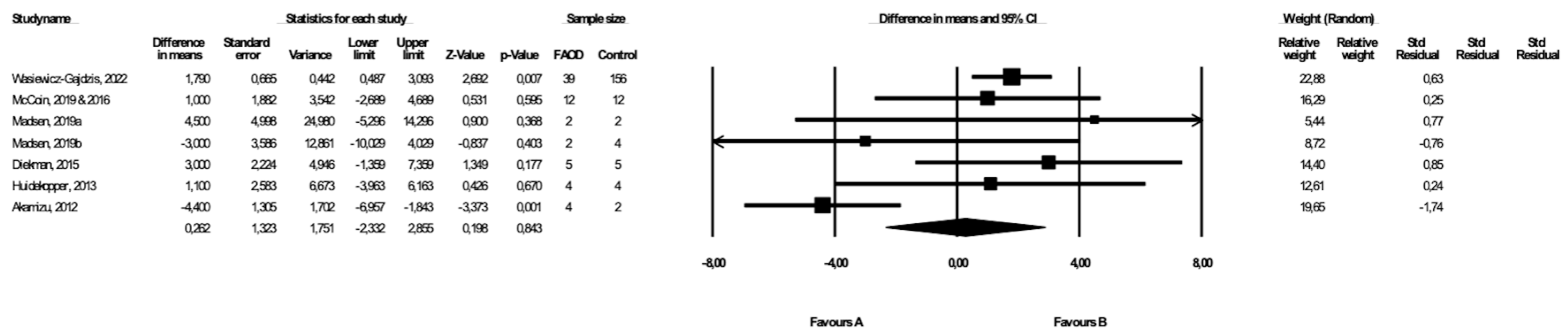
3.4. Comparison of BMI between FAOD Patients and Controls
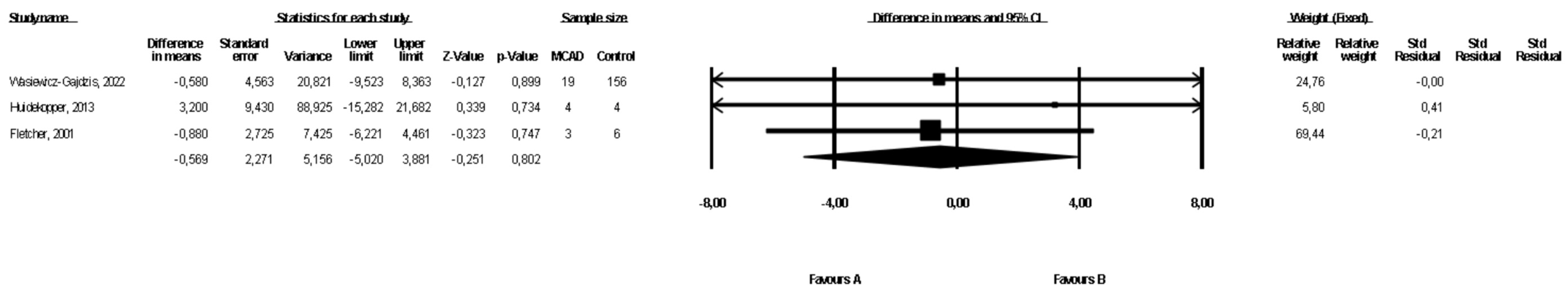
3.5. Comparison of Weight between MCAD Patients and Controls
3.6. Comparison of BMI between MCAD Patients and Controls
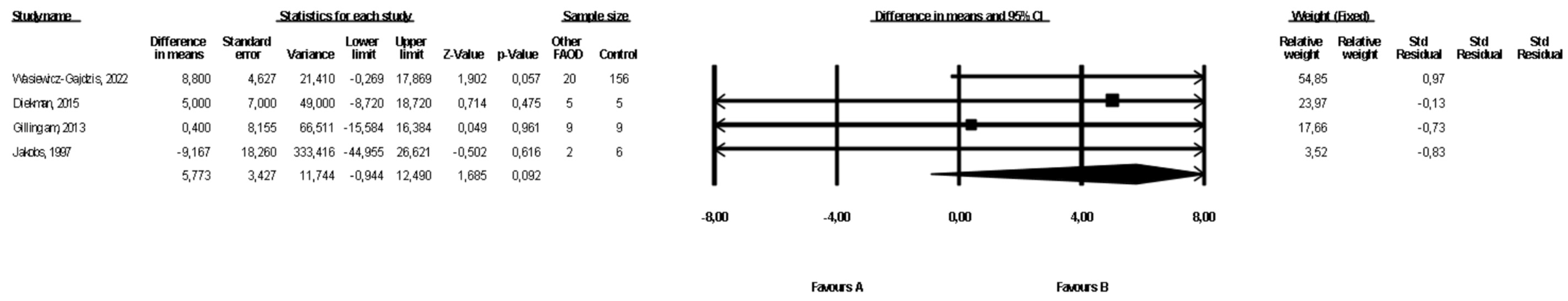
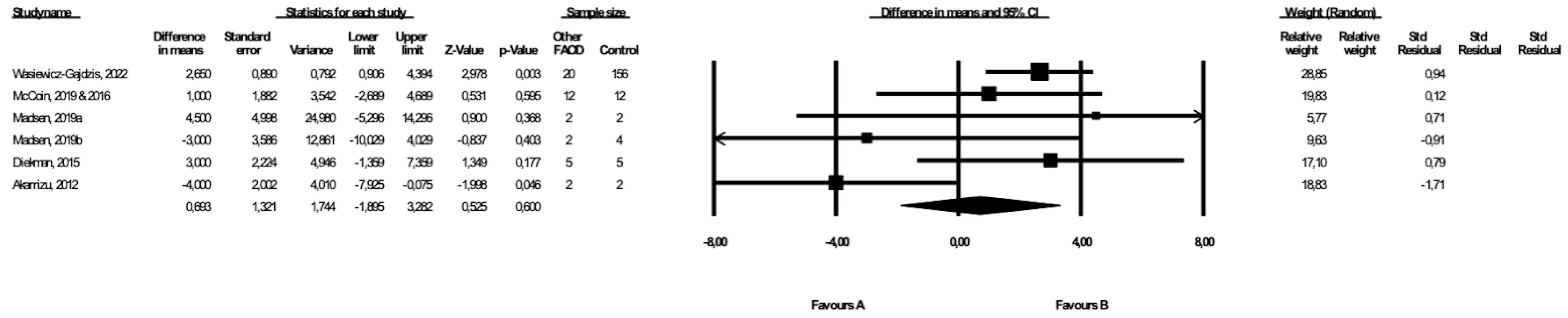
3.7. Comparison of Weight between Patients with Types of FAOD Other Than MCAD and Controls
3.8. Comparison of BMI between Patients with Types of FAOD Other Than MCAD and Controls
3.9. Sensitivity and Cumulative Meta-Analyses
3.10. Certainty of Evidence Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merritt, J.L.; Chang, I.J. Medium-Chain Acyl-Coenzyme A Dehydrogenase Deficiency. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Merritt II, J.L.; Norris, M.; Kanungo, S. Fatty Acid Oxidation Disorders. Ann. Transl. Med. 2018, 6, 473. [Google Scholar] [CrossRef] [PubMed]
- Spiekerkoetter, U.; Lindner, M.; Santer, R.; Grotzke, M.; Baumgartner, M.R.; Boehles, H.; Das, A.; Haase, C.; Hennermann, J.B.; Karall, D.; et al. Management and Outcome in 75 Individuals with Long-Chain Fatty Acid Oxidation Defects: Results from a Workshop. J. Inherit. Metab. Dis. 2009, 32, 488–497. [Google Scholar] [CrossRef]
- Saudubray, J.M.; Martin, D.; de Lonlay, P.; Touati, G.; Poggi-Travert, F.; Bonnet, D.; Jouvet, P.; Boutron, M.; Slama, A.; Vianey-Saban, C.; et al. Recognition and Management of Fatty Acid Oxidation Defects: A Series of 107 Patients. J. Inherit. Metab. Dis. 1999, 22, 488–502. [Google Scholar] [CrossRef]
- Derks, T.G.J.; Reijngoud, D.-J.; Waterham, H.R.; Gerver, W.-J.M.; van den Berg, M.P.; Sauer, P.J.J.; Smit, G.P.A. The Natural History of Medium-Chain Acyl CoA Dehydrogenase Deficiency in the Netherlands: Clinical Presentation and Outcome. J. Pediatr. 2006, 148, 665–670.e3. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, M.B.; Purnell, J.Q.; Jordan, J.; Stadler, D.; Haqq, A.M.; Harding, C.O. Effects of Higher Dietary Protein Intake on Energy Balance and Metabolic Control in Children with Long-Chain 3-Hydroxy Acyl-CoA Dehydrogenase (LCHAD) or Trifunctional Protein (TFP) Deficiency. Mol. Genet. Metab. 2007, 90, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Wszystkie Noworodki w Polsce Zostały Objęte Badaniem Przesiewowym w Kierunku Rzadkich Wad Metabolizmu—Institute of Mother and Child. Available online: https://imid.med.pl/en/409/wszystkie-noworodki-w-polsce-zostaly-objete-badaniem-przesiewowym-w-kierunku-rzadkich-wad-metabolizmu (accessed on 28 August 2022).
- Sawicka-Gutaj, N.; Gruszczyński, D.; Guzik, P.; Mostowska, A.; Walkowiak, J. Publication Ethics of Human Studies in the Light of the Declaration of Helsinki—A Mini-Review. J. Med. Sci. 2022, 91, e700. [Google Scholar] [CrossRef]
- Field, N.; Cohen, T.; Struelens, M.J.; Palm, D.; Cookson, B.; Glynn, J.R.; Gallo, V.; Ramsay, M.; Sonnenberg, P.; MacCannell, D.; et al. Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases (STROME-ID): An Extension of the STROBE Statement. Lancet Infect. Dis. 2014, 14, 341–352. [Google Scholar] [CrossRef]
- Chou, J.H.; Roumiantsev, S.; Singh, R. PediTools Electronic Growth Chart Calculators: Applications in Clinical Care, Research, and Quality Improvement. J. Med. Internet Res. 2020, 22, e16204. [Google Scholar] [CrossRef]
- The WHO Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards/standards (accessed on 15 August 2022).
- Growth Charts—Clinical Growth Charts. Available online: https://www.cdc.gov/growthcharts/clinical_charts.htm (accessed on 15 August 2022).
- Puhan, M.A.; Schunemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H.; for the GRADE Working Group. A GRADE Working Group Approach for Rating the Quality of Treatment Effect Estimates from Network Meta-Analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef]
- McCoin, C.S.; Piccolo, B.D.; Knotts, T.A.; Matern, D.; Vockley, J.; Gillingham, M.B.; Adams, S.H. Unique Plasma Metabolomic Signatures of Individuals with Inherited Disorders of Long-Chain Fatty Acid Oxidation. J. Inherit. Metab. Dis. 2016, 39, 399–408. [Google Scholar] [CrossRef]
- McCoin, C.S.; Gillingham, M.B.; Knotts, T.A.; Vockley, J.; Ono-Moore, K.D.; Blackburn, M.L.; Norman, J.E.; Adams, S.H. Blood Cytokine Patterns Suggest a Modest Inflammation Phenotype in Subjects with Long-chain Fatty Acid Oxidation Disorders. Physiol. Rep. 2019, 7, e14037. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, M.B.; Harding, C.O.; Schoeller, D.A.; Matern, D.; Purnell, J.Q. Altered Body Composition and Energy Expenditure but Normal Glucose Tolerance among Humans with a Long-Chain Fatty Acid Oxidation Disorder. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E1299–E1308. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L.; Stemmerik, M.G.; Buch, A.E.; Poulsen, N.S.; Lund, A.M.; Vissing, J. Impaired Fat Oxidation During Exercise in Long-Chain Acyl-CoA Dehydrogenase Deficiency Patients and Effect of IV-Glucose. J. Clin. Endocrinol. Metab. 2019, 104, 3610–3613. [Google Scholar] [CrossRef] [PubMed]
- Diekman, E.F.; Visser, G.; Schmitz, J.P.J.; Nievelstein, R.A.J.; de Sain-van der Velden, M.; Wardrop, M.; Van der Pol, W.L.; Houten, S.M.; van Riel, N.A.W.; Takken, T.; et al. Altered Energetics of Exercise Explain Risk of Rhabdomyolysis in Very Long-Chain Acyl-CoA Dehydrogenase Deficiency. PLoS ONE 2016, 11, e0147818. [Google Scholar] [CrossRef]
- Huidekoper, H.H.; Ackermans, M.T.; Koopman, R.; van Loon, L.J.C.; Sauerwein, H.P.; Wijburg, F.A. Normal Rates of Whole-Body Fat Oxidation and Gluconeogenesis after Overnight Fasting and Moderate-Intensity Exercise in Patients with Medium-Chain Acyl-CoA Dehydrogenase Deficiency. J. Inherit. Metab. Dis. 2013, 36, 831–840. [Google Scholar] [CrossRef]
- Akamizu, T.; Sakura, N.; Shigematsu, Y.; Tajima, G.; Ohtake, A.; Hosoda, H.; Iwakura, H.; Ariyasu, H.; Kangawa, K. Analysis of Plasma Ghrelin in Patients with Medium-Chain Acyl-CoA Dehydrogenase Deficiency and Glutaric Aciduria Type II. Eur. J. Endocrinol. 2012, 166, 235–240. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Pitt, J.J. Fasting Medium Chain Acyl-Coenzyme a Dehydrogenase-Deficient Children Can Make Ketones. Metabolism 2001, 50, 161–165. [Google Scholar] [CrossRef]
- Jakobs, C.; Kneer, J.; Martin, D.; Boulloche, J.; Brivet, M.; Poll-The, B.T.; Saudubray, J.M. In Vivo Stable Isotope Studies in Three Patients Affected with Mitochondrial Fatty Acid Oxidation Disorders: Limited Diagnostic Use of 1-13C Fatty Acid Breath Test Using Bolus Technique. Eur. J. Pediatr. 1997, 156, S78–S82. [Google Scholar] [CrossRef]
- de Castro, M.-J.; Sánchez-Pintos, P.; Abdelaziz-Salem, N.; Leis, R.; Couce, M.L. Evaluation of Body Composition, Physical Activity, and Food Intake in Patients with Inborn Errors of Intermediary Metabolism. Nutrients 2021, 13, 2111. [Google Scholar] [CrossRef]
- Knottnerus, S.J.G.; Bleeker, J.C.; Ferdinandusse, S.; Houtkooper, R.H.; Langeveld, M.; Nederveen, A.J.; Strijkers, G.J.; Visser, G.; Wanders, R.J.A.; Wijburg, F.A.; et al. Subclinical Effects of Long-chain Fatty Acid Β-oxidation Deficiency on the Adult Heart: A Case-control Magnetic Resonance Study. J. Inherit. Metab. Dis. 2020, 43, 969–980. [Google Scholar] [CrossRef]
- Madsen, K.L.; Preisler, N.; Buch, A.E.; Stemmerik, M.G.; Laforêt, P.; Vissing, J. Impaired Fat Oxidation during Exercise in Multiple Acyl-CoA Dehydrogenase Deficiency. JIMD Rep. 2019, 46, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Orngreen, M.C.; Madsen, K.L.; Preisler, N.; Andersen, G.; Vissing, J.; Laforet, P. Bezafibrate in Skeletal Muscle Fatty Acid Oxidation Disorders: A Randomized Clinical Trial. Neurology 2014, 82, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Rücklová, K.; Hrubá, E.; Pavlíková, M.; Hanák, P.; Farolfi, M.; Chrastina, P.; Vlášková, H.; Kousal, B.; Smolka, V.; Foltenová, H.; et al. Impact of Newborn Screening and Early Dietary Management on Clinical Outcome of Patients with Long Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency and Medium Chain Acyl-CoA Dehydrogenase Deficiency—A Retrospective Nationwide Study. Nutrients 2021, 13, 2925. [Google Scholar] [CrossRef] [PubMed]
- Rovelli, V.; Manzoni, F.; Viau, K.; Pasquali, M.; Longo, N. Clinical and Biochemical Outcome of Patients with Very Long-Chain Acyl-CoA Dehydrogenase Deficiency. Mol. Genet. Metab. 2019, 127, 64–73. [Google Scholar] [CrossRef]
- Anderson, D.R.; Viau, K.; Botto, L.D.; Pasquali, M.; Longo, N. Clinical and Biochemical Outcomes of Patients with Medium-Chain Acyl-CoA Dehydrogenase Deficiency. Mol. Genet. Metab. 2020, 129, 13–19. [Google Scholar] [CrossRef] [PubMed]
- De Biase, I.; Viau, K.S.; Liu, A.; Yuzyuk, T.; Botto, L.D.; Pasquali, M.; Longo, N. Diagnosis, Treatment, and Clinical Outcome of Patients with Mitochondrial Trifunctional Protein/Long-Chain 3-Hydroxy Acyl-CoA Dehydrogenase Deficiency. JIMD Rep. 2016, 31, 63–71. [Google Scholar] [CrossRef]
- Iafolla, A.K.; Thompson, R.J.; Roe, C.R. Medium-Chain Acyl-Coenzyme A Dehydrogenase Deficiency: Clinical Course in 120 Affected Children. J. Pediatr. 1994, 124, 409–415. [Google Scholar] [CrossRef]
- Haglind, C.B.; Stenlid, M.H.; Ask, S.; Alm, J.; Nemeth, A.; Döbeln, U.; Nordenström, A. Growth in Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency. JIMD Rep.—Case Res. Rep. 2012/5 2012, 8, 81–90. [Google Scholar] [CrossRef]
- Evans, M.; Andresen, B.S.; Nation, J.; Boneh, A. VLCAD Deficiency: Follow-up and Outcome of Patients Diagnosed through Newborn Screening in Victoria. Mol. Genet. Metab. 2016, 118, 282–287. [Google Scholar] [CrossRef]
| FAOD n = 39 | Controls n = 156 | p | |
|---|---|---|---|
| Age (years) | |||
| mean ± SD | 7.1 ± 4.4 | 7.1 ± 4.4 | p = 1.000 |
| median | 5.8 | 5.6 | |
| interquartile range | (3.3–10.5) | (3.2–10.2) | |
| Sex (n (%)) | |||
| Females | 16 (41.0%) | 64 (41.0%) | p = 1.000 |
| Males | 23 (59.0%) | 92 (59.0%) | |
| Weight (kg) | |||
| mean ± SD | 32.1 ± 21.1 | 27.9 ± 18.9 | p = 0.145 |
| median | 23.8 | 19.3 | |
| interquartile range | (16.3–41.8) | (15.0–34.0) | |
| Weight percentile | |||
| median | 83 | 54 | p = 0.001 |
| interquartile range | (67–96) | (32–74) | |
| BMI (kg/m2) | |||
| mean ± SD | 18.3 ± 4.2 | 16.5 ± 3.6 | p = 0.022 |
| median | 17.9 | 15.9 | |
| interquartile range | (15.3–20.5) | (14.6–17.9) | |
| BMI percentile | |||
| median | 76 | 48 | p = 0.003 |
| interquartile range | (40–95) | (16–74) | |
| BMI z-score | |||
| median | 0.71 | −0.07 | p = 0.001 |
| interquartile range | (−0.2–1.7) | (−1.0–0.6) |
| Author | Year | Country | Study Design | Number of Participants | Types of FAOD | Age (Mean Range) | Sex (%) |
|---|---|---|---|---|---|---|---|
| de Castro et al. [23] | 2021 | Spain | observational | FAOD = 10 Control = 20 | MCADD = 6 SCADD = 4 | 5–19 1 Control NI | FAOD F = 70% M = 30% Control NI |
| Knottnerus et al. [24] | 2020 | Netherlands | observational | FAOD = 14 Control = 14 | VLCADD = 8 LCHADD = 2 CPT2D = 4 | FAOD 41 (18–57) Control 38 (18–60) | FAOD F = 21% M = 79% Control F = 21% M = 79% |
| Madsen et al. [17] | 2019 | Denmark | observational | FAOD = 2 Control = 4 | LCHADD = 2 | FAOD 20.5 (15–26) Control 24.8 (19–30) | FAOD F = 50% M = 50% Control F = 75% M = 25% |
| Madsen et al. [25] | 2019 | Denmark | observational | FAOD = 2 Control = 10 | MADD = 2 | FAOD 35 (20–50) Control 32 (18–65) | FAOD F = 100% Control F = 70% M = 30% |
| McCoin et al. (s) [15] | 2019 | USA | observational | FAOD = 12 Control = 12 | CPT2D = 2 LCHAD = 10 | FAOD 14.7 (7–37) Control 15.3 (9–34) | FAOD F = 42% M = 58% Control F = 42% M = 58% |
| McCoin et al. (s) [14] | 2016 | USA | observational | FAOD = 12 Control = 11 | CPT2D = 2 LCHAD = 10 | FAOD 28 (13–37) Control 26 (NI) | FAOD F = 42% M = 58% Control F = 45% M = 55% |
| Diekman et al. [18] | 2015 | Netherlands | observational | FAOD = 5 Control = 5 | VLCADD = 5 | FAOD 14.7 (NI) Control 15.7 (NI) | FAOD F = 42% M = 58% Control F = 45% M = 55% |
| Gillingham et al. (s) [16] | 2013 | USA | observational | FAOD = 9 Control = 9 | LCHADD = 9 | FAOD 12.7 (7–17) Control 13.7 (8–22) | FAOD F = 33% M = 67% Control F = 33% M = 67% |
| Huidekopper et al. [19] | 2013 | Netherlands | observational | FAOD = 4 Control = 4 | MCADD = 4 | FAOD 27.3 (21–41) Control 27 (21–32) | FAOD F = 25% M = 75% Control F = 25% M = 75% |
| Akamizu et al. [20] | 2012 | Japan | observational | FAOD = 4 Control = 20 | CPT2D = 1 MCADD = 2 VLCADD = 1 | FAOD 8 (5–11) Control 32.6 (NI) | FAOD F = 100% Control NI |
| Fletcher et al. [21] | 2001 | Australia | observational | FAOD = 3 Control = 6 | MCADD = 3 | FAOD 2.7 (0.9–6) Control 3.7 (3.1–4.3) | FAOD F = 67% M = 33% Control NI |
| Jakobs et al. [22] | 1997 | Netherlands | observational | FAOD = 3 Control = 6 | CPT1D = 1 MCADD = 1 MADD = 1 | FAOD 5.4 (2.6–9) Control 14.3 (2.1–34.8) | FAOD F = 33% M = 67% Control F = 50% M = 50% |
| Study | No of Patients/Controls | Weight [kg] | BMI [kg/m 2] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Z-Score Median | Z-Score SD | Mean | SD | Median | Z-Score Median | Z-Score SD | Percentile Median | ||
| de Castro et al., 2021 [23] | FAOD = 10 | - | - | - | 0.9 | 1.0 | - | - | - | 0.6 | 1.3 | - |
| CG = 20 | - | - | - | 0.3 | 0.9 | - | - | - | −0.7 | 1.0 | - | |
| Knottnerus et al., 2020 [24] | FAOD = 14 | - | - | - | - | - | 25.31 | 4.01 | 25.41 | - | - | - |
| CG = 14 | - | - | - | - | - | - | - | 25 | - | - | - | |
| McCoin el.al., 2019 [15] | LCHAD = 10 | - | - | - | - | - | 22.81 | 4.11 | 23.21 | - | - | - |
| CPT2 = 2 | - | - | - | - | - | 26.31 | 5.11 | 26.31 | - | - | - | |
| CG = 12 | - | - | - | - | - | 22.41 | 4.91 | 21.71 | - | - | - | |
| Madsen et al., 2019 [25] | FAOD = 2 | - | - | - | - | - | 291 | 6.41 | 291 | - | - | - |
| CG = 10 | - | - | - | - | - | 32 | 14 | - | - | - | - | |
| Madsen et al., 2019 [17] | FAOD = 2 | - | - | - | - | - | 201 | 2.81 | 201 | - | - | - |
| CG = 4 | - | - | - | - | - | 231 | 4. 51 | 241 | - | - | - | |
| Diekman et al., 2016 [18] | FAOD = 5 | 761 | 141 | 791 | - | - | 24.81 | 4.81 | 24.91 | - | - | - |
| CG = 5 | 71 | 161 | - | - | - | 21.8 | 2.91 | - | - | - | - | |
| McCoin et al., 2016 (s) [14] | LCHAD = 10 | 55.9 | 16.41 | - | - | - | 22.8 | 4.11 | - | 1.1 | - | 84.6 |
| CPT2 = 2 | 73.4 | 17.51 | - | - | - | 26.3 | 5.11 | - | 0.5 2 | - | 70 2 | |
| FAOD = 12 | 58.81 | 17.21 | - | - | - | 23.41 | 4.21 | - | 1.0 | - | 83.31 | |
| CG = 11 | 61 | 21.21 | - | - | - | 23.1 | 4.61 | - | 0.7 3 | - | 72.3 3 | |
| Gillingham et al., 2013 (s) [16] | FAOD = 9 | 55.71 | 17.41 | 64.71 | - | - | 22.31 | 4.01 | 22.61 | 11 | - | 831 |
| CG = 9 | 55.01 | 24.41 | 48.91 | - | - | 21.41 | 5.11 | 19.71 | 0.41 | - | 631 | |
| Huidekopper et al., 2013 [19] | FAOD = 4 | 81.31 | 15.051 | 79.71 | - | - | 24.51 | 3.81 | 24.11 | - | - | - |
| Akamizu et al., 2012 [20] | FAOD = 4 | - | - | - | - | - | 15.91 | 1.41 | 15.61 | - | - | - |
| CG = 20 | - | - | - | - | - | 20.3 | 1.9 | - | - | - | - | |
| Fletcher et al., 2001 [21] | MCADD = 3 | 22.51 | 7.01 | 11.71 | - | - | - | - | - | - | - | - |
| CG = 6 | 15.31 | 1.01 | 15.81 | - | - | - | - | - | - | - | - | |
| Jakobs et al., 1997 [22] | FAOD = 3 | 21.51 | 10.51 | 191 | - | - | - | - | - | - | - | - |
| CG = 6 | 31.91 | 23.61 | 22.31 | - | - | - | - | - | - | - | - | |
| Outcome | Group | No of Studies | Patients | Controls | MD (95% CI) | p-Value | I2 | Risk of Bias | Inconsistency | Indirections | Imprecision | Grade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight | FAOD | 6 | 63 | 186 | 1.23 (−2.55, 5.00) | 0.53 | 0 | Downgrade 1 level | No downgrade | Downgrade 1 level | No downgrade | ⨁◯◯◯ Very low |
| MCADD | 3 | 46 | 166 | −0.51 (−5.02, 3.88) | 0.97 | 0 | Downgrade 1 level | No downgrade | No downgrade | No downgrade | ⨁◯◯◯ Very low | |
| Other FAOD | 3 | 56 | 176 | 6.3 (−0.94, 12.49) | 0.09 | 0 | Downgrade 1 level | No downgrade | Downgrade 1 level | Downgrade 1 level | ⨁◯◯◯ Very low | |
| BMI | FAOD | 7 | 68 | 216 | 0.26 (−2.33, 2.85) | 0.84 | 71.05 | Downgrade 1 level | Downgrade 1 level | Downgrade 1 level | Downgrade 1 level | ⨁◯◯◯ Very low |
| MCADD | 3 | 47 | 180 | −1.1 (−5.21, 3.09) | 0.62 | 83.6 | Downgrade 1 level | Downgrade 2 levels | No downgrade | Downgrade 1 level | ⨁◯◯◯ Very low | |
| Other FAOD | 6 | 64 | 212 | 0.70 (−1.90, 3.26) | 0.60 | 57.2 | Downgrade 1 level | Downgrade 1 level | No downgrade | Downgrade 1 level | ⨁◯◯◯ Very low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasiewicz-Gajdzis, M.; Jamka, M.; Geltz, J.; Bokayeva, K.; Kałużny, Ł.; Jagłowska, J.; Walkowiak, J. Anthropometric Parameters in Patients with Fatty Acid Oxidation Disorders: A Case–Control Study, Systematic Review and Meta-Analysis. Healthcare 2022, 10, 2405. https://doi.org/10.3390/healthcare10122405
Wasiewicz-Gajdzis M, Jamka M, Geltz J, Bokayeva K, Kałużny Ł, Jagłowska J, Walkowiak J. Anthropometric Parameters in Patients with Fatty Acid Oxidation Disorders: A Case–Control Study, Systematic Review and Meta-Analysis. Healthcare. 2022; 10(12):2405. https://doi.org/10.3390/healthcare10122405
Chicago/Turabian StyleWasiewicz-Gajdzis, Maria, Małgorzata Jamka, Jakub Geltz, Kamila Bokayeva, Łukasz Kałużny, Joanna Jagłowska, and Jarosław Walkowiak. 2022. "Anthropometric Parameters in Patients with Fatty Acid Oxidation Disorders: A Case–Control Study, Systematic Review and Meta-Analysis" Healthcare 10, no. 12: 2405. https://doi.org/10.3390/healthcare10122405
APA StyleWasiewicz-Gajdzis, M., Jamka, M., Geltz, J., Bokayeva, K., Kałużny, Ł., Jagłowska, J., & Walkowiak, J. (2022). Anthropometric Parameters in Patients with Fatty Acid Oxidation Disorders: A Case–Control Study, Systematic Review and Meta-Analysis. Healthcare, 10(12), 2405. https://doi.org/10.3390/healthcare10122405