Fighting Cancer around the World: A Framework for Action
Abstract
1. Introduction
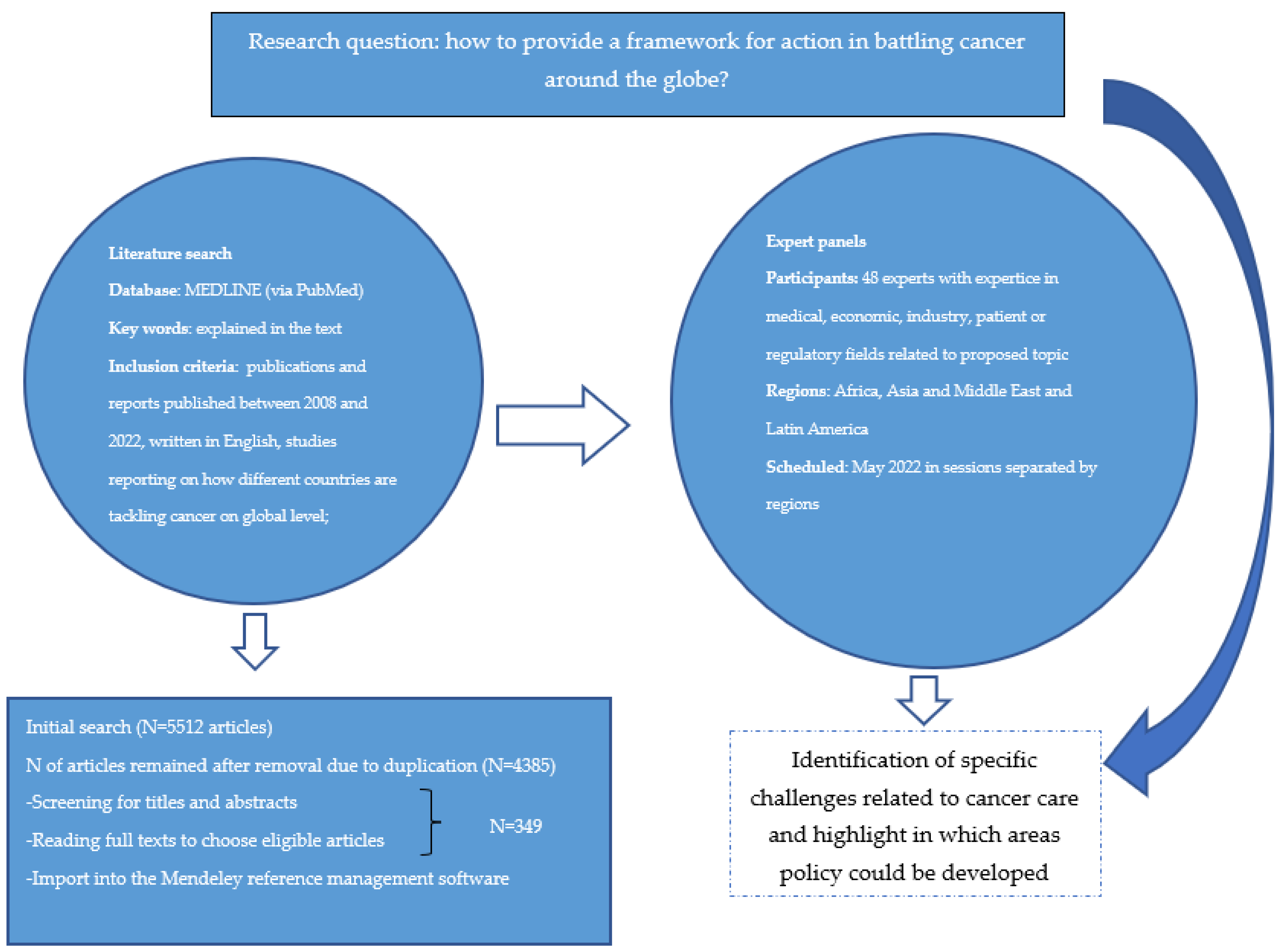
2. Materials and Methods
3. Results
3.1. The Regions’ Perspectives
3.1.1. Asia
Malaysia
The Philippines
India
China
Nepal
United Arab Emirates
Qatar
Lebanon
Kingdom of Saudi Arabia
3.1.2. Africa
Tunisia
Angola
South Africa
Cameroon
Kenya
Nigeria
3.1.3. LATAM Perspective
Brazil
Colombia
Mexico
Chile
Peru
Venezuela
4. The Tools and Conditions for the Job
4.1. Uptake of Molecular Diagnostics
4.2. Uptake of Biomarkers
4.3. Uptake of Liquid Biopsy (LB)
4.4. Uptake of Real-World Evidence (RWE)
4.5. Reimbursement and Other Regulatory Issues
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Cancer Report Cancer Research for Cancer Prevention [WWW Document]. 2020. Available online: https://www.iccpportal.org/system/files/resources/IARC%20World%20Cancer%20Report%202020.pdf (accessed on 21 July 2022).
- Cagan, R.; Meyer, P. Rethinking cancer: Current challenges and opportunities in cancer research. Dis. Model. Mech. 2017, 10, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.; Curigliano, G.; Rieß, O.; Hofman, P.; Büttner, R.; Conte, P.; Cufer, T.; Gallagher, W.M.; Georges, N.; Kerr, K.; et al. Identifying the Steps Required to Effectively Implement Next-Generation Sequencing in Oncology at a National Level in Europe. J. Pers. Med. 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Weller, D. “Yes, I have cancer, but I’m also lonely”; tackling a common problem in cancer care. Eur. J. Cancer Care 2018, 27, e12844. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D. Keeping the Person in Personalised Healthcare. Biomed. Hub 2017, 2, 63–71. [Google Scholar] [CrossRef]
- Horgan, D.; Lal, J.A. Making the Most of Innovation in Personalised Medicine: An EU Strategy for a Faster Bench to Bedside and Beyond Process. Public Health Genom. 2018, 21, 101–120. [Google Scholar] [CrossRef]
- Treviño-Sáenz, D.L.; Sánchez-Ibarra, H.E.; Fernández-Garza, L.E.; Barrera-Saldaña, H.A. Pharmacoeconomics of Metastatic Colorectal Cancer Treatment with Targeted Therapies Guided by Companion Molecular Diagnostics. J. Pharm. Res. 2020, 1, 124–129, [WWW Document]. Available online: http://jprtonline.com/articles_pdfs/pharmacoeconomics-of-metastatic-colorectal-cancer-treatment-with-targeted-therapies-guided-by-companion-molecular-diagno.pdf (accessed on 25 July 2022).
- Gorman, L.M. Psychosocial Impact of Cancer on Individual, Family and Society. Psychosoc. B. 2018, 3, 3–23. [Google Scholar]
- Horgan, D.; Baker, M.; Riegman, P.; Bernini, C. Personalised Medicine—Bringing Innovation to the Healthcare System. Biomed. Hub 2017, 2, 16–21. [Google Scholar] [CrossRef]
- Saxén, S.; Saxén, H. Implementing Personalized Genetic Medicine. Voices Bioeth. 2021, 7. [Google Scholar] [CrossRef]
- Barrera-Saldaña, H.A. Origin of personalized medicine in pioneering, passionate, genomic research. Genomics 2019, 112, 721–728. [Google Scholar] [CrossRef]
- Horgan, D.; Ciliberto, G.; Conte, P.; Baldwin, D.; Seijo, L.; Montuenga, L.M.; Paz-Ares, L.; Garassino, M.; Penault-Llorca, F.; Galli, F.; et al. Bringing Greater Accuracy to Europe’s Healthcare Systems: The Unexploited Potential of Biomarker Testing in Oncology. Biomed. Hub 2020, 5, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.; Smand, C.; Negrouk, A.; Lacombe, D. Making Change Happen in Health. Biomed. Hub 2017, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, A.A.; Dulal, S.; Martin, M.G. Personalized Medicine in Oncology in the Developing World: Barriers and Concepts to Improve Status Quo. World J. Oncol. 2021, 12, 50–60. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.L.; Settersten, R.A.; Juengst, E.T.; Fishman, J.R. Integrating genomics into clinical oncology: Ethical and social challenges from proponents of personalized medicine. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 187–192. [Google Scholar] [CrossRef]
- Bentley, A.R.; Callier, S.; Rotimi, C.N. Diversity and inclusion in genomic research: Why the uneven progress? J. Community Genet. 2017, 8, 255–266. [Google Scholar] [CrossRef]
- Landry, L.G.; Ali, N.; Williams, D.R.; Rehm, H.L.; Bonham, V.L. Lack Of Diversity In Genomic Databases Is A Barrier To Translating Precision Medicine Research Into Practice. Health Aff. 2018, 37, 780–785. [Google Scholar] [CrossRef]
- Sirugo, G.; Williams, S.M.; Tishkoff, S.A. The Missing Diversity in Human Genetic Studies. Cell 2019, 572, 573. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament and the Council—Europe’s Beating Cancer Plan; European Commission: Brussels, Belgium, 2021.
- Hickman, J.A.; Tannock, I.F.; Meheus, L.; Hutchinson, L. The European Union and personalised cancer medicine. Eur. J. Cancer 2021, 150, 95–98. [Google Scholar] [CrossRef]
- Lawler, M.; De Lorenzo, F.; Lagergren, P.; Mennini, F.S.; Narbutas, S.; Scocca, G.; Meunier, F.; the European Academy of Cancer Sciences. Challenges and solutions to embed cancer survivorship research and innovation within the EU Cancer Mission. Mol. Oncol. 2021, 15, 1750–1758. [Google Scholar] [CrossRef]
- Mateo, J.; Steuten, L.; Aftimos, P.; André, F.; Davies, M.; Garralda, E.; Geissler, J.; Husereau, D.; Martinez-Lopez, I.; Normanno, N.; et al. Delivering precision oncology to patients with cancer. Nat. Med. 2022, 28, 658–665. [Google Scholar] [CrossRef]
- Horgan, D.; Borisch, B.; Richer, E.; Bernini, C.; Kalra, D.; Lawler, M.; Ciliberto, G.; Van Poppel, H.; Paradiso, A.; Riegman, P.; et al. Propelling Health Care into the Twenties. Biomed. Hub 2020, 5, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Gebbia, V.; Guarini, A.; Piazza, D.; Bertani, A.; Spada, M.; Verderame, F.; Sergi, C.; Potenza, E.; Fazio, I.; Blasi, L.; et al. Virtual Multidisciplinary Tumor Boards: A Narrative Review Focused on Lung Cancer. Pulm. Ther. 2021, 7, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Pitel, B.; Wagner, A.H.; Boca, S.M.; McCoy, M.; King, I.; Gupta, S.; Park, B.H.; Warner, J.L.; Chen, J.; et al. Collaborative, Multidisciplinary Evaluation of Cancer Variants Through Virtual Molecular Tumor Boards Informs Local Clinical Practices. JCO Clin. Cancer Informatics 2020, 4, 602–613. [Google Scholar] [CrossRef]
- Frappe, E. Liquid Biopsy Technology—Changing the Paradigm in Clinical Diagnostics and IVD Regulatory Approaches [WWW Document]. 2021. Available online: https://www.iqvia.com/blogs/2021/04/liquid-biopsy-technology-changing-the-paradigm-in-clinical-diagnostics-and-ivd-regulatory-approaches (accessed on 22 July 2022).
- Ng, C.J.; Teo, C.H.; Abdullah, N.; Tan, W.P.; Tan, H.M. Relationships between cancer pattern, country income and geographical region in Asia. BMC Cancer 2015, 15, 613. [Google Scholar] [CrossRef]
- WHO. Health at a Glance: Asia/Pacific 2020 Measuring Progress Towards Universal Health Coverage: Measuring Progress Towards Universal Health Coverage; WHO: Geneva, Switzerland, 2020.
- United Nations. Addressing the Challenges of Population Ageing in Asia and the Pacific. In Implementation of the Madrid International Plan of Action on Ageing; UN iLibrary: San Francisco, CA, USA, 2017. [Google Scholar] [CrossRef]
- Eniu, A.; Cherny, N.I.; Bertram, M.; Thongprasert, S.; Douillard, J.-Y.; Bricalli, G.; Vyas, M.; Trapani, D. Cancer medicines in Asia and Asia-Pacific: What is available, and is it effective enough? ESMO Open 2019, 4, e000483. [Google Scholar] [CrossRef]
- Nakamura, Y.; Shitara, K.; Lee, J. The Right Treatment of the Right Patient: Integrating Genetic Profiling Into Clinical Decision Making in Advanced Gastric Cancer in Asia. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e166–e173. [Google Scholar] [CrossRef]
- Arafa, M.A.; Rabah, D.M.; Farhat, K.H. Rising cancer rates in the Arab World: Now is the time for action. East. Mediterr. Health J. 2020, 26, 638–640. [Google Scholar] [CrossRef]
- WHO. Regional Framework for Action on Cancer Prevention and Control [WWW Document]. 2017. Available online: https://applications.emro.who.int/docs/RC_technical_papers_2017_3_20037_en.pdf (accessed on 22 July 2022).
- Getting to Personalised Health Care in APAC. [WWW Document], n.d.]. 2022. Available online: https://futureproofinghealthcare.com/sites/default/files/2021-02/Getting%20to%20Personalised%20healthcare%20in%20APAC%20whitepaper.pdf (accessed on 22 July 2022).
- National Health and Morbidity Survey (NHMS). Technical Report, Volume 1, Ministry of Health Malaysia. 2019. Available online: https://iku.moh.gov.my/images/IKU/Document/REPORT/NHMS2019/Report_NHMS2019-NCD_v2.pdf (accessed on 22 July 2022).
- Teh, H.S.; Woon, Y.L. Burden of cancers attributable to modifiable risk factors in Malaysia. BMC Public Health 2021, 21, 410. [Google Scholar] [CrossRef]
- Philippine Statistics Authority. Causes of Deaths in the Philippines (Preliminary): January to December 2020 | Philippine Statistics Authority [WWW Document]; Philippine Statistics Authority: Metro Manila, Philippines, 2021.
- Wu, T.-Y.; Lee, J. Promoting Breast Cancer Awareness and Screening Practices for Early Detection in Low-Resource Settings. Eur. J. Breast Health 2019, 15, 18–25. [Google Scholar] [CrossRef]
- Current Challenges and Evolving Strategies in Implementing Cancer and Palliative Care Services in the Philippines. Br. J. Cancer Res. 2019, 2, 257–263. [CrossRef]
- UICC. Cancer and Universal Health Coverage in the Philippines [WWW Document]. 2020. Available online: https://www.uicc.org/case-studies/cancer-and-universal-health-coverage-philippines (accessed on 22 July 2022).
- Varghese, C. Cancer Prevention and Control in India [WWW Document]. 2022. Available online: https://main.mohfw.gov.in/sites/default/files/Cancer%20Prevention%20And%20Control%20In%20India.pdf (accessed on 22 July 2022).
- Cao, M.; Chen, W. Cancer burden and control in China. Ann. Cancer Epidemiol. 2019, 3, 4. [Google Scholar] [CrossRef]
- Wang, W. Genomics and Public Health: China’s Perspective. In Genomic Medicine in Emerging Economies; Academic Press: Cambridge, MA, USA, 2018; pp. 27–48. [Google Scholar] [CrossRef]
- IC2PerMed. A Short and Comprehensive Guide for Personalised Medicine Strategies and Programmes in China [WWW Document]. 2021. Available online: https://www.ic2permed.eu/2022/03/02/a-short-and-comprehensive-guide-for-personalised-medicine-strategies-and-programmes-in-china/ (accessed on 22 July 2022).
- Rahma, A.T.; Elbarazi, I.; Ali, B.R.; Patrinos, G.P.; Ahmed, L.A.; Al-Maskari, F. Stakeholders’ Interest and Attitudes toward Genomic Medicine and Pharmacogenomics Implementation in the United Arab Emirates: A Qualitative Study. Public Health Genom. 2021, 24, 99–109. [Google Scholar] [CrossRef]
- Al-Shamsi, H.; Darr, H.; Abu-Gheida, I.; Ansari, J.; McManus, M.C.; Jaafar, H.; Tirmazy, S.H.; ElKhoury, M.; Azribi, F.; Jelovac, D.; et al. The State of Cancer Care in the United Arab Emirates in 2020: Challenges and Recommendations, A report by the United Arab Emirates Oncology Task Force. Gulf J. Oncol. 2020, 1, 71–87. [Google Scholar]
- Qoronfleh, M.W. Pathway to excellence in cancer care: Learning from Qatar’s experience. Precis. Med. Sci. 2020, 9, 51–61. [Google Scholar] [CrossRef]
- Qoronfleh, M.W.; Chouchane, L.; Mifsud, B.; Al Emadi, M.; Ismail, S. THE FUTURE OF MEDICINE, healthcare innovation through precision medicine: Policy case study of Qatar. Life Sci. Soc. Policy 2020, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Precision Health in Qatar [WWW Document] QF Stories RSS. Available online: https://www.qf.org.qa/research/precision-health (accessed on 25 July 2022).
- Qatar Biobank [WWW Document]. Available online: https://www.qatarbiobank.org.qa/ (accessed on 25 July 2022).
- Admin. Precision Medicine and Functional Genomics 2022 [WWW Document]. Sidra Medicine. 2022. Available online: https://www.sidra.org/events/research/precision-medicine-conferences/fg2022/ (accessed on 25 July 2022).
- Elias, F. Financial burden of cancer drug treatment in Lebanon. Eur. J. Cancer 2017, 72, S117. [Google Scholar] [CrossRef]
- Abusanad, A.; Alghamdi, M.; Bakkar, M.; Jazieh, A.R. General Oncology Care in the Kingdom of Saudi Arabia. In Cancer in the Arab World; Al-Shamsi, H.O., Abu-Gheida, I.H., Iqbal, F., Al-Awadhi, A., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Alghamdi, S.M.; Alqahtani, J.S.; Aldhahir, A.M. Current status of telehealth in Saudi Arabia during COVID-19. J. Fam. Community Med. 2020, 27, 208–211. [Google Scholar] [CrossRef]
- Althubiti, M.A.; Eldein, M.M.N. Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med. J. 2018, 39, 1259–1262. [Google Scholar] [CrossRef]
- Glover, L. Australia: International Health Care System Profiles [WWW Document]; Nuffield Trust: London, UK, 2017. [Google Scholar]
- Mukai, Y.; Ueno, H. Establishment and implementation of Cancer Genomic Medicine in Japan. Cancer Sci. 2020, 112, 970–977. [Google Scholar] [CrossRef]
- OECD. Public health genomics in Korea. In OECD Reviews of Public Health: Korea: A Healthier Tomorrow; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Sylla, B.S.; Wild, C.P. A million africans a year dying from cancer by 2030: What can cancer research and control offer to the continent? Int. J. Cancer 2011, 130, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Azubuike, S.O.; Muirhead, C.; Hayes, L.; McNally, R. Rising global burden of breast cancer: The case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: A review. World J. Surg. Oncol. 2018, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.C.; Tishkoff, S.A. African Genetic Diversity: Implications for Human Demographic History, Modern Human Origins, and Complex Disease Mapping. Annu. Rev. Genom. Hum. Genet. 2008, 9, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, S.N.; Francis, V.; Tambo, E.; Diallo, S.H.; Landouré, G.; Nembaware, V.; Dareng, E.; Muhamed, B.; Odutola, M.; Akeredolu, T.; et al. Implementation of genomics research in Africa: Challenges and recommendations. Glob. Health Action 2018, 11, 1419033. [Google Scholar] [CrossRef] [PubMed]
- ASP Policy Paper 1. A Framework for the Implementation of Genomic Medicine for Public Health in Africa [WWW Document]. 2020. Available online: https://www.aasciences.africa/sites/default/files/Publications/A%20Framework%20for%20the%20Implementation%20of%20Genomic%20Medicine%20for%20Public%20Health%20in%20Africa.pdf (accessed on 25 July 2022).
- Hamdi, Y.; Abdeljaoued-Tej, I.; Zatchi, A.A.; Abdelhak, S.; Boubaker, S.; Brown, J.S.; Benkahla, A. Cancer in Africa: The Untold Story. Front. Oncol. 2021, 11, 650117. [Google Scholar] [CrossRef]
- Maphumulo, W.T.; Bhengu, B.R. Challenges of quality improvement in the healthcare of South Africa post-apartheid: A critical review. Curationis 2019, 42, e1–e9. [Google Scholar] [CrossRef]
- Tapera, O.; Nyakabau, A.M. Limited knowledge and access to palliative care among women with cervical cancer: An opportunity for integrating oncology and palliative care in Zimbabwe. BMC Palliat. Care 2020, 19, 20. [Google Scholar] [CrossRef]
- Mwakigonja, A.R.; Lushina, N.E.; Mwanga, A. Characterization of hormonal receptors and human epidermal growth factor receptor-2 in tissues of women with breast cancer at Muhimbili National Hospital, Dar es salaam, Tanzania. Infect. Agents Cancer 2017, 12, 60. [Google Scholar] [CrossRef]
- EU-Africa PerMed. 2022. Available online: https://www.euafrica-permed.eu/ (accessed on 25 July 2022).
- Wanjau, M.N.; Kivuti-Bitok, L.W.; Aminde, L.N.; Veerman, L. Stakeholder perceptions of current practices and challenges in priority setting for non-communicable disease control in Kenya: A qualitative study. BMJ Open 2021, 11, e043641. [Google Scholar] [CrossRef]
- Jedy-Agba, E.; Curado, M.P.; Ogunbiyi, O.; Oga, E.; Fabowale, T.; Igbinoba, F.; Osubor, G.; Otu, T.; Kumai, H.; Koechlin, A.; et al. Cancer incidence in Nigeria: A report from population-based cancer registries. Cancer Epidemiology 2012, 36, e271–e278. [Google Scholar] [CrossRef]
- Alemayehu, C.; Mitchell, G.; Nikles, J. Barriers for conducting clinical trials in developing countries- a systematic review. Int. J. Equity Health 2018, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Aparicio, A.; Orue, A. Precision oncology in Latin America: Current situation, challenges and perspectives. ecancermedicalscience 2019, 13, 920. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.H.; Werutsky, G.; Mohar, A.; Ferrigno, A.S.; Müller, B.G.; Bychkovsky, B.L.; Castro, E.C.J.; Uribe, C.J.; Villarreal-Garza, C.; Soto-Perez-De-Celis, E.; et al. Cancer control in Latin America and the Caribbean: Recent advances and opportunities to move forward. Lancet Oncol. 2021, 22, e474–e487. [Google Scholar] [CrossRef]
- Salvo, M.; González-Feliú, E.; Toro, J.; Gallegos, I.; Maureira, I.; Miranda-González, N.; Barajas, O.; Bustamante, E.; Ahumada, M.; Colombo, A.; et al. Validation of an NGS Panel Designed for Detection of Actionable Mutations in Tumors Common in Latin America. J. Pers. Med. 2021, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Kanavos, P.; Parkin, G.C.; Kamphuis, B.; Gill, J. Latin America health care System Overview: A comparative analysis of fiscal space in health care. Lond. Sch. Econ. Polit. Sci. 2019. Available online: https://www.lse.ac.uk/business/consulting/assets/documents/latam/Latin-America-Fiscal-Space-PPT-PK.pdf (accessed on 25 July 2022).
- Raez, L.E.; Nogueira, A.; Santos, E.S.; dos Santos, R.S.; Franceschini, J.; Ron, D.A.; Block, M.; Yamaguchi, N.; Rolfo, C. Challenges in Lung Cancer Screening in Latin America. J. Glob. Oncol. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Fonseca, B.D.P.; Albuquerque, P.C.; Saldanha, R.D.F.; Zicker, F. Geographic accessibility to cancer treatment in Brazil: A network analysis. Lancet Reg. Health-Am. 2021, 7, 100153. [Google Scholar] [CrossRef]
- da Silva, M.J.S.; Bergmann, A. Novos Rumos da Política de Controle do Câncer no Brasil. Rev. Bras. Cancerol. 2021, 68, e.002668. [Google Scholar] [CrossRef]
- Caleffi, M.; Crivelatti, I.; Burchardt, N.A.; Ribeiro, R.A.; Acevedo, Y.; Job, L.G.; Nonnemacher, N.; Rosa, D.D. Breast cancer survival in Brazil: How much health care access impact on cancer outcomes? Breast 2020, 54, 155–159. [Google Scholar] [CrossRef]
- Galduróz, J.C.; Tomita, N.; Bezerra, A.G. Measures to reduce smoking: Brazil takes the lead. Rev. Bras. Psiquiatr. 2020, 42, 456–457. [Google Scholar] [CrossRef]
- Santos, M.; A Coudry, R.; Gil Ferreira, C.; Stefani, S.; Cunha, I.W.; Zalis, M.G.; Araujo, L.H. Increasing access to next-generation sequencing in oncology for Brazil. Lancet Oncol. 2019, 20, 20–23. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef]
- ICCI-LA. Addressing the Rising Burden of Cancer in Colombia: Challenges & Opportunities [WWW Document]. 2021. Available online: https://www.uicc.org/resources/addressing-rising-burden-cancer-colombia-challenges-opportunities (accessed on 25 July 2022).
- Murillo, R.; González, A.; Galvis, J.C.; Hidalgo, I.; Marín, A.; Muñoz, J.E.; Sánchez, R. Radiation Oncology Workforce in Colombia. JCO Glob. Oncol. 2020, 6, 190–194. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.; Buitrago, G.; Quitian-Reyes, H.; Wiesner, C.; Castillo, J.S. Access to cancer care in Colombia, a middle-income country with universal health coverage. J. Cancer Policy 2018, 15, 104–112. [Google Scholar] [CrossRef]
- Padilla-Raygoza, N.; Monroy-Torres, R.; Sandoval-Salazar, C.; Vera-Becerra, L.E.; Patiño-López, M.E.; García-Campos, M.D.L.; Campos, V.B.; Jiménez, M.D.C.O.; Delgado-Sandoval, S.D.C.; Ramírez-Gómez, X.S.; et al. Cancer prevention programmes in Mexico: Are we doing enough? ecancermedicalscience 2020, 14, 997. [Google Scholar] [CrossRef] [PubMed]
- Moye-Holz, D.; Saucedo, R.S.; Van Dijk, J.P.; A Reijneveld, S.; Hogerzeil, H.V. Access to innovative cancer medicines in a middle-income country—The case of Mexico. J. Pharm. Policy Pr. 2018, 11, 25. [Google Scholar] [CrossRef]
- The Economist. Cancer Control in Chile an Underfunded System with Pockets of Strength [WWW Document]. 2017. Available online: https://worldcancerinitiative.economist.com/pdf/Roche-Cancer-control-access-and-inequality-in-Latin-America-A-tale-of-light-and-shadow/CancerControlinChile.pdf (accessed on 25 July 2022).
- Leon, A.; Puschel, K.; Craig, H.; Are, C. Cancer on the Global Stage: Incidence and Cancer-Related Mortality in Chile [WWW Document]. The ASCO Post. 2020. Available online: https://ascopost.com/issues/november-10-2020/incidence-and-cancer-related-mortality-in-chile/ (accessed on 25 July 2022).
- Dienstmann, R. WS04.02 Access to Biomarker Testing in Latin America. J. Thorac. Oncol. 2021, 16, S842. [Google Scholar] [CrossRef]
- Duma, N.; Duran, L.D. The State of Cancer Care in Venezuela. J. Glob. Oncol. 2019, 5, 1–2. [Google Scholar] [CrossRef]
- A Phillips, K.; Douglas, M.P.; Wordsworth, S.; Buchanan, J.; A Marshall, D. Availability and funding of clinical genomic sequencing globally. BMJ Glob. Health 2021, 6, e004415. [Google Scholar] [CrossRef]
- Chong, H.Y.; Allotey, P.A.; Chaiyakunapruk, N. Current landscape of personalized medicine adoption and implementation in Southeast Asia. BMC Med Genom. 2018, 11, 94. [Google Scholar] [CrossRef]
- WHO. Molecular Diagnostics Integration Global Meeting Report, 10–12 July 2019, Geneva, Switzerland. World Health Organization. [WWW Document]. 2020. Available online: https://apps.who.int/iris/handle/10665/331708 (accessed on 25 July 2022). License: CC BY-NC-SA 3.0 IGO.
- Research and Markets. The 2022 Market for Analytical & Life Science Instrumentation in the Asia Pacific [WWW Document]. 2022. Available online: https://www.researchandmarkets.com/reports/5576304/the-2022-market-for-analytical-and-life-science (accessed on 25 July 2022).
- Discussion paper, OECD “Policy Issues for the Development and Use of Biomarkers in Health”. [WWW Document]. 2011. Available online: https://www.oecd.org/health/biotech/49023036.pdf (accessed on 25 July 2022).
- Hatta, M.; Hanif, E.M.; Chin, S.-F.; Neoh, H.-M. Pathogens and Carcinogenesis: A Review. Biology 2021, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Temilola, D.O.; Wium, M.; Coulidiati, T.H.; Adeola, H.A.; Carbone, G.M.; Catapano, C.V.; Zerbini, L.F. The Prospect and Challenges to the Flow of Liquid Biopsy in Africa. Cells 2019, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Market Data Forecast. Latin America Liquid Biopsy Market Research Report—Segmented by Cancer Type, Sample Type, Diagnostic Approach, End Users, Country (Mexico, Brazil, Argentina, Chile and Rest of Latin America)—Industry Analysis, Size, Share, Growth, Trends, Forecast (2022 to 2027) [WWW Document]. 2022. Available online: https://www.marketdataforecast.com/market-reports/latin-america-liquid-biopsy-market (accessed on 25 July 2022).
- Fierce Pharma. Singapore Researchers Close in on ‘Liquid Biopsy’ to Track Cancer Progress, Treatment [WWW Document]. 2015. Available online: https://www.fiercepharma.com/pharma-asia/singapore-researchers-close-on-liquid-biopsy-to-track-cancer-progress-treatment (accessed on 25 July 2022).
- Fierce Biotech. In a First, China Approves Amoy’s Lung Cancer Liquid Biopsy [WWW Document]. 2018. Available online: https://www.fiercebiotech.com/medtech/a-first-china-approves-amoy-s-lung-cancer-liquid-biopsy (accessed on 25 July 2022).
- Market Data Forecast. Liquid Biopsy Market Size, Share, Growth Analysis Report: 2022 to 2027 [WWW Document]. Market Data Forecast. 2022. Available online: https://www.marketdataforecast.com/market-reports/liquid-biopsy-market (accessed on 25 July 2022).
- Ispe. Ispe’s Position on Real-World Evidence (RWE) [WWW Document]. 2022. Available online: https://pharmacoepi.org/pub/?id=136DECF1-C559-BA4F-92C4-CF6E3ED16BB6 (accessed on 25 July 2022).
- Khosla, S.; White, R.; Medina, J.; Ouwens, M.; Emmas, C.; Koder, T.; Male, G.; Leonard, S. Real World Evidence (RWE)—A Disruptive Innovation or the Quiet Evolution of Medical Evidence Generation? F1000Research 2018, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Ewumi, O. How Can Big Data, RWD, and RWE Upgrade Health Care in Africa? [WWW Document]. 2021. Available online: https://www.the-yuan.com/89/How-Can-Big-Data-RWD-and-RWE-Upgrade-healthcare-in-Africa.html (accessed on 25 July 2022).
- Lou, J.; Kc, S.; Toh, K.Y.; Dabak, S.; Adler, A.; Ahn, J.; Bayani, D.B.S.; Chan, K.; Choiphel, D.; Chua, B.; et al. Real-world data for health technology assessment for reimbursement decisions in Asia: Current landscape and a way forward. Int. J. Technol. Assess. Health Care 2020, 36, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Hincapie, A.; Gilardino, R.; Tang, B.; Julian, G.; Soares, C.; Machnicki, G. PNS118 Real World Evidence (RWE) Use in Latin America Healthcare Decision Making: An Stakeholder Survey Analysis. Value Health 2021, 24, S194. [Google Scholar] [CrossRef]
- Kamphuis, B.W.; Kanavos, P. Assessing pricing and reimbursement policies for generic pharmaceuticals in the MENA region for improved efficiency, affordability and generic penetration. Health Policy OPEN 2021, 2, 100045. [Google Scholar] [CrossRef]
- Son, K.-B. Understanding the trends in international agreements on pricing and reimbursement for newly marketed medicines and their implications for access to medicines: A computational text analysis. Glob. Health 2020, 16, 98. [Google Scholar] [CrossRef]
- Augustovski, F.; Rojas, J.A.D.; Ferraz, M.B.; Hernandez, I.C.; Donato, B.M.K.; Raimundo, K.; Asche, C.V. Status Update of the Reimbursement Review Environment in the Public Sector across Four Latin American Countries. Value Health Reg. Issues 2012, 1, 223–227. [Google Scholar] [CrossRef][Green Version]
- Hill, C.V. Malaysian Genetic Test for the Detection of Breast Cancer in Asian Women [WWW Document]. Parentraide Cancer. 2022. Available online: https://parentraide-cancer.org/malaysian-genetic-test-for-the-detection-of-breast-cancer-in-asian-women/ (accessed on 26 July 2022).
- Tan, J.; Abdullah, A.; Khaw, C.; Petraeus, M.; Ooi, C.; Moghana, J.; Salim, Z. M’sian Team Creates Tool to Detect Breast Cancer Genes in Asian Women with Better Accuracy [WWW Document]. Vulcan Post. 2022. Available online: https://vulcanpost.com/780356/arica-malaysia-genetic-test-breast-cancer-detection-asian-women/ (accessed on 25 July 2022).
| Feature | Number (%) |
|---|---|
| Total Number of Experts | 48 (100%) |
| Sex | |
| Total | 48 (100%) |
| 27 (56%) |
| 21 (44%) |
| Specialty | |
| 16 (33%) |
| 11 (23%) |
| 8 (17%) |
| 13 (27%) |
| Regions | |
| 17 (35.5%) |
| 17 (35.5%) |
| 10 (21%) |
| 4 (8%) |
| Country | Cancer Situation | Recommendations |
|---|---|---|
| China |
|
|
| Nepal |
|
|
| United Arab Emirates |
|
|
| Qatar |
|
|
| Country | Cancer Situation | Recommendations |
|---|---|---|
| Tunisia |
|
|
| Cameroon |
|
|
| Kenya |
|
|
| Nigeria |
|
|
| Country | Cancer Situation | Recommendations |
|---|---|---|
| Brazil |
|
|
| Colombia |
|
|
| Mexico |
|
|
| Chile |
|
|
| Peru |
|
|
| Venezuela |
|
|
| Countries | Funding of Cancer Treatment/Research | Genomics/Biomarkers | Cancer Incidence and Risk Factors | Cancer Strategic Plans | Primary Prevention Efforts |
|---|---|---|---|---|---|
| Angola | It is highly needed to invest in diagnostic facilities, pathology, surgical capacities, chemotherapy, radiotherapy and palliative care resources | Development of genomic surveillance center and lab | Infectious diseases are still of major importance, but mortality rate of oncological diseases is very high | A national plan will be needed to effectively mobilize resources, train staff and reinforce laboratory biosafety measures | More awareness programs and preventive measures need to be put in place |
| Brazil | National Health System (SUS) subsidizes cancer treatment for approximately 75% of the population; Brazil has the highest public funding for clinical studies | The need to increase access to next-generation sequencing in oncology is recognized, so agencies and payers will be required to collaborate in building data collection infrastructure | Estimations are there were 625,000 new cancer cases in Brazil in 2020 | Brazil ranks very well in working to reduce smoking rates as a cancer prevention strategy | The primary care network is the patient’s entryway to the health system in Brazil, playing a decisive role in actions to promote health, prevention and tracking cancer |
| Cameroon | Funding is limited, and governance and political will to push personalized medicine are lacking | Genetic testing for breast cancer (BC) is out of reach for most patients, and targeted therapies are not available locally; there are unresolved issues on data sharing and on validation and regulatory approval of genetic tests and targeted therapies | Mortality of cancer is high, especially cervical cancer | Cameroon has a national strategic plan for prevention and cancer control, but it makes no reference to personalized medicine, genomics, or biomarkers | There is low patient awareness and education, and uptake of screening is low |
| Chile | Chile has, after Brazil, the second-highest public funding for clinical studies; Chile has radiotherapy coverage of more than 100% | Tumor sequencing is available in Chile; cancer genomics technologies are not fully implemented; efforts have been made to generate research in gastric, colon and breast cancer | Cancer is the second leading cause of death and accounts for 23.4% of all deaths in the country; most common are prostate, colon, breast, stomach, and lung cancers | Currently, there is no national registry of cancer in Chile, although there are five population-based provincial registries; major cancer survivorship programs are lacking | Access to the primary health-care system is universal and free in Chile. The country has invested in preventive measures such as Pap screening tests and an HPV vaccination program. It also has a program to screen those over 40 with a family history of stomach cancer and a current ulcer and is piloting a screening program for colorectal cancer |
| China | There are many investments in genome science and public health genomics-related programs and services | Research on biomarkers and big data is developing at a rapid pace within the country; technologies such as NGS and liquid biopsy are widely adopted | China is facing increased cancer rates where the top five commonly diagnosed cancer types in males are lung cancer (14.5%), prostate cancer (13.5%), colorectal cancer (10.9%), stomach cancer (7.2%) and liver cancer (6.3%) | China has national genomics policies to address a variety of genetic issues | Cancer prevention and early action measures are recognized as key projects in Chinese Government; there are many initiatives and guidelines launched |
| Colombia | There is a need to increase funding to develop innovative cancer technologies, medicines, and treatments accessible to all patients in need | Whole exome sequencing, non-invasive prena taltesting and tumor sequencing are available in Colombia; there is an urgent need to expand the use of NGS in breast, lung, and unknown primary cancers | Colombia had an age-standardized rate (ASR) of 178.8 new cases of cancer per 100,000 people in 2018 | Colombia has a National Cancer Control Plan that aims to: emphasize cancer prevention; improve early detection; improve quality of cancer care and recovery of cancer patients and survivors; strengthen national information systems; and improve the training and development of practitioners | There is a universal health care and a government-sponsored 10-year cancer control plan focused on prevention, early detection, and treatment in Colombia |
| India | Reforms have been put in place that aim to strengthen primary health care and move towards universal health coverage featuring cancer care benefit packages, with a standards-based, interoperable, national digital health information system | Some strengthening of molecular and genomic testing facilities has been triggered by the COVID-19 pandemic | The estimated prevalence of cancer is 2.5 million with an incidence of 0.7 million cases per year. There are some 800,000 new cancer cases in India every year, and tobacco is identified as the most important cause of cancer | India has created a National Cancer Programme envisaging control of tobacco | More efforts in prevention measures should be put in place since the late stage at presentation is very often the main reason for the poor survival from cancer |
| Kenya | Kenya has set universal health coverage as a priority | Patients have little to no access to genetic testing and counseling services | Cancer is the third leading cause of death after infectious and cardiovascular diseases | Kenya has developed a national cancer control strategy | Public health systems supported by modern technologies and promotion of healthier lifestyles, along with new technologies to enhance disease surveillance, prevention, early diagnosis and treatment are set as priorities |
| Kingdom of Saudi Arabia | Cancer care is offered free of charge for Saudi patients by a royal decree; more research funding on cancer screening, prevention, and care quality are needed in KSA | Within a period of 5 years, the Saudi Human Genome Program aims to sequence 100,000 samples (normal and disease) from the Saudi population | The incidence of cancer cases and costs of care are high | There are cancer screening programs for breast cancer, colorectal cancer and cervical cancer | The Ministry of Health has been advocating a healthy lifestyle with a healthy diet, physical activity, maintaining ideal body weight, and smoking cessation to decrease noncommunicable diseases, including cancer |
| Lebanon | Health and third-party payers are providing the financial coverage; cancer drugs are free of charge for uninsured patients, and this country has one of the most developed health care systems in the region | Lebanon is making progress towards implementing precision genetic and genomic research | Cancer rates are increasing and consequently the burden of cancer cost | Many programs put in place raise awareness about cancer screening and prevention through educating and counseling the population, and cancer research has been established by the health ministry | There are cigarette cessation and anti-smoking campaigns for lung cancer as part of preventive measures |
| Malaysia | Government needs to take a more robust approach to pharmaceutical companies when negotiating prices: at present nearly half of cancer patients experience financial catastrophe within a year of diagnosis | There are challenges regarding NGS testing | Tobacco and infections are reported as the principal causes of cancer deaths, which have a prevalence of close to 1/1000 | Malaysia has developed the National Strategic Plan for Cancer Control | There is a need for better awareness and early diagnosis, particularly to enable remote communities to access equitable care with targeted therapies |
| Mexico | Seguro Popular (SP) was created to provide universal health coverage, including cancer care; 8% of SP’s resources were allocated to Fondo de Protrección contra Gastos Catastróficos (FPGC), of which 28% finances cancer care; about 50–60% of cancer patients in Mexico are fully covered | Mexico has a high number of NGS platforms; a small fraction of the population in Mexico has access to genetic analyses to identify factors associated with the development of some types of cancer, for the early detection of a tumor, or to take the option of chemotherapy or prophylactic surgery | The most common types of cancer among Mexican men are prostate, colorectal, lung, gastric, and testicular at younger ages. Among Mexican women, they are breast, uterine cervix, and colorectal | In 2008, a general law was approved for the control of tobacco and in 2009, in agreement with the General Law Regulation for Control of Tobacco, pictographs and warnings were implemented on the packaging | Genetic counseling and molecular diagnosis are routinely offered by family cancer clinics in a few levels 3 government and specialized private hospitals; citizens have access to a wide range of special cancer diagnostic preventive measures, such as Pap smears offered for women aged 25 to 34 every three years |
| Nigeria | Nigeria spent only around 0.5% of its 2017 budget on health care; there is a lack of funding | The genomics capacity in Nigeria has for many years been supported by bioinformatics at various institutions across the country | Around 100,000 new cases of cancer occur every year | Nigeria developed the National Cancer Control Plan to reduce the incidence and prevalence of cancer | Prevention measures are inadequate, there is a lack of proper access to basic health care, as well as health-related impoverishment |
| Nepal | Health care is underfunded, so the population suffers from financial risk in the case of using health services for diseases such as cancer | Genetic research in Nepal heavily relies on resources from international institutes | In 2020, Nepal had an estimated total of 20,508 cancer cases | There is a partially implemented health insurance policy that has several limitations and is not available to everyone and also lacks funding | There are delays in presentation, diagnosis and treatment that need to be tackled |
| Peru | There is a need for more provision and reimbursement of liquid biopsy (LB) in lung cancer, and more funding of studies of the genomics of cancer in the highly diverse Peruvian population | There is a lack of local genomic laboratories, which means samples requiring comprehensive genomic analysis have to be sent abroad; there is little medical familiarity with biomarkers and genetic tests | In 2020, there were a total of 69,849 cancer cases in Peru | Governmental cancer control program and development of a national tumor bank are underway | There is a need for more public education programs |
| Philippines | Government is pushed to extend financial and other forms of assistance to impoverished cancer patients and to provide funds for cancer research | There are many genetic tests and services that are available and delivered to the whole country, such as cytogenetics, molecular genetics, biochemical genetics, and newborn screening | Cancer was the second leading cause of death in 2020 | Effective screening and prevention strategies exist for many cancers; in February 2019, the National Integrated Cancer Control Act was signed into law | Screening programs and public health education need more efforts |
| Qatar | Qatar is investing a lot in cancer research; they are bringing in top researchers from all over the world and establishing institutes of research | Cancer molecular genetic boards to integrate PM and genomics into cancer care are now in place. | Projections are that the cancer incidence in Qatar will triple between 2010 and 2030 due to aging and population growth | A National Cancer Research Strategy, Qatar Biobank (QBB) and the Qatar Genome Program (QGP) have been put in place | The aims for the future are to include evidence-based approaches for public engagement, prevention and early detection, especially the use of personalized approaches |
| South Africa | There are many projects in South Africa that aim to provide better allocation of research funding; the SAMRC is the largest local health research funder in South Africa | There is a lack of population-level genomic data and lack of access to targeted therapies | South Africa has a high burden of noncommunicable diseases (NCDs) | South Africa has established funding of PM, with a genome program and a PM think tank, aiming at a research strategy and product pipeline with an NCD focus on cancer | Cancer prevention guidelines have been put in place; cervical cancer prevention and control policy have been developed |
| Tunisia | PerMediNA-Precision Medicine in North Africa is linking the Instituts Pasteur in Tunisia, Algeria, Morocco and Paris with EUR 1 million funding from the French government | More sequencing and genotyping facilities along with biobanks are needed | There was a total of 19,446 cancer cases in Tunisia in 2020 | Tunisia runs human genome programs, and the Oncogenetics Unit at the Institut Pasteur in Tunis, is conducting research-based genetic diagnosis | Primary prevention strategies remain insufficient as evidenced by the high prevalence of smoking in 2018 (26%) |
| United Arab Emirates | The top-up funding scheme currently covers breast, colorectal and cervical cancer | There are genomic projects, including the genomic and pharmacogenomic research of the Emirates Genome Program | Cancer is the third leading cause of death in the UAE, right after cardiovascular disease and trauma | National Cancer Control Plan for 2022–2026 has been proposed-and will require accurate data, a reliable cancer registry and periodic monitoring and evaluation | There is a colon cancer prevention program that includes primary preventive strategies and secondary prevention by stool fit test every 2 years or colonoscopy every 10 years |
| Venezuela | Overall health care spending is around 5% of GDP in Venezuela | Infrastructure in terms of biobank organization is improving | In 2020, there were a total of 58,424 cancer cases in Venezuela | A national cancer plan or strategy needs to be developed | Greater efforts are needed in adoption and awareness of PM; availability of education, training and outreach activities are low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horgan, D.; Mia, R.; Erhabor, T.; Hamdi, Y.; Dandara, C.; Lal, J.A.; Domgue, J.F.; Ewumi, O.; Nyawira, T.; Meyer, S.; et al. Fighting Cancer around the World: A Framework for Action. Healthcare 2022, 10, 2125. https://doi.org/10.3390/healthcare10112125
Horgan D, Mia R, Erhabor T, Hamdi Y, Dandara C, Lal JA, Domgue JF, Ewumi O, Nyawira T, Meyer S, et al. Fighting Cancer around the World: A Framework for Action. Healthcare. 2022; 10(11):2125. https://doi.org/10.3390/healthcare10112125
Chicago/Turabian StyleHorgan, Denis, Rizwana Mia, Tosan Erhabor, Yosr Hamdi, Collet Dandara, Jonathan A. Lal, Joel Fokom Domgue, Oladimeji Ewumi, Teresia Nyawira, Salomé Meyer, and et al. 2022. "Fighting Cancer around the World: A Framework for Action" Healthcare 10, no. 11: 2125. https://doi.org/10.3390/healthcare10112125
APA StyleHorgan, D., Mia, R., Erhabor, T., Hamdi, Y., Dandara, C., Lal, J. A., Domgue, J. F., Ewumi, O., Nyawira, T., Meyer, S., Kondji, D., Francisco, N. M., Ikeda, S., Chuah, C., De Guzman, R., Paul, A., Reddy Nallamalla, K., Park, W.-Y., Tripathi, V., ... Barrera-Saldana, H. A. (2022). Fighting Cancer around the World: A Framework for Action. Healthcare, 10(11), 2125. https://doi.org/10.3390/healthcare10112125








