Risk Role of Breast Cancer in Association with Human Papilloma Virus among Female Population in Taiwan: A Nationwide Population-Based Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Data Source and Study Population
2.2. Main Outcome and Covariates
2.3. Statistical Analysis
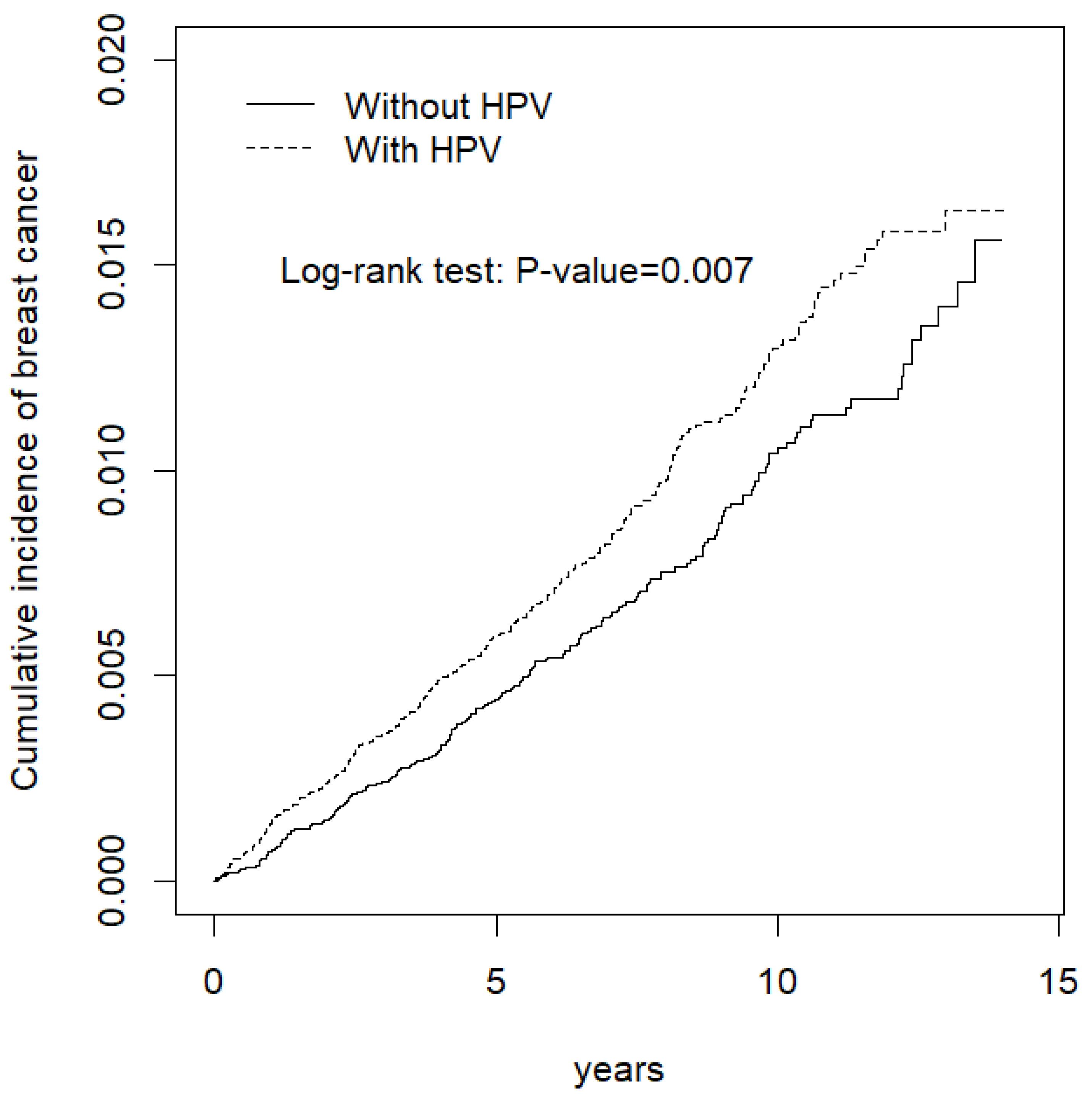
3. Results
4. Discussion
4.1. Limitations
4.2. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breast Cancer Prevelance. Available online: https://www.hpa.gov.tw/Home/Index.aspx (accessed on 25 June 2018).
- Palacios, J.; Robles-Frias, M.J.; Castilla, M.A.; Lopez-Garcia, M.A.; Benitez, J. The molecular pathology of hereditary breast cancer. Pathobiology 2008, 75, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Ljubojevic, S.; Skerlev, M. HPV-associated diseases. Clin. Dermatol. 2014, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; Forslund, O.; Ekberg, H.; Sterner, G.; Hansson, B.G. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J. Virol. 2000, 74, 11636–11641. [Google Scholar] [CrossRef] [PubMed]
- Pahud, B.A.; Ault, K.A. The Expanded Impact of Human Papillomavirus Vaccine. Infect. Dis. Clin. N. Am. 2015, 29, 715–724. [Google Scholar] [CrossRef]
- Nowińska, K.; Ciesielska, U.; Podhorska-Okołów, M.; Dzięgiel, P. The role of human papillomavirus in oncogenic transformation and its contribution to the etiology of precancerous lesions and cancer of the larynx: A review. Adv. Clin. Exp. Med. 2017, 26, 539–547. [Google Scholar] [CrossRef]
- Stokley, S.; Jeyarajah, J.; Yankey, D.; Cano, M.; Gee, J.; Roark, J.; Curtis, C.R.; Markowitz, L. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morb. Mortal Wkly. Rep. 2014, 63, 620–624. [Google Scholar]
- Li, J.; Ding, J.; Zhai, K. Detection of Human Papillomavirus DNA in Patients with Breast Tumor in China. PLoS ONE 2015, 10, e0136050. [Google Scholar] [CrossRef]
- Choi, J.; Kim, C.; Lee, H.S.; Choi, Y.J.; Kim, H.Y.; Lee, J.; Chang, H.; Kim, A. Detection of Human Papillomavirus in Korean Breast Cancer Patients by Real-Time Polymerase Chain Reaction and Meta-Analysis of Human Papillomavirus and Breast Cancer. J. Pathol. Transl. Med. 2016, 50, 442–450. [Google Scholar] [CrossRef][Green Version]
- Ngan, C.; Lawson, J.S.; Clay, R.; Delprado, W.; Whitaker, N.J.; Glenn, W.K. Early Human Papilloma Virus (HPV) Oncogenic Influences in Breast Cancer. Breast Cancer 2015, 9, 93–97. [Google Scholar] [CrossRef]
- Antonsson, A.; Spurr, T.P.; Chen, A.C.; Francis, G.D.; McMillan, N.A.; Saunders, N.A.; Law, M.; Bennett, I.C. High prevalence of human papillomaviruses in fresh frozen breast cancer samples. J. Med. Virol. 2011, 83, 2157–2163. [Google Scholar] [CrossRef]
- Atique, S.; Hsieh, C.H.; Hsiao, R.T.; Iqbal, U.; Nguyen, P.A.A.; Islam, M.M.; Li, Y.C.J.; Hsu, C.Y.; Chuang, T.W.; Syed-Abdul, S. Viral warts (Human Papilloma Virus) as a potential risk for breast cancer among younger females. Comput. Methods Programs Biomed. 2017, 144, 203–207. [Google Scholar] [CrossRef]
- Tsai, J.H.; Tsai, C.H.; Cheng, M.H.; Lin, S.J.; Xu, F.L.; Yang, C.C. Association of viral factors with non-familial breast cancer in Taiwan by comparison with non-cancerous, fibroadenoma, and thyroid tumor tissues. J. Med. Virol. 2005, 75, 276–281. [Google Scholar] [CrossRef]
- Gumus, M.A.H.M.U.T.; Yumuk, P.F.; Salepci, T.; Aliustaoglu, M.; Dane, F.A.Y.S.A.L.; Ekenel, M.; Basaran, G.; Kaya, H.; Barisik, N.; Turhal, N.S. HPV DNA frequency and subset analysis in human breast cancer patients’ normal and tumoral tissue samples. J. Exp. Clin. Cancer Res. 2006, 25, 515–521. [Google Scholar]
- Choi, Y.L.; Cho, E.Y.; Kim, J.H.; Nam, S.J.; Oh, Y.L.; Song, S.Y.; Yang, J.H.; Kim, D.S. Detection of human papillomavirus DNA by DNA chip in breast carcinomas of Korean women. Tumour Biol. 2007, 28, 327–332. [Google Scholar] [CrossRef]
- Cantu de León, D.; Pérez Montiel, D.; Nemcova, J.; Mykyskova, I.; Turcios, E.; Villavicencio, V.; Cetina, L.; Coronel, A.; Hes, O. Human papillomavirus (HPV) in breast tumors: Prevalence in a group of Mexican patients. BMC Cancer 2009, 9, 26. [Google Scholar] [CrossRef]
- He, Q.; Zhang, S.Q.; Chu, Y.L.; Jia, X.L.; Wang, X.L. The correlations between HPV16 infection and expressions of c-erbB-2 and bcl-2 in breast carcinoma. Mol. Biol. Rep. 2009, 36, 807–812. [Google Scholar] [CrossRef]
- De Villiers, E.M.; Sandstrom, R.E.; Hausen, H.z.; Buck, C.E. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res. 2005, 7, R1–R11. [Google Scholar] [CrossRef]
- Frega, A.; Lorenzon, L.; Bononi, M.; De Cesare, A.; Ciardi, A.; Lombardi, D.; Assorgi, C.; Gentile, M.; Moscarini, M.; Torrisi, M.R.; et al. Evaluation of E6 and E7 mRNA expression in HPV DNA positive breast cancer. Eur. J. Gynaecol. Oncol. 2012, 33, 164–167. [Google Scholar]
- Glenn, W.K.; Heng, B.; Delprado, W.; Iacopetta, B.; Whitaker, N.J.; Lawson, J.S. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS ONE 2012, 7, e48788. [Google Scholar] [CrossRef]
- Sigaroodi, A.; Nadji, S.A.; Naghshvar, F.; Nategh, R.; Emami, H.; Velayati, A.A. Human papillomavirus is associated with breast cancer in the north part of Iran. Sci. World J. 2012, 2012, 837191. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; Al-Alwan, N.A.; Al-Alwany, S.H. Detection and genotyping of human papillomavirus in breast cancer tissues from Iraqi patients. East Mediterr. Health J. 2014, 20, 372–377. [Google Scholar] [PubMed]
- Aguayo, F.; Khan, N.; Koriyama, C.; González, C.; Ampuero, S.; Padilla, O.; Solís, L.; Eizuru, Y.; Corvalán, A.; Akiba, S. Human papillomavirus and Epstein-Barr virus infections in breast cancer from chile. Infect. Agents Cancer 2011, 6, 7. [Google Scholar] [CrossRef]
- Lawson, J.S.; Glenn, W.K.; Salyakina, D.; Delprado, W.; Clay, R.; Antonsson, A.; Heng, B.; Miyauchi, S.; Tran, D.D.; Ngan, C.C. Human Papilloma Viruses and Breast Cancer. Front. Oncol. 2015, 5, 298. [Google Scholar] [CrossRef]
- Bønløkke, S.; Blaakær, J.; Steiniche, T.; Høgdall, E.; Jensen, S.G.; Hammer, A.; Balslev, E.; Strube, M.L.; Knakkergaard, H.; Lenz, S. Evidence of No Association Between Human Papillomavirus and Breast Cancer. Front Oncol. 2018, 8, 209. [Google Scholar] [CrossRef]
- Vernet-Tomas, M.; Mena, M.; Alemany, L.; Bravo, I.; De Sanjosé, S.; Nicolau, P.; Bergueiro, A.; Corominas, J.M.; Serrano, S.; Carreras, R.; et al. Human Papillomavirus and Breast Cancer: No Evidence of Association in a Spanish Set of Cases. Anticancer Res. 2015, 35, 851–856. [Google Scholar]
- Bratthauer, G.L.; Tavassoli, F.A.; O’Leary, T.J. Etiology of Breast Carcinoma: No Apparent Role for Papillomavirus Types 6/11/16/18. Pathol. Res. Pract. 1992, 188, 384–386. [Google Scholar] [CrossRef]
- Manzouri, L.; Salehi, R.; Shariatpanahi, S.; Rezaie, P. Prevalence of human papilloma virus among women with breast cancer since 2005-2009 in Isfahan. Adv. Biomed. Res. 2014, 3, 75. [Google Scholar] [CrossRef]
- Mendizabal-Ruiz, A.P.; Morales, J.A.; Ramirez-Jirano, L.J.; Padilla-Rosas, M.; Moran-Moguel, M.C.; Montoya-Fuentes, H. Low frequency of human papillomavirus DNA in breast cancer tissue. Breast Cancer Res. Treat 2009, 114, 189–194. [Google Scholar] [CrossRef]
- Mou, X.; Chen, L.; Liu, F.; Shen, Y.; Wang, H.; Li, Y.; Yuan, L.; Lin, J.; Lin, J.; Teng, L. Low prevalence of human papillomavirus (HPV) in Chinese patients with breast cancer. J. Int. Med. Res. 2011, 39, 1636–1644. [Google Scholar] [CrossRef]
- Ahangar-Oskouee, M.; Shahmahmoodi, S.; Jalilvand, S.; Mahmoodi, M.; Ziaee, A.A.; Esmaeili, H.A.; Keshtvarz, M.; Pishraft-Sabet, L.; Yousefi, M.; Mollaei-Kandelous, Y.; et al. No detection of ‘high-risk’ human papillomaviruses in a group of Iranian women with breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4061–4065. [Google Scholar] [CrossRef]
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C.; et al. HPV Typing of Cancers Workgroup US assessment of HPV types in cancers: Implications for current and 9-valent HPV vaccines. J. Natl. Cancer Inst. 2015, 107, djv086. [Google Scholar] [CrossRef]
- Spence, A.R.; Goggin, P.; Franco, E.L. Process of care failures in invasive cervical cancer: Systematic review and meta-analysis. Prev. Med. 2007, 45, 93–106. [Google Scholar] [CrossRef]
- De Oliveira, T.H.A.; do Amaral, C.M.; de França São Marcos, B.; Nascimento, K.C.G.; de Miranda Rios, A.C.; Quixabeira, D.C.A.; Muniz, M.T.C.; Silva Neto, J.D.C.; de Freitas, A.C. Presence and activity of HPV in primary lung cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 2367–2376. [Google Scholar] [CrossRef]
- Tsyganov, M.M.; Pevzner, A.M.; Ibragimova, M.K.; Deryusheva, I.V.; Litviakov, N.V. Human papillomavirus and lung cancer: An overview and a meta-analysis. J. Cancer Res. Clin. Oncol. 2019, 145, 1919–1937. [Google Scholar] [CrossRef]
| HPV | |||||
|---|---|---|---|---|---|
| No | Yes | ||||
| n = 30,936 | n = 30,936 | ||||
| n | % | n | % | p-Value | |
| Age, year | 0.99 | ||||
| 20−49 | 22,560 | 72.9 | 22,560 | 72.9 | |
| 50−64 | 5583 | 18.1 | 5583 | 18.1 | |
| ≥65 | 2793 | 9.03 | 2793 | 9.03 | |
| Mean (SD) † | 41.5 | 15.3 | 41.5 | 15.4 | 0.76 |
| Comorbidity | |||||
| Hypertension | 5102 | 16.5 | 5102 | 16.5 | 0.99 |
| Coronary artery disease (CAD) | 2681 | 8.67 | 2681 | 8.67 | 0.99 |
| Chronic obstructive pulmonary disease (COPD) | 1798 | 5.81 | 1798 | 5.81 | 0.99 |
| Stroke | 259 | 0.84 | 259 | 0.84 | 0.99 |
| Diabetes mellitus | 808 | 2.61 | 808 | 2.61 | 0.99 |
| Hyperlipidemia | 4697 | 15.2 | 4697 | 15.2 | 0.99 |
| Obesity | 560 | 1.81 | 560 | 1.81 | 0.99 |
| Event | PY | Rate # | Crude HR (95% CI) | Adjusted HR & (95% CI) | |
|---|---|---|---|---|---|
| HPV | |||||
| No | 217 | 221,132 | 9.81 | 1.00 | 1.00 |
| Yes | 280 | 223,329 | 12.5 | 1.28(1.21, 1.35) ** | 1.27(1.20, 1.34) ** |
| Age, years | |||||
| 20−49 | 302 | 334,656 | 9.02 | 1.00 | 1.00 |
| 50−64 | 134 | 75,192 | 17.8 | 1.97(1.86, 2.10) ** | 1.67(1.56, 1.80) ** |
| ≥65 | 61 | 34,612 | 17.6 | 1.95(1.80, 2.12) ** | 1.36(1.23, 1.52) ** |
| Comorbidity | |||||
| Hypertension | |||||
| No | 368 | 376,435 | 9.78 | 1.00 | 1.00 |
| Yes | 129 | 68,026 | 19.0 | 1.94(1.83, 2.06) ** | 1.30(1.20, 1.41) ** |
| Coronary artery disease (CAD) | |||||
| No | 430 | 408,266 | 10.5 | 1.00 | 1.00 |
| Yes | 67 | 36,195 | 18.5 | 1.76(1.63, 1.90) ** | 1.02(0.93, 1.12) |
| Chronic obstructive pulmonary disease (COPD) | |||||
| No | 457 | 421,435 | 10.8 | 1.00 | 1.00 |
| Yes | 40 | 23,026 | 17.4 | 1.60(1.45, 1.76) ** | 1.12(1.01, 1.24) * |
| Stroke | |||||
| No | 491 | 441,751 | 11.1 | 1.00 | 1.00 |
| Yes | 6 | 2710 | 22.1 | 1.99(1.57, 2.54) ** | 1.04(0.82, 1.34) |
| Diabetes mellitus | |||||
| No | 465 | 434,513 | 10.7 | 1.00 | 1.00 |
| Yes | 32 | 9948 | 32.2 | 3.01(2.70, 3.35) ** | 1.90(1.69, 2.14) ** |
| Hyperlipidemia | |||||
| No | 390 | 381,171 | 10.2 | 1.00 | 1.00 |
| Yes | 107 | 62,690 | 17.1 | 1.67(1.57, 1.78) ** | 1.03(0.95, 1.11) |
| Obesity | |||||
| No | 488 | 437,968 | 11.1 | 1.00 | 1.00 |
| Yes | 9 | 6493 | 13.9 | 1.24(1.02, 1.52) * | 1.14(0.94, 1.39) |
| HPV | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Event | PY | Rate # | Event | PY | Rate # | Crude HR (95% CI) | Adjusted HR & (95% CI) | |
| Age, years | ||||||||
| 20−49 | 136 | 166,722 | 8.16 | 166 | 167,934 | 9.88 | 1.21(1.14, 1.29) ** | 1.21(1.14, 1.29) ** |
| 50−64 | 56 | 37,616 | 14.9 | 78 | 37,576 | 20.8 | 1.39(1.24, 1.57) ** | 1.39(1.24, 1.57) ** |
| ≥65 | 25 | 16,793 | 14.9 | 36 | 17,819 | 20.2 | 1.36(1.14, 1.61) ** | 1.36(1.15, 1.61) ** |
| Comorbidity § | ||||||||
| No | 129 | 164,589 | 7.84 | 179 | 165,767 | 10.8 | 1.38(1.29, 1.47) ** | 1.38(1.29, 1.47) ** |
| Yes | 88 | 56,543 | 15.6 | 101 | 57,562 | 17.6 | 1.13(1.03, 1.24) * | 1.12(1.02, 1.24) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-H.; Liao, C.-Y.; Yeh, M.-H.; Wei, J.C.-C. Risk Role of Breast Cancer in Association with Human Papilloma Virus among Female Population in Taiwan: A Nationwide Population-Based Cohort Study. Healthcare 2022, 10, 2235. https://doi.org/10.3390/healthcare10112235
Liu C-H, Liao C-Y, Yeh M-H, Wei JC-C. Risk Role of Breast Cancer in Association with Human Papilloma Virus among Female Population in Taiwan: A Nationwide Population-Based Cohort Study. Healthcare. 2022; 10(11):2235. https://doi.org/10.3390/healthcare10112235
Chicago/Turabian StyleLiu, Chia-Hsin, Chi-You Liao, Ming-Hsin Yeh, and James Cheng-Chung Wei. 2022. "Risk Role of Breast Cancer in Association with Human Papilloma Virus among Female Population in Taiwan: A Nationwide Population-Based Cohort Study" Healthcare 10, no. 11: 2235. https://doi.org/10.3390/healthcare10112235
APA StyleLiu, C.-H., Liao, C.-Y., Yeh, M.-H., & Wei, J. C.-C. (2022). Risk Role of Breast Cancer in Association with Human Papilloma Virus among Female Population in Taiwan: A Nationwide Population-Based Cohort Study. Healthcare, 10(11), 2235. https://doi.org/10.3390/healthcare10112235






