Blood Pressure Measurement: From Cuff-Based to Contactless Monitoring
Abstract
1. Introduction
2. Biophysical Theory
3. Contact-Based BP Measurement from PPG Signals
| Year | Ref. | Dataset | Data Description | (1) | (2) | (3) | Preprocessing | Models | SBP | DBP |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-machine learning (non-ML) methods | ||||||||||
| 2018 | [105] | Private | 32 subjects | • | Oscillometry | Error | Error | |||
| 2018 | [58] | Private | 18 subjects | • | Oscillometry | Error | Error | |||
| 2016 | [106] | Test: Private | 85 subjects Smartphone data | • | - | AuraLife [107] | AE | AE | ||
| 2018 | [108] | Private | 32 pregnant women Smartphone data | - | Preventicus | Error | - | |||
| 2021 | [109] | Private | 965 subjects Smartphone data | - | Preventicus | Error | - | |||
| Traditional machine learning (TML) methods | ||||||||||
| 2012 | [110] | Private | 5 subjects Smartphone data | • | • | - | Regression analysis | Acc. | Acc. | |
| 2013 | [111] | Private | 17 subjects Smartphone data | • | • | - | SVM | Acc. | Acc. | |
| • | • | - | Linear regression | Acc. | Acc. | |||||
| 2014 | [112] | UQVS [113] | 32 subjects | • | • | MIC feature selection | SVM | Acc. = | Acc. = % | |
| Private | 156 subjects Smartphone dataset | • | • | Acc. = | Acc. = % | |||||
| 2016 | [114] | Private | 65 subjects (age , SBP , DBP ) | • | • | Discrete wavelet transform, forward feature selection [115] | Nonlinear SVM | Error | Error | |
| 2016 | [116] | UQVS [113] | 32 subjects | • | - | SV Regression | AE | AE | ||
| 2017 | [117] | UQVS [113] | 32 subjects | • | - | SVM | AE | AE | ||
| 2017 | [118] | Private | 68 subjects (age , SBP , DBP ) | • | - | Linear regression | MAPD | MAPD | ||
| 2018 | [119] | Private | 7 subjects | • | - | Regression analysis | - | RMSE | ||
| 2018 | [120] | Private | 205 subjects Smartphone data (independent splitting) | • | - | Lasso regression | AE | AE | ||
| 2019 | [121] | MIMIC | 441 subjects | • | FFT, FFT,PCA | A series of 4 regressions | AE | AE | ||
| 2020 | [122] | [122] | 15 subjects | • | • | - | Regression analysis | MAE | MAE | |
| Deep learning (DL) methods | ||||||||||
| 2013 | [123] | MIMIC [124] | - | • | - | ANN | AE | AE | ||
| 2013 | [125] | Train: MIMIC [124] Test: private (phone) | 5 test subjects (SBP , DBP | • | - | ANN | MAE | MAE | ||
| 2015 | [126] | MIMIC-II | 4254 records | • | - | ANN | AE | AE | ||
| 2016 | [127] | Train: MIMIC-II Test: private | Train: 69 subjects Test: 23 subjects | • | - | ANN | Error | Error | ||
| 2016 | [128] | MIMIC-II | 3000 subjects | • | - | ANN | AE | AE | ||
| 2018 | [129] | Private | 84 subjects | • | • | - | LSTM | RMSE | RMSE | |
| 2018 | [130] | Private | No subject description Exclude BMI | • | Activity features (this paper also considered the input features from the tri-axial accelerometer and not pure PPG methods) | LSTM | Median± IQR | Median± IQR | ||
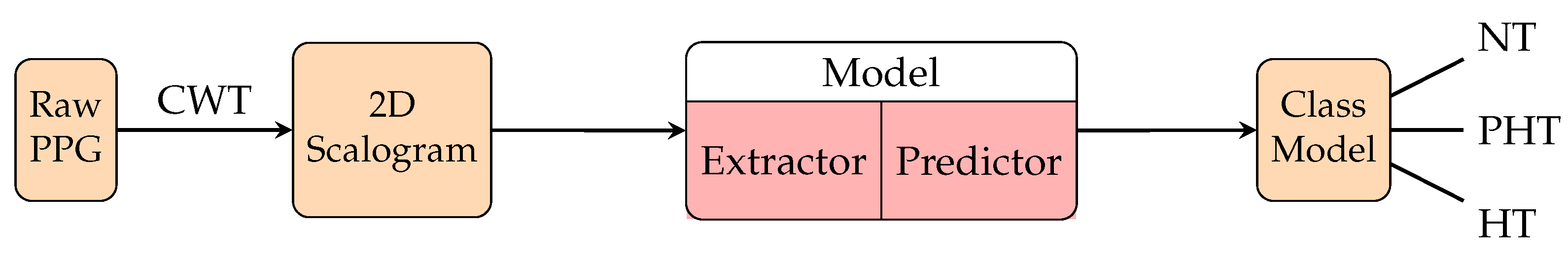
| 2018 | [131] | MIMIC | 120 subjects | • | Scalogram from CWT | GoogleNet | F1 score for hypertension | |||
| 2019 | [132] | MIMIC [124] | 39 subjects | • | • | ANN-LSTM | ||||
| 2019 | [133] | MIMIC-II | 510 patients | • | ResNet [134] +GRU | MAE | MAE | |||
| (independent splitting) | • | • | MAE | MAE | ||||||
| 2019 | [135] | MIMIC-II [126] | 942 subjects (independent splitting) | • | - | CNN | AE | AE | ||
| • | • | AE | AE | |||||||
| • | • | • | AE | AE | ||||||
| 2020 | [136] | MIMIC-II | 500 records | • | - | GRU | AE | AE | ||
| • | LSTM | AE | AE | |||||||
| 2020 | [137] | Private | 26 subjectsSmartphone data (various conditions) | • | - | CNN | AE | AE | ||
| 2020 | [138] | Figshare [139] | 116 subjects(independent splitting, uniform subject distribution) | • | • | CNN | F1 score for hypertension | |||
| 2020 | [140] | UCI, MIMIC-II | 1557 subjects | • | • | - | LRCN | AE | AE | |
| 2020 | [141] | MIMIC-II [35] | 942 subjects | • | - | PPG2ABP | MAE = | MAE = | ||
| 2021 | [142] | MIMIC-III | 200,000 records (no description) | • | • | - | CNN-LSTM | AE | AE | |
| 2021 | [143] | MIMIC-II | 200 subjects (114 men, 86 women, age ) | • | • | - | VGG19-LSTM | AE | AE | |
| 2021 | [144] | Train [126] Test: UQVS [113] | 5 train records (no description) 1 test subject (no description) | • | • | - | T2T-GAN | Error of AP | ||
| 2021 | [145] | MIMIC-II UQVS [113] | 20 subjects 32 subjects (subject independent splitting) | • | - | CNN-LSTM | AE AE | AE AE | ||
| 2021 | [146] | MIMIC [124] | 48 subjects test split: of total | • | • | - | CNN-LSTM | AE | AE | |
| 2021 | [147] | MIMIC-III | Mixed: (12,000) records Non-mixed: (4000) records | • | - | AlexNet [148] | MAE MAE | MAE MAE | ||
| 2021 | [147] | MIMIC-III | Mixed: (12,000) records Non-mixed: (4000) records | • | - | ResNet [134] | MAE MAE | MAE MAE | ||
| 2021 | [147] | MIMIC-III | Mixed: (12,000) records Non-mixed: (4000) records | • | - | LSTM | MAE MAE | MAE MAE | ||
| 2021 | [149] | MIMIC I, III | 100 subjects | • | - | U-Net | AE | AE | ||
| 2021 | [150] | MIMIC II [35] | 942 subjects | • | - | U-Net | MAE | MAE | ||
| 2021 | [151] | (dataset) | (subjects) | - | LASSO-LSTM | MAE | MAE | |||
| 2021 | [152] | MIMIC-II [126] | 5289 subjects | • | - | LSTM Autoencoder | AE | AE | ||
| 2022 | [89] | Train: MIMIC-II Test: MIMIC-II Test: UQVS [113] | MIMIC-II: (12,000) records Test: random, 3000 records UQVS: 32 subjects | • | • | MFMC filter | MLPlstm-BP | AE AE | AE AE | |
| 2022 | [89] | train:MIMIC-II test: MIMIC-II test: UQVS [113] | MIMIC-II: (12000) records Test: random 3000 records UQVS: 32 subjects | • | • | MFMC filter | gMLP-BP | AE AE | AE AE | |
| 2022 | [153] | [147] Non-mixed [126] | [147]: 1,250,000 samples [147]: subject uniform distribution [126]: 4254 records | • | - | InfoGAN [154] enc-dec | MAE AE | MAE AE | ||
3.1. Non-Machine Learning (Non-ML) Methods
3.1.1. Mathematical Modeling
3.1.2. Direct Verification Methods
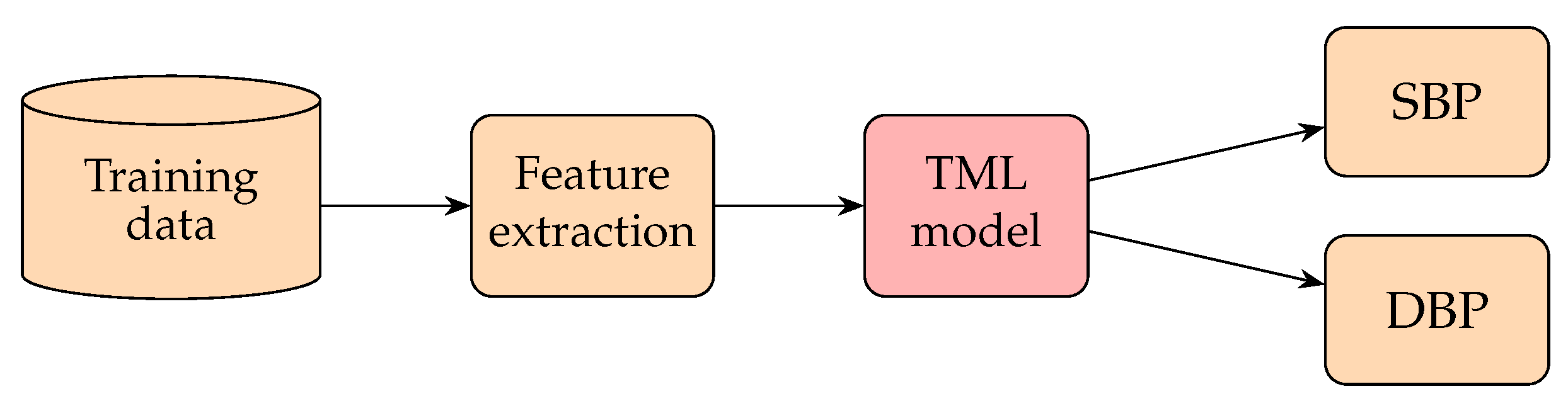
3.2. Traditional Machine Learning (TML) Methods
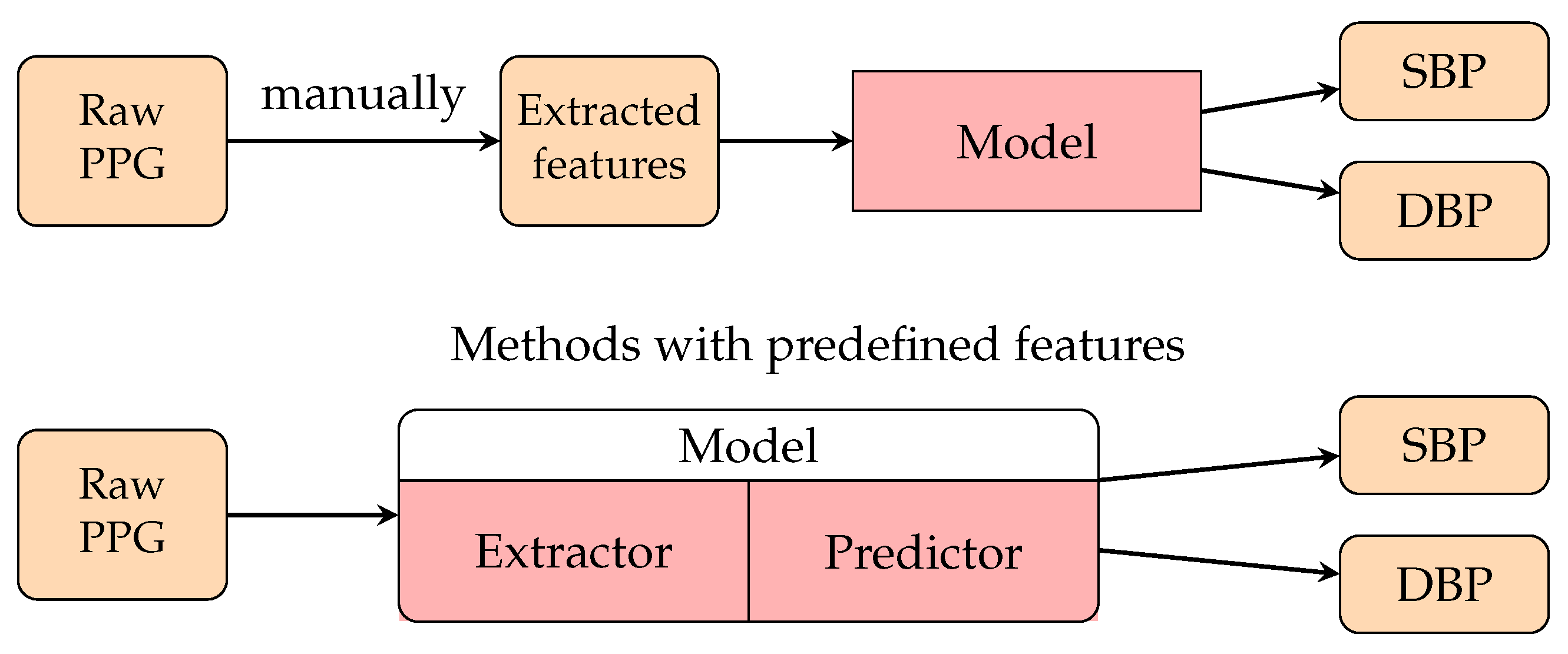
3.3. Deep Learning (DL) Methods
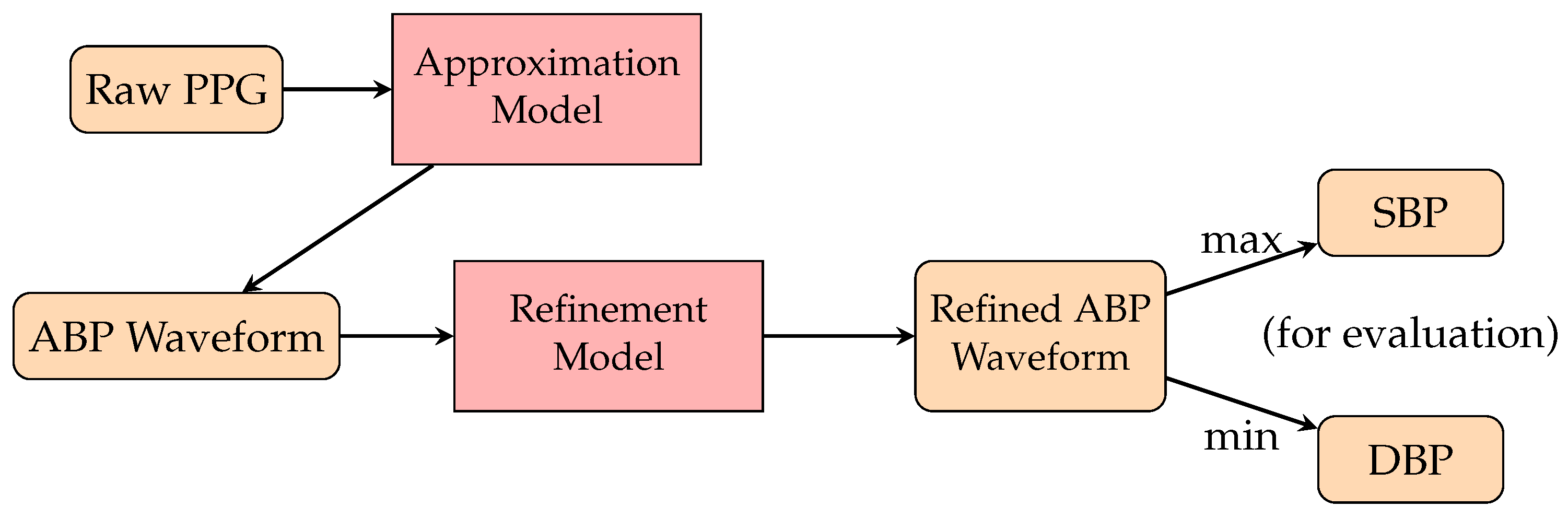
3.3.1. PPG Waveform-Based Methods
3.3.2. PTT-Based Method
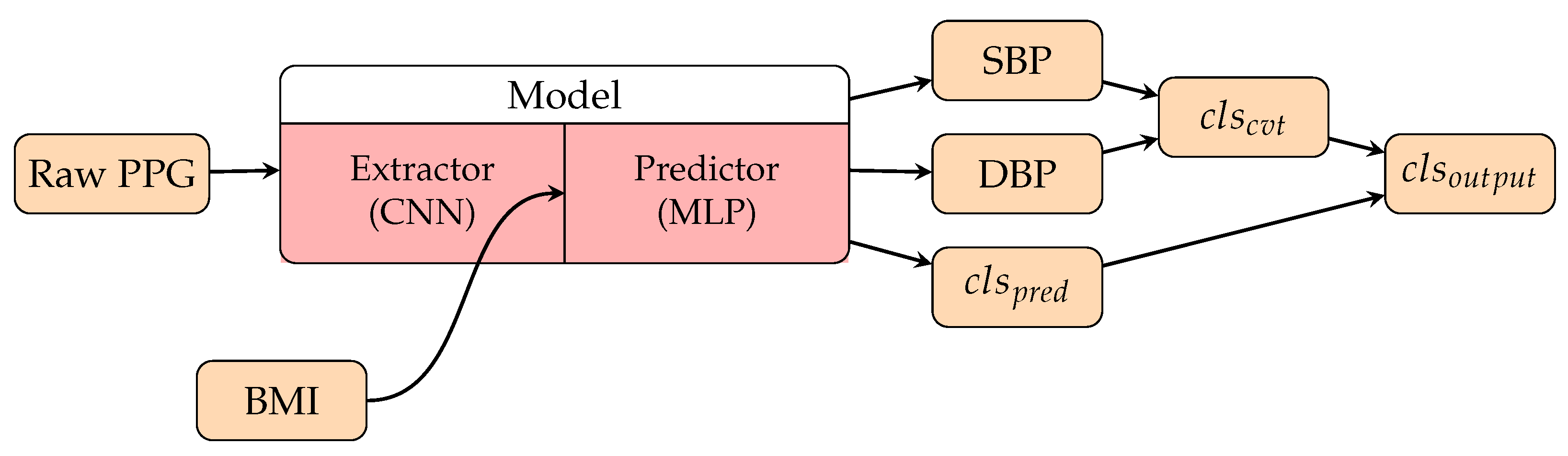
3.3.3. Personalization Factors in Deep Learning Methods
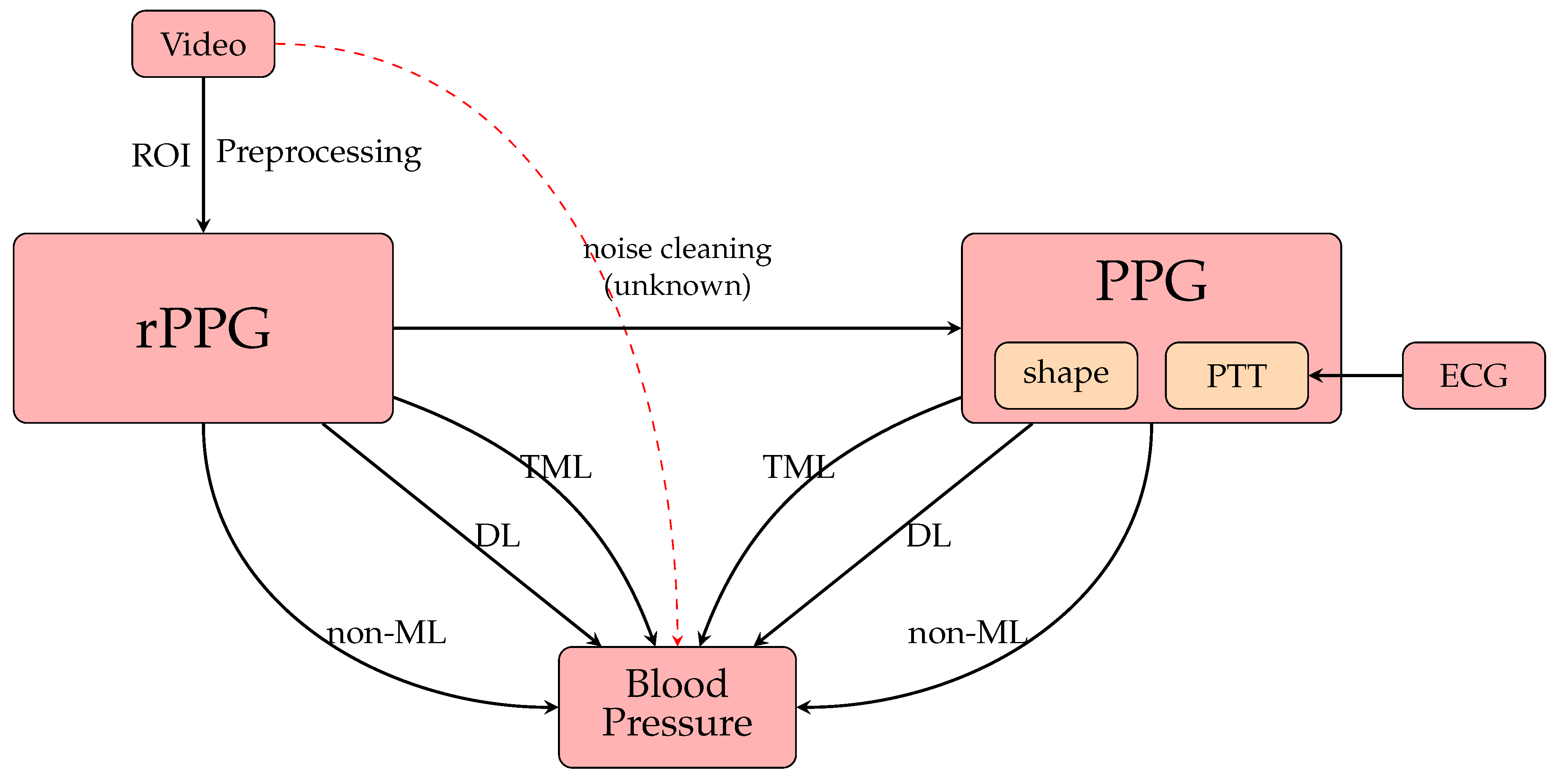
4. Contactless BP Measurement from rPPG Signals
| Year | Ref. | Dataset | Data Description | (1) | (2) | (3) | Preprocessing | Models | SBP | DBP |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-machine learning (non-ML) methods | ||||||||||
| 2015 | [214] | Private | 10 subjects | • | - | - | - | |||
| 2016 | [215] | Private | 7 subjects | • | iPTT | correlation estimation | - | |||
| 2019 | [216] | Private | 20 subjects | • | - | Time difference between 2 waveforms from a palm | - | |||
| 2020 | [217] | Private | 6 subjects | • | Modeling rPPG by Gaussian curves, pair filtering | Regression | AE | AE | ||
| Traditional machine learning (TML) methods | ||||||||||
| 2016 | [218] | Private | 45 subjects | • | PCA [219] | Regression | AE | AE | ||
| 2016 | [220] | Private | 3 subjects | • | ICA | Linear regression | AE | AE | ||
| 2017 | [221] | Private | 13 subjects (SBP , DBP | • | iPTT | KNN model with transfer learning | RMSE | RMSE | ||
| 2017 | [222] | Private | 45 subjects | • | PWV formula | 2nd order polynomial regression | AE | AE | ||
| 2018 | [223] | Private | 8 subjects having individual models | • | • | ICA | Linear regression | MAE of MBP | ||
| 2019 | [224] | Private | 10 subjects | • | Pulse wave detection | Lasso regression | Error of BP | |||
| 2019 | [225] | Private | 100 subjects (70 men and 30 women, age ) | • | • | JADE algorithm [226,227] | multiple linear regression | RMSE | RMSE | |
| 2021 | [228] | Private | 191 subjects (141 men and 50 women, age ) | • | Green channel, cheek and nose areas, Mallat algorithm, peak extraction | Support vector regression | AE | AE | ||
| Deep learning (DL) methods | ||||||||||
| 2017 | [229] | Private | 20 subjects without known blood pressure disease | • | ICA | Feedforward neural network | AER (afternoon) AER (evening) | AER (afternoon) AER (evening) | ||
| 2019 | [230] | Private | 1328 subjects (SBP , DBP ) | • | • | • | TOI, PCA | ANN | Error | Error |
| 2021 | [147] | Private | 50 subjects, subject independent splitting | • | • | Pretrained by PPG | AlexNet [148] | MAE | MAE | |
| 2021 | [147] | Private | 50 subjects, subject independent splitting | • | • | Pretrained by PPG | ResNet [134] | MAE | MAE | |
| 2021 | [147] | Private | 50 subjects, subject independent splitting | • | • | Pretrained by PPG | LSTM | MAE | MAE | |
| 2022 | [153] | Private | Train: 961 subjects Test: 177 subjects | • | • | CHROM [231] | InfoGAN [154] Encoder-decoder | AE | AE | |
| 2022 | [232] | Private | 10 subjects, subject mixed splitting | • | 2 spatial descriptors | ResNet [134] +CBAM [233] | MAE | MAE | ||
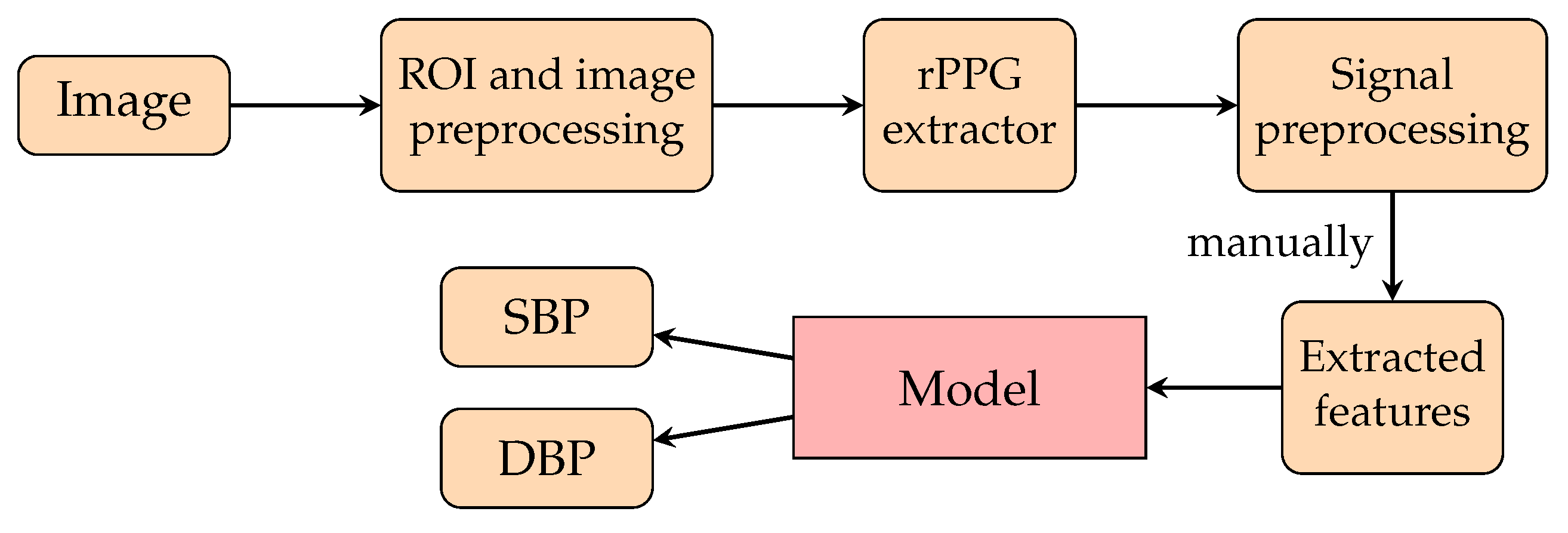
4.1. Non-Machine Learning (Non-ML) Methods
4.2. Traditional Machine Learning (TML) Methods
4.3. Deep Learning (DL) Methods
5. Discussion
6. Future Directions
6.1. Satisfactory Signal Quality
6.2. Public Dataset Enlargement
6.3. Effective Calibration by Personalization
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Abbreviation(s) | Full name(s) |
| AAMI | Association for the Advancement of Medical Instrumentation |
| ABP | Arterial blood pressure |
| Acc. | Accuracy |
| AE | Absolute error |
| ANN | Artificial neural network |
| ANSI | American National Standards Institute |
| BCG | Ballistocardiography |
| BHS | British Hypertension Society |
| BMI | Body mass index |
| BP | Blood pressure |
| CHD | Coronary heart disease |
| CNN | Convolutional neural network |
| CO | Cardiac output |
| CP | Cardiac period |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| CWT | Continuous wavelet transform |
| DBP | Diastolic blood pressure |
| DL | Deep learning |
| DNN | Deep neural network |
| DT | Diastolic time |
| DWT | Discrete wavelet transform |
| ECG | Electrocardiography |
| FCNN | Fully connected neural network |
| FFT | Fast Fourier transform |
| An inverse of the fast Fourier transform | |
| GAN | Generative adversarial network |
| GCG | Gyrocardiography |
| GRU | Gated recurrent unit |
| HT | Hypertension |
| IBP | Instant blood pressure |
| ICG | Impedance cardiography |
| IEEE | Institute of Electrical and Electronics Engineers |
| IMAR | Iterative metal artifact reduction |
| IPG | Impedance photoplethysmography |
| iPPG | Imaging photoplethysmography |
| IR | Infrared |
| JADE | Joint Approximation Diagonalisation of Eigen-matrices |
| JHS | Jackson Heart Study |
| LASSO | Least absolute shrinkage and selection operator |
| LMS filter | Least mean squares filter |
| LSTM | Long short-term memory |
| MAE | Mean absolute error |
| MAP | Mean arterial pressure |
| MAPD | Minimum absolute percentage difference |
| ME | Mean error |
| MERS | Middle East respiratory syndrome |
| MI | Myocardial infarction |
| MIC | Maximal information coefficient |
| MIMIC | Medical Information Mart for Intensive Care |
| ML | Machine learning |
| mNPV | Modified normalised pulse volume |
| NT | Normotension |
| OD | Oscillometric device |
| OLE | Ordinary least squares |
| PAT | Pulse arrival time |
| PCA | Principal component analysis |
| PCG | Phonocardiogram |
| PD | Phase difference |
| PEP | Pulse ejection period |
| PHT | Prehypertension |
| PIR | Photoplethysmogram intensity ratio |
| PPG | Photoplethysmography |
| PTT | Pulse transit time |
| PWA | Pulse wave analysis |
| PWV | Pulse wave velocity |
| RMSE | Root mean square error |
| RNN | Recurrent neural network |
| ROI | Regions of interest |
| rPPG | Remote photoplethysmography |
| RZS | Random zero sphygmomanometer |
| SARS | Severe acute respiratory syndrome |
| SBP | Systolic blood pressure |
| SBS | Strain-based sensor |
| SCG | Seismocardiography |
| Oxygen saturation | |
| SUT | Systolic upstroke time |
| SV | Support vector |
| SVM | Support vector machine |
| TML | Traditional machine learning |
| TOI | Transdermal optical imaging |
| TPR | Total peripheral resistance |
| UQVS | The University of Queensland vital signs |
References
- Shahoud, J.S.; Sanvictores, T.; Aeddula, N.R. Physiology, arterial pressure regulation. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Das, B.; Neupane, D.; Singh Gill, S.; Bir Singh, G. Factors affecting non-adherence to medical appointments among patients with hypertension at public health facilities in Punjab, India. J. Clin. Hypertens. 2021, 23, 713–719. [Google Scholar] [CrossRef]
- Albarwani, S.; Al-Siyabi, S.; Tanira, M.O. Prehypertension: Underlying pathology and therapeutic options. World J. Cardiol. 2014, 6, 728. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; Materson, B.J. Treating stage 2 hypertension. J. Clin. Hypertens. 2005, 7, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Salkic, S.; Batic-Mujanovic, O.; Ljuca, F.; Brkic, S. Clinical presentation of hypertensive crises in emergency medical services. Mater.-Socio-Med. 2014, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Alley, W.D.; Copelin, I.E. Hypertensive Urgency; StatPearls Publishing: Tampa, FL, USA, 2018. [Google Scholar]
- Renata, A.; Renata, C.; Suzanne, O.; Maria, A.; George, B.; Dan, B.; Anna, D.; Guido, G.; Jens, J.; Neil, P.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef]
- Sharma, S.; Hashmi, M.F.; Bhattacharya, P.T. Hypotension. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Koya, H.H.; Paul, M. Shock. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Verhaeverbeke, I.; Mets, T. Drug-induced orthostatic hypotension in the elderly. Drug Saf. 1997, 17, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Tewelde, S.Z.; Liu, S.S.; Winters, M.E. Cardiogenic shock. Cardiol. Clin. 2018, 36, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.M.; Jamal, S.F. Essential hypertension. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Oparil, S.; Zaman, M.A.; Calhoun, D.A. Pathogenesis of hypertension. Ann. Intern. Med. 2003, 139, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.A.; Kumar, S.K.; De Caro, M. Hypertensive crisis. Cardiol. Rev. 2010, 18, 102–107. [Google Scholar] [CrossRef]
- Booth, J. A short history of blood pressure measurement. Proc. R. Soc. Med. 1977, 70, 793–799. [Google Scholar] [CrossRef]
- Pickering, D.; Stevens, S. How to measure and record blood pressure. Community Eye Health 2013, 26, 76. [Google Scholar] [PubMed]
- Rennie, A.; McGregor-Schuerman, M.; Dale, I.; Robinson, C.; McWilliam, R. Mercury poisoning after spillage at home from a sphygmomanometer on loan from hospital. BMJ 1999, 319, 366–367. [Google Scholar] [CrossRef]
- Nunn, D.E.; Beveridge, R.W. Apparatus and Method for Measuring Blood Pressure. U.S. Patent 4,427,013, 24 January 1984. [Google Scholar]
- Steinman, J.; Barszczyk, A.; Sun, H.S.; Lee, K.; Feng, Z.P. Smartphones and Video Cameras: Future Methods for Blood Pressure Measurement. Front. Digit. Health 2021, 3, 770096. [Google Scholar] [CrossRef] [PubMed]
- Viera, A.J.; Lingley, K.; Hinderliter, A.L. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: A cross-sectional repeated measures study. BMC Med. Res. Methodol. 2011, 11, 59. [Google Scholar] [CrossRef]
- Ellis, D.; Miyashita, Y. Primary hypertension and special aspects of hypertension in older children and adolescents. Adolesc. Health Med. Ther. 2011, 2, 45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yarows, S.A.; Julius, S.; Pickering, T.G. Home blood pressure monitoring. Arch. Intern. Med. 2000, 160, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Yarows, S.A. What is the Cost of Measuring a Blood Pressure? Ann. Clin. Hypertens. 2018, 2, 59–66. [Google Scholar]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef]
- Reisner, A.; Shaltis, P.A.; McCombie, D.; Asada, H.H.; Warner, D.S.; Warner, M.A. Utility of the photoplethysmogram in circulatory monitoring. J. Am. Soc. Anesthesiol. 2008, 108, 950–958. [Google Scholar] [CrossRef]
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef]
- Hosanee, M.; Chan, G.; Welykholowa, K.; Cooper, R.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Menon, C.; Lovell, N.H.; et al. Cuffless single-site photoplethysmography for blood pressure monitoring. J. Clin. Med. 2020, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Athaya, T.; Choi, S. A Review of Noninvasive Methodologies to Estimate the Blood Pressure Waveform. Sensors 2022, 22, 3953. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.; Wei, X. Monitoring heart and respiratory rates at radial artery based on PPG. Optik 2013, 124, 3954–3956. [Google Scholar] [CrossRef]
- Fuster, B.; Arana-Iñiguez, R.; Folle, J.; Ferreiro, C.; Coleman, W. Circulation patterns during clamping of the carotid arteries as determined by cutaneous carotid photoplethysmography. Acta Neurol. Latinoam. 1971, 17, 273–293. [Google Scholar] [PubMed]
- Magro, G.; Mantovani, A. Exploration of the peripheral arteriolar circulation by calibrated photoplethysmography. Minerva Med. 1952, 43, 1153–1160. [Google Scholar] [PubMed]
- Abramowitz, H.B.; Queral, L.A.; Flinn, W.R.; Nora, P.F.; Peterson, L.K.; Bergan, J.J.; Yao, J.S. The use of photoplethysmography in the assessment of venous insufficiency: A comparison to venous pressure measurements. Surgery 1979, 86, 434–441. [Google Scholar]
- Kaufmann, S.; Malhotra, A.; Ardelt, G.; Hunsche, N.; Bresslein, K.; Kusche, R.; Ryschka, M. A System for in-Ear Pulse Wave Measurements. arXiv 2020, arXiv:2008.03105. [Google Scholar]
- Heydari, F.; Ebrahim, M.P.; Redoute, J.M.; Joe, K.; Walker, K.; Yuce, M.R. A chest-based continuous cuffless blood pressure method: Estimation and evaluation using multiple body sensors. Inf. Fusion 2020, 54, 119–127. [Google Scholar] [CrossRef]
- Kachuee, M.; Kiani, M.M.; Mohammadzade, H.; Shabany, M. Cuffless blood pressure estimation algorithms for continuous health-care monitoring. IEEE Trans. Biomed. Eng. 2016, 64, 859–869. [Google Scholar] [CrossRef]
- Wong, M.Y.M.; Pickwell-MacPherson, E.; Zhang, Y.T.; Cheng, J.C. The effects of pre-ejection period on post-exercise systolic blood pressure estimation using the pulse arrival time technique. Eur. J. Appl. Physiol. 2011, 111, 135–144. [Google Scholar] [CrossRef]
- Zhang, G.; Cottrell, A.C.; Henry, I.C.; McCombie, D.B. Assessment of pre-ejection period in ambulatory subjects using seismocardiogram in a wearable blood pressure monitor. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 3386–3389. [Google Scholar]
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.H.; Abbott, D.; Lim, K.; Ward, R. The use of photoplethysmography for assessing hypertension. NPJ Digit. Med. 2019, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Abbott, D.; Howard, N.; Lim, K.; Ward, R.; Elgendi, M. How effective is pulse arrival time for evaluating blood pressure? Challenges and recommendations from a study using the MIMIC database. J. Clin. Med. 2019, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gao, M.; Xu, D.; Olivier, N.B.; Mukkamala, R. Pulse arrival time is not an adequate surrogate for pulse transit time as a marker of blood pressure. J. Appl. Physiol. 2011, 111, 1681–1686. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Hypertension assessment via ECG and PPG signals: An evaluation using MIMIC database. Diagnostics 2018, 8, 65. [Google Scholar] [CrossRef]
- Poon, C.; Zhang, Y. Cuff-less and noninvasive measurements of arterial blood pressure by pulse transit time. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 5877–5880. [Google Scholar]
- Chen, Y.; Wen, C.; Tao, G.; Bi, M. A new methodology of continuous and noninvasive blood pressure measurement by pulse wave velocity. In Proceedings of the 2010 11th International Conference on Control Automation Robotics & Vision, Singapore, 7–10 December 2010; pp. 1018–1023. [Google Scholar]
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Carek, A.M.; Mukkamala, R.; Inan, O.T.; Hahn, J.O. Ballistocardiogram as proximal timing reference for pulse transit time measurement: Potential for cuffless blood pressure monitoring. IEEE Trans. Biomed. Eng. 2015, 62, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.O.; Carek, A.M.; Kim, C.S.; Ashouri, H.; Inan, O.T.; Hahn, J.O.; Mukkamala, R. Weighing scale-based pulse transit time is a superior marker of blood pressure than conventional pulse arrival time. Sci. Rep. 2016, 6, 39273. [Google Scholar] [CrossRef]
- Yang, C.; Tavassolian, N. Pulse transit time measurement using seismocardiogram, photoplethysmogram, and acoustic recordings: Evaluation and comparison. IEEE J. Biomed. Health Inform. 2017, 22, 733–740. [Google Scholar] [CrossRef]
- Inan, O.T.; Migeotte, P.F.; Park, K.S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and seismocardiography: A review of recent advances. IEEE J. Biomed. Health Inform. 2014, 19, 1414–1427. [Google Scholar] [CrossRef]
- Yang, C.; Dong, Y.; Chen, Y.; Tavassolian, N. A Low-cost, Smartphone-only Pulse Transit Time Measurement System Using Cardio-mechanical Signals and Optical Sensors. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 1–4. [Google Scholar]
- Ibrahim, B.; Nathan, V.; Jafari, R. Exploration and validation of alternate sensing methods for wearable continuous pulse transit time measurement using optical and bioimpedance modalities. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 2051–2055. [Google Scholar]
- Huynh, T.H.; Jafari, R.; Chung, W.Y. Noninvasive cuffless blood pressure estimation using pulse transit time and impedance plethysmography. IEEE Trans. Biomed. Eng. 2018, 66, 967–976. [Google Scholar] [CrossRef]
- Welykholowa, K.; Hosanee, M.; Chan, G.; Cooper, R.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Menon, C.; Lovell, N.H.; et al. Multimodal photoplethysmography-based approaches for improved detection of hypertension. J. Clin. Med. 2020, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Wong, K.L.; Chin, J.W.; Chan, T.T.; So, R.H. Deep learning methods for remote heart rate measurement: A review and future research agenda. Sensors 2021, 21, 6296. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, K.H.; Abedi, G.R.; Midgley, C.M.; Alkhamis, A.; Alsaqer, T.; Almoaddi, A.; Algwizani, A.; Ghazal, S.S.; Assiri, A.M.; Jokhdar, H.; et al. Diabetes mellitus, hypertension, and death among 32 patients with MERS-CoV infection, Saudi Arabia. Emerg. Infect. Dis. 2020, 26, 166. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, B.; Grimm, D.; Krüger, M.; Kopp, S.; Infanger, M.; Wehland, M. SARS-CoV-2 and hypertension. Physiol. Rep. 2021, 9, e14800. [Google Scholar] [CrossRef]
- Dasari, A.; Prakash, S.K.A.; Jeni, L.A.; Tucker, C.S. Evaluation of biases in remote photoplethysmography methods. NPJ Digit. Med. 2021, 4, 91. [Google Scholar] [CrossRef] [PubMed]
- Lokendra, B.; Puneet, G. AND-rPPG: A novel denoising-rPPG network for improving remote heart rate estimation. Comput. Biol. Med. 2022, 141, 105146. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, A.; Natarajan, K.; Yavarimanesh, M.; Mukkamala, R. An iPhone application for blood pressure monitoring via the oscillometric finger pressing method. Sci. Rep. 2018, 8, 13136. [Google Scholar] [CrossRef]
- Kumar, R.; Dubey, P.; Zafer, A.; Kumar, A.; Yadav, S. Past, present and future of blood pressure measuring instruments and their calibration. Measurement 2021, 172, 108845. [Google Scholar] [CrossRef]
- Sun, Y.; Thakor, N. Photoplethysmography revisited: From contact to noncontact, from point to imaging. IEEE Trans. Biomed. Eng. 2015, 63, 463–477. [Google Scholar] [CrossRef]
- Pickering, T.G. Principles and techniques of blood pressure measurement. Cardiol. Clin. 2002, 20, 207–223. [Google Scholar] [CrossRef]
- Ogedegbe, G.; Pickering, T. Principles and techniques of blood pressure measurement. Cardiol. Clin. 2010, 28, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Young, T. Hydraulic investigations, subservient to an intended Croonian Lecture on the motion of the blood. In Abstracts of the Papers Printed in the Philosophical Transactions of the Royal Society of London; The Royal Society London: London, UK, 1832; pp. 298–300. [Google Scholar]
- Moens, A. Over de Voortplantingssnelheid van den Pols [On the Speed of Propagation of the Pulse]. Ph.D. Thesis, University of Leiden, Leiden, The Netherlands, 1877. [Google Scholar]
- Moens, A.I. Die Pulscurve; Brill: Leiden, The Netherlands, 1878. [Google Scholar]
- Korteweg, V.D. Ueber die Fortpflanzungsgeschwindigkeit des Schalles in elastischen Röhren. Ann. Der Phys. 1878, 241, 525–542. [Google Scholar] [CrossRef]
- Atabek, H.; Lew, H. Wave propagation through a viscous incompressible fluid contained in an initially stressed elastic tube. Biophys. J. 1966, 6, 481–503. [Google Scholar] [CrossRef]
- Lazović, B.; Mazić, S.; Zikich, D.; Žikić, D. The mathematical model of the radial artery blood pressure waveform through monitoring of the age-related changes. Wave Motion 2015, 56, 14–21. [Google Scholar] [CrossRef]
- Žikić, D.; Žikić, K. Wave propagation through a viscous fluid-filled elastic tube under initial pressure: Theoretical and biophysical model. Eur. Biophys. J. 2022, 51, 365–374. [Google Scholar] [CrossRef]
- Eigler, N. Hemodynamics; Milnor, W.R., Ed.; Williams & Wilkins: Baltimore, MD, USA, 1989; 417p, ISBN 0-683-06049-X. [Google Scholar]
- Mukkamala, R.; Hahn, J.O.; Inan, O.T.; Mestha, L.K.; Kim, C.S.; Töreyin, H.; Kyal, S. Toward ubiquitous blood pressure monitoring via pulse transit time: Theory and practice. IEEE Trans. Biomed. Eng. 2015, 62, 1879–1901. [Google Scholar] [CrossRef]
- Frank, O. Die Elastizität der Blutgefäße; Oldenbourg: Saint Petersburg, Russia, 1920. [Google Scholar]
- Bramwell, J.C.; Hill, A. Velocity of transmission of the pulse-wave: And elasticity of arteries. Lancet 1922, 199, 891–892. [Google Scholar] [CrossRef]
- Wesseling, K.; Jansen, J.; Settels, J.; Schreuder, J. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J. Appl. Physiol. 1993, 74, 2566–2573. [Google Scholar] [CrossRef]
- Wesseling, K. A simple device for the continuous measurement of cardiac output. Its model basis and experimental varification. Adv. Cardiovasc. Phys. 1983, 5, 16–52. [Google Scholar]
- Langewouters, G.; Wesseling, K.; Goedhard, W. The static elastic properties of 45 human thoracic and 20 abdominal aortas in vitro and the parameters of a new model. J. Biomech. 1984, 17, 425–435. [Google Scholar] [CrossRef]
- Esmaili, A.; Kachuee, M.; Shabany, M. Nonlinear cuffless blood pressure estimation of healthy subjects using pulse transit time and arrival time. IEEE Trans. Instrum. Meas. 2017, 66, 3299–3308. [Google Scholar]
- Natarajan, K.; Yavarimanesh, M.; Wang, W.; Mukkamala, R. Camera-based blood pressure monitoring. In Contactless Vital Signs Monitoring; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–148. [Google Scholar]
- Gao, M.; Cheng, H.M.; Sung, S.H.; Chen, C.H.; Olivier, N.B.; Mukkamala, R. Estimation of pulse transit time as a function of blood pressure using a nonlinear arterial tube-load model. IEEE Trans. Biomed. Eng. 2016, 64, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, T.; Doering, K.; Kropf-Sanchen, C.; Rüdiger, S.; Blanta, I.; Stoiber, K.; Rottbauer, W.; Schumann, C. Pulse transit time and blood pressure during cardiopulmonary exercise tests. Physiol. Res. 2014, 63, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Geddes, L.; Voelz, M.; Babbs, C.; Bourland, J.; Tacker, W. Pulse transit time as an indicator of arterial blood pressure. Psychophysiology 1981, 18, 71–74. [Google Scholar]
- Mühlsteff, J.; Aubert, X.L.; Schuett, M. Cuffless estimation of systolic blood pressure for short effort bicycle tests: The prominent role of the pre-ejection period. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 5088–5092. [Google Scholar]
- Gesche, H.; Grosskurth, D.; Küchler, G.; Patzak, A. Continuous blood pressure measurement by using the pulse transit time: Comparison to a cuff-based method. Eur. J. Appl. Physiol. 2012, 112, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.S.; Nathan, V.; Zong, C.; Akinbola, E.; Aroul, A.L.P.; Philipose, L.; Soundarapandian, K.; Shi, X.; Jafari, R. BioWatch—A wrist watch based signal acquisition system for physiological signals including blood pressure. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2286–2289. [Google Scholar]
- Mukherjee, R.; Ghosh, S.; Gupta, B.; Chakravarty, T. A literature review on current and proposed technologies of noninvasive blood pressure measurement. Telemed. E-Health 2018, 24, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T. Cuffless blood pressure monitors: Principles, standards and approval for medical use. IEICE Trans. Commun. 2021, 104, 580–586. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Pan, L.; Zhang, J.; Xiang, Y. SoK: An Overview of PPG’s Application in Authentication. arXiv 2022, arXiv:2201.11291. [Google Scholar]
- Ding, X.R.; Zhao, N.; Yang, G.Z.; Pettigrew, R.I.; Lo, B.; Miao, F.; Li, Y.; Liu, J.; Zhang, Y.T. Continuous blood pressure measurement from invasive to unobtrusive: Celebration of 200th birth anniversary of Carl Ludwig. IEEE J. Biomed. Health Inform. 2016, 20, 1455–1465. [Google Scholar] [CrossRef]
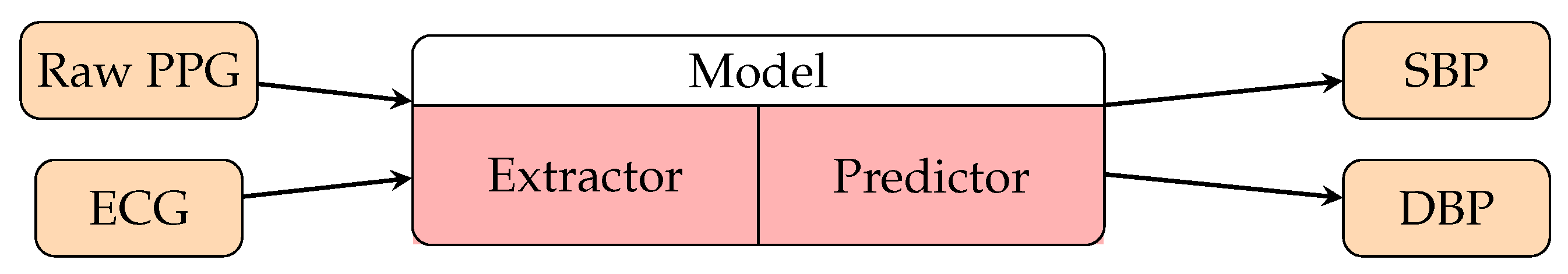
- Huang, B.; Chen, W.; Lin, C.L.; Juang, C.F.; Wang, J. MLP-BP: A novel framework for cuffless blood pressure measurement with PPG and ECG signals based on MLP-Mixer neural networks. Biomed. Signal Process. Control 2022, 73, 103404. [Google Scholar] [CrossRef]
- Mishra, B.; Nirala, N.S. A Survey on Denoising Techniques of PPG Signal. In Proceedings of the 2020 IEEE International Conference for Innovation in Technology (INOCON), Bangluru, India, 6–8 November 2020; pp. 1–8. [Google Scholar]
- Chan, K.; Zhang, Y. Adaptive reduction of motion artifact from photoplethysmographic recordings using a variable step-size LMS filter. In Proceedings of the SENSORS, Orlando, FL, USA, 12–14 June 2002; Volume 2, pp. 1343–1346. [Google Scholar]
- Lee, H.W.; Lee, J.W.; Jung, W.G.; Lee, G.K. The periodic moving average filter for removing motion artifacts from PPG signals. Int. J. Control. Autom. Syst. 2007, 5, 701–706. [Google Scholar]
- Ram, M.R.; Madhav, K.V.; Krishna, E.H.; Komalla, N.R.; Reddy, K.A. On the performance of AS-LMS based adaptive filter for reduction of motion artifacts from PPG signals. In Proceedings of the 2011 IEEE International Instrumentation and Measurement Technology Conference, Hangzhou, China, 10–12 May 2011; pp. 1–4. [Google Scholar]
- Islam, M.T.; Tanvir Ahmed, S.; Zabir, I.; Shahnaz, C.; Fattah, S.A. Cascade and parallel combination (CPC) of adaptive filters for estimating heart rate during intensive physical exercise from photoplethysmographic signal. Healthc. Technol. Lett. 2018, 5, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Han, J.; Baek, H.J.; Shin, J.H.; Park, K.S.; Yi, W.J. Improved elimination of motion artifacts from a photoplethysmographic signal using a Kalman smoother with simultaneous accelerometry. Physiol. Meas. 2010, 31, 1585. [Google Scholar] [CrossRef]
- Lee, Y.K.; Jo, J.; Lee, Y.; Shin, H.S.; Kwon, O.W. Particle filter-based noise reduction of PPG signals for robust emotion recognition. In Proceedings of the 2012 IEEE International Conference on Consumer Electronics (ICCE), Las Vegas, NV, USA, 13–16 January 2012; pp. 598–599. [Google Scholar]
- Salehizadeh, S.; Dao, D.K.; Chong, J.W.; McManus, D.; Darling, C.; Mendelson, Y.; Chon, K.H. Photoplethysmograph signal reconstruction based on a novel motion artifact detection-reduction approach. Part II: Motion and noise artifact removal. Ann. Biomed. Eng. 2014, 42, 2251–2263. [Google Scholar] [CrossRef]
- Joseph, G.; Joseph, A.; Titus, G.; Thomas, R.M.; Jose, D. Photoplethysmogram (PPG) signal analysis and wavelet de-noising. In Proceedings of the 2014 Annual International Conference on Emerging Research Areas: Magnetics, Machines and Drives (AICERA/iCMMD), Kottayam, India, 24–26 July 2014; pp. 1–5. [Google Scholar]
- Joseph, G.; Titus, G. ICA based System with WPT for Removal of Motion Artifacts in Photoplethysmogram (PPG) Signal. Int. J. Eng. Res. Technol. 2014, 3, 897–900. [Google Scholar]
- Bai, T.; Li, D.; Wang, H.; Pang, Y.; Li, G.; Lin, J.; Zhou, Q.; Jeon, G. A PPG signal de-noising method based on the DTCWT and the morphological filtering. In Proceedings of the 2016 12th International Conference on Signal-Image Technology & Internet-Based Systems (SITIS), Naples, Italy, 28 November–1 December 2016; pp. 503–506. [Google Scholar]
- Awodeyi, A.E.; Alty, S.R.; Ghavami, M. Median filter approach for removal of baseline wander in photoplethysmography signals. In Proceedings of the 2013 European Modelling Symposium, Manchester, UK, 20–22 November 2013; pp. 261–264. [Google Scholar]
- Timimi, A.A.; Ali, M.M.; Chellappan, K. A novel AMARS technique for baseline wander removal applied to photoplethysmogram. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 627–639. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Wu, J.; Tang, B.; Li, D. Comparison and noise suppression of the transmitted and reflected photoplethysmography signals. BioMed Res. Int. 2018, 2018, 4523593. [Google Scholar] [CrossRef]
- Park, C.; Shin, H.; Lee, B. Blockwise PPG enhancement based on time-variant zero-phase harmonic notch filtering. Sensors 2017, 17, 860. [Google Scholar] [CrossRef]
- Chandrasekhar, A.; Kim, C.S.; Naji, M.; Natarajan, K.; Hahn, J.O.; Mukkamala, R. Smartphone-based blood pressure monitoring via the oscillometric finger-pressing method. Sci. Transl. Med. 2018, 10, eaap8674. [Google Scholar] [CrossRef]
- Plante, T.B.; Urrea, B.; MacFarlane, Z.T.; Blumenthal, R.S.; Miller, E.R.; Appel, L.J.; Martin, S.S. Validation of the instant blood pressure smartphone app. JAMA Intern. Med. 2016, 176, 700–702. [Google Scholar] [CrossRef]
- AuraLife: Instant Blood Pressure; Apple Inc.: Cupertino, CA, USA, 2022.
- Raichle, C.J.; Eckstein, J.; Lapaire, O.; Leonardi, L.; Brasier, N.; Vischer, A.S.; Burkard, T. Performance of a blood pressure smartphone app in pregnant women: The iPARR Trial (iPhone app compared with standard RR measurement). Hypertension 2018, 71, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Doerr, M.; Weber, S.; Birkemeyer, R.; Leonardi, L.; Winterhalder, C.; Raichle, C.J.; Brasier, N.; Burkard, T.; Eckstein, J. iPhone App compared with standard blood pressure measurement—The iPARR trial. Am. Heart J. 2021, 233, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.; Dantu, R.; Jonnada, S.; Thiyagaraja, S.; Subbu, K.P. Cuffless differential blood pressure estimation using smart phones. IEEE Trans. Biomed. Eng. 2012, 60, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, A.; Sinha, A.; Pal, A. Estimation of blood pressure levels from reflective photoplethysmograph using smart phones. In Proceedings of the 13th IEEE International Conference on BioInformatics and BioEngineering, Chania, Greece, 10–13 November 2013; pp. 1–5. [Google Scholar]
- Visvanathan, A.; Banerjee, R.; Dutta Choudhury, A.; Sinha, A.; Kundu, S. Smart phone based blood pressure indicator. In Proceedings of the 4th ACM MobiHoc Workshop on Pervasive Wireless Healthcare, Philadelphia, PA, USA, 11 August 2014; pp. 19–24. [Google Scholar]
- Liu, D.; Görges, M.; Jenkins, S.A. University of Queensland vital signs dataset: Development of an accessible repository of anesthesia patient monitoring data for research. Anesth. Analg. 2012, 114, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.C.; Wittek, P.; Zhao, L.; Jiang, W.J. Data-driven estimation of blood pressure using photoplethysmographic signals. In Proceedings of the 2016 38th Annual International Conference of the IEEE (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 766–769. [Google Scholar]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Duan, K.; Qian, Z.; Atef, M.; Wang, G. A feature exploration methodology for learning based cuffless blood pressure measurement using photoplethysmography. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 6385–6388. [Google Scholar]
- Zhang, Y.; Feng, Z. A SVM method for continuous blood pressure estimation from a PPG signal. In Proceedings of the 9th International Conference on Machine Learning and Computing, Singapore, 24–26 February 2017; pp. 128–132. [Google Scholar]
- Datta, S.; Choudhury, A.D.; Chowdhury, A.; Banerjee, T.; Banerjee, R.; Bhattacharya, S.; Pal, A.; Mandana, K.M. Novel statistical post processing to improve blood pressure estimation from smartphone photoplethysmogram. In Proceedings of the First International Workshop on Human-Centered Sensing, Networking, and Systems, Delft, The Netherlands, 5 November 2017; pp. 31–36. [Google Scholar]
- Wang, E.J.; Zhu, J.; Jain, M.; Lee, T.J.; Saba, E.; Nachman, L.; Patel, S.N. Seismo: Blood pressure monitoring using built-in smartphone accelerometer and camera. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems, Montreal, QC, Canada, 21–26 April 2018; pp. 1–9. [Google Scholar]
- Dey, J.; Gaurav, A.; Tiwari, V.N. InstaBP: Cuff-less blood pressure monitoring on smartphone using single PPG sensor. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5002–5005. [Google Scholar]
- Mousavi, S.S.; Firouzmand, M.; Charmi, M.; Hemmati, M.; Moghadam, M.; Ghorbani, Y. Blood pressure estimation from appropriate and inappropriate PPG signals using A whole-based method. Biomed. Signal Process. Control 2019, 47, 196–206. [Google Scholar] [CrossRef]
- Nemcova, A.; Jordanova, I.; Varecka, M.; Smisek, R.; Marsanova, L.; Smital, L.; Vitek, M. Monitoring of heart rate, blood oxygen saturation, and blood pressure using a smartphone. Biomed. Signal Process. Control 2020, 59, 101928. [Google Scholar] [CrossRef]
- Kurylyak, Y.; Lamonaca, F.; Grimaldi, D. A Neural Network-based method for continuous blood pressure estimation from a PPG signal. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Minneapolis, MN, USA, 6–9 May 2013; pp. 280–283. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Lamonaca, F.; Barbe, K.; Kurylyak, Y.; Grimaldi, D.; Van Moer, W.; Furfaro, A.; Spagnuolo, V. Application of the artificial neural network for blood pressure evaluation with smartphones. In Proceedings of the 2013 IEEE 7th International Conference on Intelligent Data Acquisition and Advanced Computing Systems (IDAACS), Berlin, Germany, 12–14 September, 2013; Volume 1, pp. 408–412. [Google Scholar]
- Kachuee, M.; Kiani, M.M.; Mohammadzade, H.; Shabany, M. Cuff-less high-accuracy calibration-free blood pressure estimation using pulse transit time. In Proceedings of the 2015 IEEE International Symposium on Circuits and Systems (ISCAS), Lisbon, Portugal, 24–27 May 2015; pp. 1006–1009. [Google Scholar]
- Xing, X.; Sun, M. Optical blood pressure estimation with photoplethysmography and FFT-based neural networks. Biomed. Opt. Express 2016, 7, 3007–3020. [Google Scholar] [CrossRef]
- Gaurav, A.; Maheedhar, M.; Tiwari, V.N.; Narayanan, R. Cuff-less PPG based continuous blood pressure monitoring—A smartphone based approach. In Proceedings of the 2016 38th annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 607–610. [Google Scholar]
- Su, P.; Ding, X.R.; Zhang, Y.T.; Liu, J.; Miao, F.; Zhao, N. Long-term blood pressure prediction with deep recurrent neural networks. In Proceedings of the 2018 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018; pp. 323–328. [Google Scholar]
- Radha, M.; de Groot, K.; Rajani, N.; Wong, C.; Kobold, N.; Vos, V.; Fonseca, P.; Mastellos, N.; Wark, P.A.; Velthoven, N.; et al. Wrist-Worn Blood Pressure Tracking in Healthy Free-Living Individuals Using Neural Networks. arXiv 2018, arXiv:1805.09121. [Google Scholar]
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Photoplethysmography and deep learning: Enhancing hypertension risk stratification. Biosensors 2018, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.S.; Hasan, M.K. Cuffless blood pressure estimation from electrocardiogram and photoplethysmogram using waveform based ANN-LSTM network. Biomed. Signal Process. Control 2019, 51, 382–392. [Google Scholar] [CrossRef]
- Slapničar, G.; Mlakar, N.; Luštrek, M. Blood pressure estimation from photoplethysmogram using a spectro-temporal deep neural network. Sensors 2019, 19, 3420. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Baek, S.; Jang, J.; Yoon, S. End-to-end blood pressure prediction via fully convolutional networks. IEEE Access 2019, 7, 185458–185468. [Google Scholar] [CrossRef]
- El Hajj, C.; Kyriacou, P.A. Cuffless and continuous blood pressure estimation from ppg signals using recurrent neural networks. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 4269–4272. [Google Scholar]
- Baek, S.; Jang, J.; Cho, S.H.; Choi, J.M.; Yoon, S. Blood pressure prediction by a smartphone sensor using fully convolutional networks. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 188–191. [Google Scholar]
- Han, C.; Gu, M.; Yu, F.; Huang, R.; Huang, X.; Cui, L. Calibration-free Blood Pressure Assessment Using An Integrated Deep Learning Method. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Korea, 16–19 December 2020; pp. 1001–1005. [Google Scholar]
- Liang, Y.; Chen, Z.; Liu, G.; Elgendi, M. A new, short-recorded photoplethysmogram dataset for blood pressure monitoring in China. Sci. Data 2018, 5, 180020. [Google Scholar] [CrossRef]
- Panwar, M.; Gautam, A.; Biswas, D.; Acharyya, A. PP-Net: A deep learning framework for PPG-based blood pressure and heart rate estimation. IEEE Sens. J. 2020, 20, 10000–10011. [Google Scholar] [CrossRef]
- Ibtehaz, N.; Rahman, M.S. Ppg2abp: Translating Photoplethysmogram (ppg) Signals to Arterial Blood Pressure (abp) Waveforms Using Fully Convolutional Neural Networks. arXiv 2020, arXiv:2005.01669. [Google Scholar]
- Baker, S.; Xiang, W.; Atkinson, I. A hybrid neural network for continuous and non-invasive estimation of blood pressure from raw electrocardiogram and photoplethysmogram waveforms. Comput. Methods Programs Biomed. 2021, 207, 106191. [Google Scholar] [CrossRef]
- Pu, Y.; Xie, X.; Xiong, L.; Zhang, H. Cuff-Less Blood Pressure Estimation from Electrocardiogram and Photoplethysmography Based on VGG19-LSTM Network. In Computer Methods in Medicine and Health Care; IOS Press: Amsterdam, The Netherlands, 2021; pp. 33–46. [Google Scholar]
- Brophy, E.; De Vos, M.; Boylan, G.; Ward, T. Estimation of Continuous Blood Pressure from PPG via a Federated Learning Approach. Sensors 2021, 21, 6311. [Google Scholar] [CrossRef]
- Tazarv, A.; Levorato, M. A deep learning approach to predict blood pressure from ppg signals. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual Conference, 1–5 November 2021; pp. 5658–5662. [Google Scholar]
- Jeong, D.U.; Lim, K.M. Combined deep CNN–LSTM network-based multitasking learning architecture for noninvasive continuous blood pressure estimation using difference in ECG-PPG features. Sci. Rep. 2021, 11, 13539. [Google Scholar] [CrossRef]
- Schrumpf, F.; Frenzel, P.; Aust, C.; Osterhoff, G.; Fuchs, M. Assessment of Non-Invasive Blood Pressure Prediction from PPG and rPPG Signals Using Deep Learning. Sensors 2021, 21, 6022. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Athaya, T.; Choi, S. An estimation method of continuous non-invasive arterial blood pressure waveform using photoplethysmography: A U-Net architecture-based approach. Sensors 2021, 21, 1867. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.R.; Vedanth, S.; Poojah, G.; Abhishek, K.; Kumar, M.N.; Vijayaraghavan, V. BP-Net: Efficient Deep Learning for Continuous Arterial Blood Pressure Estimation using Photoplethysmogram. In Proceedings of the 2021 20th IEEE International Conference on Machine Learning and Applications (ICMLA), virtually online, 13–15 December 2021; pp. 1495–1500. [Google Scholar]
- Wang, D.; Yang, X.; Liu, X.; Ma, L.; Li, L.; Wang, W. Photoplethysmography-based blood pressure estimation combining filter-wrapper collaborated feature selection with LASSO-LSTM model. IEEE Trans. Instrum. Meas. 2021, 70, 4006914. [Google Scholar] [CrossRef]
- Harfiya, L.N.; Chang, C.C.; Li, Y.H. Continuous blood pressure estimation using exclusively photopletysmography by LSTM-based signal-to-signal translation. Sensors 2021, 21, 2952. [Google Scholar] [CrossRef]
- Wu, B.F.; Chiu, L.W.; Wu, Y.C.; Lai, C.C.; Chu, P.H. Contactless Blood Pressure Measurement via Remote Photoplethysmography With Synthetic Data Generation Using Generative Adversarial Network. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR) Workshops, New Orleans, LA, USA, 18–24 June 2022; pp. 2130–2138. [Google Scholar]
- Chen, X.; Duan, Y.; Houthooft, R.; Schulman, J.; Sutskever, I.; Abbeel, P. Infogan: Interpretable representation learning by information maximizing generative adversarial nets. Adv. Neural Inf. Process. Syst. 2016, 29. [Google Scholar]
- Jager, G.N.; Westerhof, N.; Noordergraaf, A. Oscillatory flow impedance in electrical analog of arterial system: Representation of sleeve effect and non-Newtonian properties of blood. Circ. Res. 1965, 16, 121–133. [Google Scholar] [CrossRef]
- Fogliardi, R.; Burattini, R.; Shroff, S.; Campbell, K. Fit to diastolic arterial pressure by third-order lumped model yields unreliable estimates of arterial compliance. Med. Eng. Phys. 1996, 18, 225–233. [Google Scholar] [CrossRef]
- Pietrabissa, R.; Mantero, S.; Marotta, T.; Menicanti, L. A lumped parameter model to evaluate the fluid dynamics of different coronary bypasses. Med. Eng. Phys. 1996, 18, 477–484. [Google Scholar] [CrossRef]
- Hellevik, L.; Segers, P.; Stergiopulos, N.; Irgens, F.; Verdonck, P.; Thompson, C.; Lo, K.; Miyagishima, R.; Smiseth, O. Mechanism of pulmonary venous pressure and flow waves. Heart Vessel. 1999, 14, 67–71. [Google Scholar] [CrossRef]
- Segers, P.; Stergiopulos, N.; Schreuder, J.J.; Westerhof, B.E.; Westerhof, N. Left ventricular wall stress normalization in chronic pressure-overloaded heart: A mathematical model study. Am. J. Physiol.-Heart Circ. Physiol. 2000, 279, H1120–H1127. [Google Scholar] [CrossRef]
- Olufsen, M.S.; Nadim, A.; Lipsitz, L.A. Dynamics of cerebral blood flow regulation explained using a lumped parameter model. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 282, R611–R622. [Google Scholar] [CrossRef]
- Liang, F.; Liu, H. A closed-loop lumped parameter computational model for human cardiovascular system. JSME Int. J. Ser. C Mech. Syst. Mach. Elem. Manuf. 2005, 48, 484–493. [Google Scholar] [CrossRef]
- Huberts, W.; Bosboom, E.M.H.; van de Vosse, F.N. A lumped model for blood flow and pressure in the systemic arteries based on an approximate velocity profile function. Math. Biosci. Eng. 2009, 6, 27. [Google Scholar] [PubMed]
- Kokalari, I.; Karaja, T.; Guerrisi, M. Review on lumped parameter method for modeling the blood flow in systemic arteries. Sci. Res. 2013, 60, 27458. [Google Scholar] [CrossRef]
- Frank, O.; des arteriellen Pulses, D.G. Erste Abhandlung. Mathematische Analyse. Z. Fur Biol. 1899, 37, 485–526. [Google Scholar]
- Westerhof, N.; Elzinga, G.; Sipkema, P. An artificial arterial system for pumping hearts. J. Appl. Physiol. 1971, 31, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Stergiopulos, N.; Meister, J.J.; Westerhof, N. Simple and accurate way for estimating total and segmental arterial compliance: The pulse pressure method. Ann. Biomed. Eng. 1994, 22, 392–397. [Google Scholar] [CrossRef]
- Stergiopulos, N.; Westerhof, B.E.; Westerhof, N. Total arterial inertance as the fourth element of the windkessel model. Am. J. Physiol.-Heart Circ. Physiol. 1999, 276, H81–H88. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, N.; Bosman, F.; De Vries, C.J.; Noordergraaf, A. Analog studies of the human systemic arterial tree. J. Biomech. 1969, 2, 121–143. [Google Scholar] [CrossRef]
- Burattini, R.; Natalucci, S. Complex and frequency-dependent compliance of viscoelastic windkessel resolves contradictions in elastic windkessels. Med. Eng. Phys. 1998, 20, 502–514. [Google Scholar] [CrossRef]
- Womersley, J.R. Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J. Physiol. 1955, 127, 553. [Google Scholar] [CrossRef] [PubMed]
- Womersley, J.R. An Elastic Tube Theory of Pulse Transmission and Oscillatory Flow in Mammalian Arteries; Technical Report; Aerospace Research Labs Wright-Patterson AFB: Dayton, OH, USA, 1957. [Google Scholar]
- Papageorgiou, G.; Jones, N. Physical modelling of the arterial wall. Part 1: Testing of tubes of various materials. J. Biomed. Eng. 1987, 9, 153–156. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Jones, N. Physical modelling of the arterial wall. Part 2: Simulation of the non-linear elasticity of the arterial wall. J. Biomed. Eng. 1987, 9, 216–221. [Google Scholar] [CrossRef]
- Olufsen, M.S.; Peskin, C.S.; Kim, W.Y.; Pedersen, E.M.; Nadim, A.; Larsen, J. Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann. Biomed. Eng. 2000, 28, 1281–1299. [Google Scholar] [CrossRef]
- Sherwin, S.J.; Formaggia, L.; Peiro, J.; Franke, V. Computational modelling of 1D blood flow with variable mechanical properties and its application to the simulation of wave propagation in the human arterial system. Int. J. Numer. Methods Fluids 2003, 43, 673–700. [Google Scholar] [CrossRef]
- Alastruey, J.; Parker, K.; Peiró, J.; Sherwin, S.J. Can the modified Allen’s test always detect sufficient collateral flow in the hand? A computational study. Comput. Methods Biomech. Biomed. Eng. 2006, 9, 353–361. [Google Scholar] [CrossRef]
- Bessems, D.; Rutten, M.; Van De Vosse, F. A wave propagation model of blood flow in large vessels using an approximate velocity profile function. J. Fluid Mech. 2007, 580, 145–168. [Google Scholar] [CrossRef]
- Alastruey, J.; Passerini, T.; Formaggia, L.; Peiró, J. Physical determining factors of the arterial pulse waveform: Theoretical analysis and calculation using the 1-D formulation. J. Eng. Math. 2012, 77, 19–37. [Google Scholar] [CrossRef]
- Taylor, C.A.; Hughes, T.J.; Zarins, C.K. Computational investigations in vascular disease. Comput. Phys. 1996, 10, 224–232. [Google Scholar] [CrossRef]
- Formaggia, L.; Gerbeau, J.F.; Nobile, F.; Quarteroni, A. On the coupling of 3D and 1D Navier–Stokes equations for flow problems in compliant vessels. Comput. Methods Appl. Mech. Eng. 2001, 191, 561–582. [Google Scholar] [CrossRef]
- Karamanoglu, M.; Gallagher, D.E.; Avolio, A.P.; O’Rourke, M.F. Functional origin of reflected pressure waves in a multibranched model of the human arterial system. Am. J. Physiol.-Heart Circ. Physiol. 1994, 267, H1681–H1688. [Google Scholar] [CrossRef]
- Karamanoglu, M.; Gallagher, D.E.; Avolio, A.P.; O’Rourke, M.F. Pressure wave propagation in a multibranched model of the human upper limb. Am. J. Physiol.-Heart Circ. Physiol. 1995, 269, H1363–H1369. [Google Scholar] [CrossRef]
- John, L. Forward electrical transmission line model of the human arterial system. Med. Biol. Eng. Comput. 2004, 42, 312–321. [Google Scholar] [CrossRef]
- Žikić, D.; Stojadinović, B.; Nestorović, Z. Biophysical modeling of wave propagation phenomena: Experimental determination of pulse wave velocity in viscous fluid-filled elastic tubes in a gravitation field. Eur. Biophys. J. 2019, 48, 407–411. [Google Scholar] [CrossRef]
- Nagasawa, T.; Iuchi, K.; Takahashi, R.; Tsunomura, M.; de Souza, R.P.; Ogawa-Ochiai, K.; Tsumura, N.; Cardoso, G.C. Blood Pressure Estimation by Photoplethysmogram Decomposition into Hyperbolic Secant Waves. Appl. Sci. 2022, 12, 1798. [Google Scholar] [CrossRef]
- Liu, H.; Ivanov, K.; Wang, Y.; Wang, L. Toward a smartphone application for estimation of pulse transit time. Sensors 2015, 15, 27303–27321. [Google Scholar] [CrossRef]
- Junior, A.D.; Murali, S.; Rincon, F.; Atienza, D. Methods for reliable estimation of pulse transit time and blood pressure variations using smartphone sensors. Microprocess. Microsyst. 2016, 46, 84–95. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, Y. Continuous and noninvasive estimation of arterial blood pressure using a photoplethysmographic approach. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No. 03CH37439), Cancun, Mexico, 17–21 September 2003; Volume 4, pp. 3153–3156. [Google Scholar]
- Suzuki, S.; Oguri, K. Cuffless and non-invasive systolic blood pressure estimation for aged class by using a photoplethysmograph. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 1327–1330. [Google Scholar]
- Peng, R.C.; Yan, W.R.; Zhang, N.L.; Lin, W.H.; Zhou, X.L.; Zhang, Y.T. Cuffless and continuous blood pressure estimation from the heart sound signals. Sensors 2015, 15, 23653–23666. [Google Scholar] [CrossRef]
- Yoon, Y.Z.; Kang, J.M.; Kwon, Y.; Park, S.; Noh, S.; Kim, Y.; Park, J.; Hwang, S.W. Cuff-less blood pressure estimation using pulse waveform analysis and pulse arrival time. IEEE J. Biomed. Health Inform. 2017, 22, 1068–1074. [Google Scholar] [CrossRef]
- Matsumura, K.; Rolfe, P.; Toda, S.; Yamakoshi, T. Cuffless blood pressure estimation using only a smartphone. Sci. Rep. 2018, 8, 7298. [Google Scholar] [CrossRef] [PubMed]
- Tabei, F.; Gresham, J.M.; Askarian, B.; Jung, K.; Chong, J.W. Cuff-less blood pressure monitoring system using smartphones. IEEE Access 2020, 8, 11534–11545. [Google Scholar] [CrossRef]
- Chowdhury, M.H.; Shuzan, M.N.I.; Chowdhury, M.E.; Mahbub, Z.B.; Uddin, M.M.; Khandakar, A.; Reaz, M.B.I. Estimating blood pressure from the photoplethysmogram signal and demographic features using machine learning techniques. Sensors 2020, 20, 3127. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Petrie, J.; Littler, W.; de Swiet, M.; Padfield, P.L.; O’Malley, K.; Jamieson, M.; Altman, D.; Bland, M.; Atkins, N. The British Hypertension Society protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systems. J. Hypertens. 1990, 8, 607–619. [Google Scholar] [CrossRef]
- ANSI/AAMI SP 10-1987; American National Standards for Electronic or Automated Sphygmomanometers. Association for the Advancement of Medical Instrumentation: Arlington, VA, USA, 1987.
- Kim, J.Y.; Cho, B.H.; Im, S.M.; Jeon, M.J.; Kim, I.Y.; Kim, S.I. Comparative study on artificial neural network with multiple regressions for continuous estimation of blood pressure. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 6942–6945. [Google Scholar]
- Donoho, D.L.; Johnstone, J.M. Ideal spatial adaptation by wavelet shrinkage. biometrika 1994, 81, 425–455. [Google Scholar] [CrossRef]
- Donoho, D.L. De-noising by soft-thresholding. IEEE Trans. Inf. Theory 1995, 41, 613–627. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X. Contactless Vital Signs Monitoring; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Lu, Y.; Wang, C.; Meng, M.Q.H. Video-based contactless blood pressure estimation: A review. In Proceedings of the 2020 IEEE International Conference on Real-time Computing and Robotics (RCAR), Asahikawa, Japan, 28–29 September 2020; pp. 62–67. [Google Scholar]
- Bajraktari, F.; Liu, J.; Pott, P.P. Methods of Contactless Blood Pressure Measurement. Curr. Dir. Biomed. Eng. 2022, 8, 439–442. [Google Scholar] [CrossRef]
- Wang, W.; Shan, C. Impact of makeup on remote-ppg monitoring. Biomed. Phys. Eng. Express 2020, 6, 035004. [Google Scholar] [CrossRef]
- Kumar, M.; Veeraraghavan, A.; Sabharwal, A. DistancePPG: Robust non-contact vital signs monitoring using a camera. Biomed. Opt. Express 2015, 6, 1565–1588. [Google Scholar] [CrossRef]
- Nowara, E.M.; McDuff, D.; Veeraraghavan, A. A meta-analysis of the impact of skin tone and gender on non-contact photoplethysmography measurements. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops, Seattle, WA, USA, 14–19 June 2020; pp. 284–285. [Google Scholar]
- Moço, A.V.; Stuijk, S.; de Haan, G. New insights into the origin of remote PPG signals in visible light and infrared. Sci. Rep. 2018, 8, 8501. [Google Scholar] [CrossRef]
- Nowara, E.M.; Marks, T.K.; Mansour, H.; Veeraraghavan, A. Near-infrared imaging photoplethysmography during driving. IEEE Trans. Intell. Transp. Syst. 2020, 23, 3589–3600. [Google Scholar] [CrossRef]
- Amelard, R.; Scharfenberger, C.; Kazemzadeh, F.; Pfisterer, K.J.; Lin, B.S.; Clausi, D.A.; Wong, A. Feasibility of long-distance heart rate monitoring using transmittance photoplethysmographic imaging (PPGI). Sci. Rep. 2015, 5, 14637. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Y.; Zhao, Y.; Dong, L.; Hui, M.; Liu, M.; Liu, X. Robust Imaging Photoplethysmography in Long-Distance Motion. IEEE Photonics J. 2020, 12, 3900512. [Google Scholar] [CrossRef]
- Mironenko, Y.; Kalinin, K.; Kopeliovich, M.; Petrushan, M. Remote photoplethysmography: Rarely considered factors. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops, Seattle, WA, USA, 14–19 June 2020; pp. 296–297. [Google Scholar]
- Tohma, A.; Nishikawa, M.; Hashimoto, T.; Yamazaki, Y.; Sun, G. Evaluation of Remote Photoplethysmography Measurement Conditions toward Telemedicine Applications. Sensors 2021, 21, 8357. [Google Scholar] [CrossRef]
- Estepp, J.R.; Blackford, E.B.; Meier, C.M. Recovering pulse rate during motion artifact with a multi-imager array for non-contact imaging photoplethysmography. In Proceedings of the 2014 IEEE International Conference on Systems, Man, and Cybernetics (SMC), San Diego, CA, USA, 5–8 October 2014; pp. 1462–1469. [Google Scholar]
- Van Gastel, M.; Stuijk, S.; de Haan, G. Motion robust remote-PPG in infrared. IEEE Trans. Biomed. Eng. 2015, 62, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yoshioka, M.; Ozawa, J. Non-contact pulse transit time measurement using imaging camera, and its relation to blood pressure. In Proceedings of the 2015 14th IAPR International Conference on Machine Vision Applications (MVA), Tokyo, Japan, 18–22 May 2015; pp. 414–417. [Google Scholar]
- Jeong, I.C.; Finkelstein, J. Introducing contactless blood pressure assessment using a high speed video camera. J. Med. Syst. 2016, 40, 77. [Google Scholar] [CrossRef]
- Sugita, N.; Yoshizawa, M.; Abe, M.; Tanaka, A.; Homma, N.; Yambe, T. Contactless technique for measuring blood-pressure variability from one region in video plethysmography. J. Med. Biol. Eng. 2019, 39, 76–85. [Google Scholar] [CrossRef]
- Fan, X.; Ye, Q.; Yang, X.; Choudhury, S.D. Robust blood pressure estimation using an RGB camera. J. Ambient Intell. Humaniz. Comput. 2020, 11, 4329–4336. [Google Scholar] [CrossRef]
- Jain, M.; Deb, S.; Subramanyam, A.V. Face video based touchless blood pressure and heart rate estimation. In Proceedings of the 2016 IEEE 18th International Workshop on Multimedia Signal Processing (MMSP), Montreal, QC, Canada, 21–23 September 2016; pp. 1–5. [Google Scholar]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Secerbegovic, A.; Bergsland, J.; Halvorsen, P.S.; Suljanovic, N.; Mujcic, A.; Balasingham, I. Blood pressure estimation using video plethysmography. In Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, Czech Republic, 13–16 April 2016; pp. 461–464. [Google Scholar]
- Huang, P.W.; Lin, C.H.; Chung, M.L.; Lin, T.M.; Wu, B.F. Image based contactless blood pressure assessment using Pulse Transit Time. In Proceedings of the 2017 International Automatic Control Conference (CACS), Pingtung, Taiwan, 12–15 November 2017; pp. 1–6. [Google Scholar]
- Khong, W.L.; Rao, N.S.V.K.; Mariappan, M. Blood pressure measurements using non-contact video imaging techniques. In Proceedings of the 2017 IEEE 2nd International Conference on Automatic Control and Intelligent Systems (I2CACIS), Kota Kinabalu, Malaysia, 21 October 2017; pp. 35–40. [Google Scholar]
- Oiwa, K.; Bando, S.; Nozawa, A. Contactless blood pressure sensing using facial visible and thermal images. Artif. Life Robot. 2018, 23, 387–394. [Google Scholar] [CrossRef]
- Adachi, Y.; Edo, Y.; Ogawa, R.; Tomizawa, R.; Iwai, Y.; Okumura, T. Noncontact blood pressure monitoring technology using facial photoplethysmograms. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 2411–2415. [Google Scholar]
- Zhou, Y.; Ni, H.; Zhang, Q.; Wu, Q. The noninvasive blood pressure measurement based on facial images processing. IEEE Sens. J. 2019, 19, 10624–10634. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Q.; Zhou, Y.; Wu, X.; Ou, Y.; Zhou, H. Webcam-based, non-contact, real-time measurement for the physiological parameters of drivers. Measurement 2017, 100, 311–321. [Google Scholar] [CrossRef]
- Huang, X. An improved FastICA algorithm for blind signal separation and its application. In Proceedings of the 2012 International Conference on Image Analysis and Signal Processing, Huangzhou, China, 9–11 November 2012; pp. 1–4. [Google Scholar]
- Rong, M.; Li, K. A blood pressure prediction method based on imaging photoplethysmography in combination with machine learning. Biomed. Signal Process. Control 2021, 64, 102328. [Google Scholar] [CrossRef]
- Patil, O.R.; Gao, Y.; Li, B.; Jin, Z. CamBP: A camera-based, non-contact blood pressure monitor. In Proceedings of the 2017 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2017 ACM International Symposium on Wearable Computers, Maui, HI, USA, 11–15 September 2017; pp. 524–529. [Google Scholar]
- Luo, H.; Yang, D.; Barszczyk, A.; Vempala, N.; Wei, J.; Wu, S.J.; Zheng, P.P.; Fu, G.; Lee, K.; Feng, Z.P. Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ. Cardiovasc. Imaging 2019, 12, e008857. [Google Scholar] [CrossRef]
- De Haan, G.; Jeanne, V. Robust pulse rate from chrominance-based rPPG. IEEE Trans. Biomed. Eng. 2013, 60, 2878–2886. [Google Scholar] [CrossRef]
- Iuchi, K.; Miyazaki, R.; Cardoso, G.C.; Ogawa-Ochiai, K.; Tsumura, N. Remote Estimation of Continuous Blood Pressure by a Convolutional Neural Network Trained on Spatial Patterns of Facial Pulse Waves. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR) Workshops, New Orleans, Louisiana, 19–24 June 2022; pp. 2139–2145. [Google Scholar]
- Woo, S.; Park, J.; Lee, J.Y.; Kweon, I.S. CBAM: Convolutional Block Attention Module. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018. [Google Scholar]
- Sugita, N.; Obara, K.; Yoshizawa, M.; Abe, M.; Tanaka, A.; Homma, N. Techniques for estimating blood pressure variation using video images. In Proceedings of the 2015 37th annual international conference of the IEEE International Conference of the IEEE (EMBC), Milan, Italy, 25–29 August 2015; pp. 4218–4221. [Google Scholar]
- Poh, M.Z.; McDuff, D.J.; Picard, R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express 2010, 18, 10762–10774. [Google Scholar] [CrossRef]
- Lewandowska, M.; Rumiński, J.; Kocejko, T.; Nowak, J. Measuring pulse rate with a webcam—A non-contact method for evaluating cardiac activity. In Proceedings of the 2011 Federated Conference on Computer Science and Information Systems (FedCSIS), Szczecin, Poland, 18–21 September 2011; pp. 405–410. [Google Scholar]
- Kwon, S.; Kim, J.; Lee, D.; Park, K. ROI analysis for remote photoplethysmography on facial video. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Szczecin, Poland, 18–21 September 2015; pp. 4938–4941. [Google Scholar]
- Kim, D.Y.; Lee, K.; Sohn, C.B. Assessment of ROI Selection for Facial Video-Based rPPG. Sensors 2021, 21, 7923. [Google Scholar] [CrossRef]
- Wong, K.L.; Chin, J.W.; Chan, T.T.; Odinaev, I.; Suhartono, K.; Tianqu, K.; So, R.H. Optimising rPPG Signal Extraction by Exploiting Facial Surface Orientation. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, Louisiana, 19–24 June 2022; pp. 2165–2171. [Google Scholar]
- Johnson, A.E.; Pollard, T.J.; Shen, L.; Lehman, L.w.H.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Anthony Celi, L.; Mark, R.G. MIMIC-III, a freely accessible critical care database. Sci. Data 2016, 3, 160035. [Google Scholar] [CrossRef] [PubMed]
- Němcová, A. Monitoring of heart rate, blood oxygen saturation and blood pressure using smartphone. Biomed. Signal Process. Control. 2020, 59, 101928. [Google Scholar] [CrossRef]
- Soleymani, M.; Lichtenauer, J.; Pun, T.; Pantic, M. A multimodal database for affect recognition and implicit tagging. IEEE Trans. Affect. Comput. 2011, 3, 42–55. [Google Scholar] [CrossRef]
- Bobbia, S.; Macwan, R.; Benezeth, Y.; Mansouri, A.; Dubois, J. Unsupervised skin tissue segmentation for remote photoplethysmography. Pattern Recognit. Lett. 2019, 124, 82–90. [Google Scholar] [CrossRef]
- Salvi, P. Pulse Waves; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Ding, X.; Yan, B.P.; Zhang, Y.T.; Liu, J.; Zhao, N.; Tsang, H.K. Pulse transit time based continuous cuffless blood pressure estimation: A new extension and a comprehensive evaluation. Sci. Rep. 2017, 7, 11554. [Google Scholar] [CrossRef] [PubMed]
- Seals, S.R.; Colantonio, L.D.; Tingle, J.V.; Shimbo, D.; Correa, A.; Griswold, M.E.; Muntner, P. Calibration of blood pressure measurements in the Jackson Heart Study. Blood Press. Monit. 2019, 24, 130. [Google Scholar] [CrossRef] [PubMed]
- Barvik, D.; Cerny, M.; Penhaker, M.; Noury, N. Noninvasive Continuous Blood Pressure Estimation from Pulse Transit Time: A review of the calibration models. IEEE Rev. Biomed. Eng. 2021, 15, 138–151. [Google Scholar] [CrossRef]
- Chen, J.W.; Huang, H.K.; Fang, Y.T.; Lin, Y.T.; Li, S.Z.; Chen, B.W.; Lo, Y.C.; Chen, P.C.; Wang, C.F.; Chen, Y.Y. A Data-Driven Model with Feedback Calibration Embedded Blood Pressure Estimator Using Reflective Photoplethysmography. Sensors 2022, 22, 1873. [Google Scholar] [CrossRef]
- Leitner, J.; Chiang, P.H.; Dey, S. Personalized blood pressure estimation using photoplethysmography: A transfer learning approach. IEEE J. Biomed. Health Inform. 2021, 26, 218–228. [Google Scholar] [CrossRef]
- Bresch, E.; Derkx, R.; Paulussen, I.; Noordergraaf, G.J.; Schmitt, L.; Muehlsteff, J. Personalization of pulse arrival time based blood pressure surrogates through single spot check measurements. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 5437–5440. [Google Scholar]
- Liu, X.; Jiang, Z.; Fromm, J.; Xu, X.; Patel, S.; McDuff, D. MetaPhys: Few-shot adaptation for non-contact physiological measurement. In Proceedings of the Conference on Health, Inference, and Learning, Virtual Event USA, 8–10 April 2021; pp. 154–163. [Google Scholar]
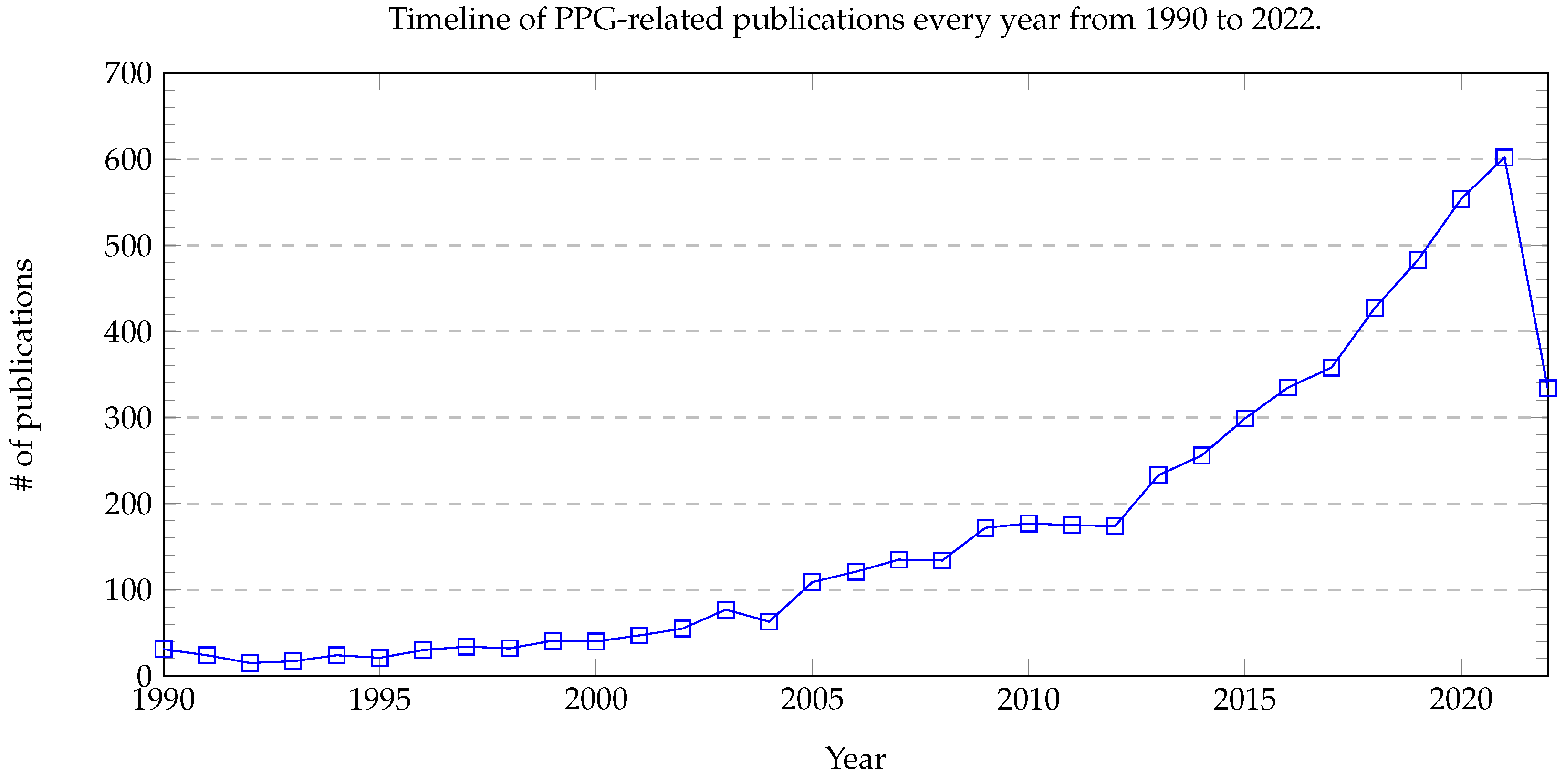
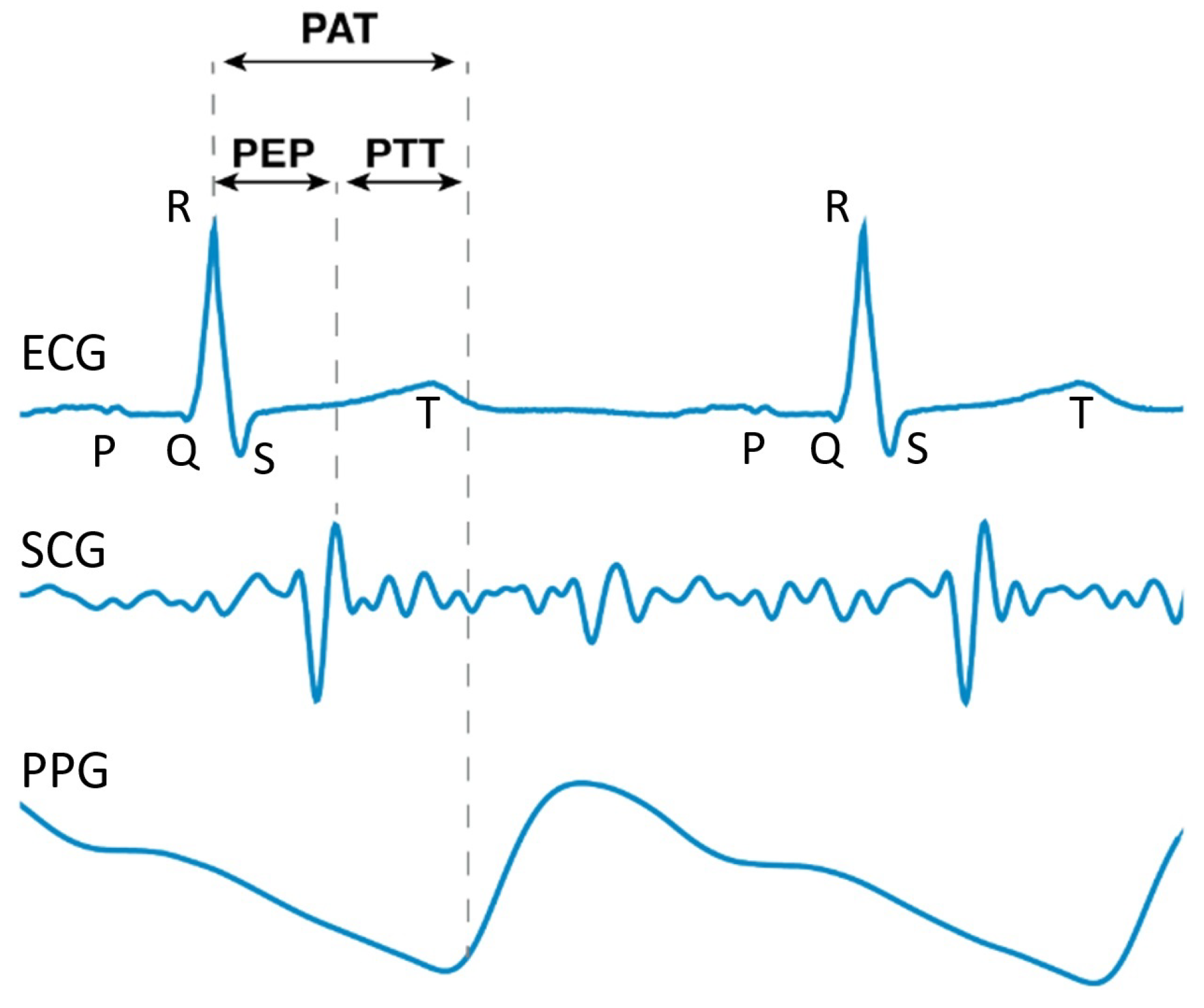
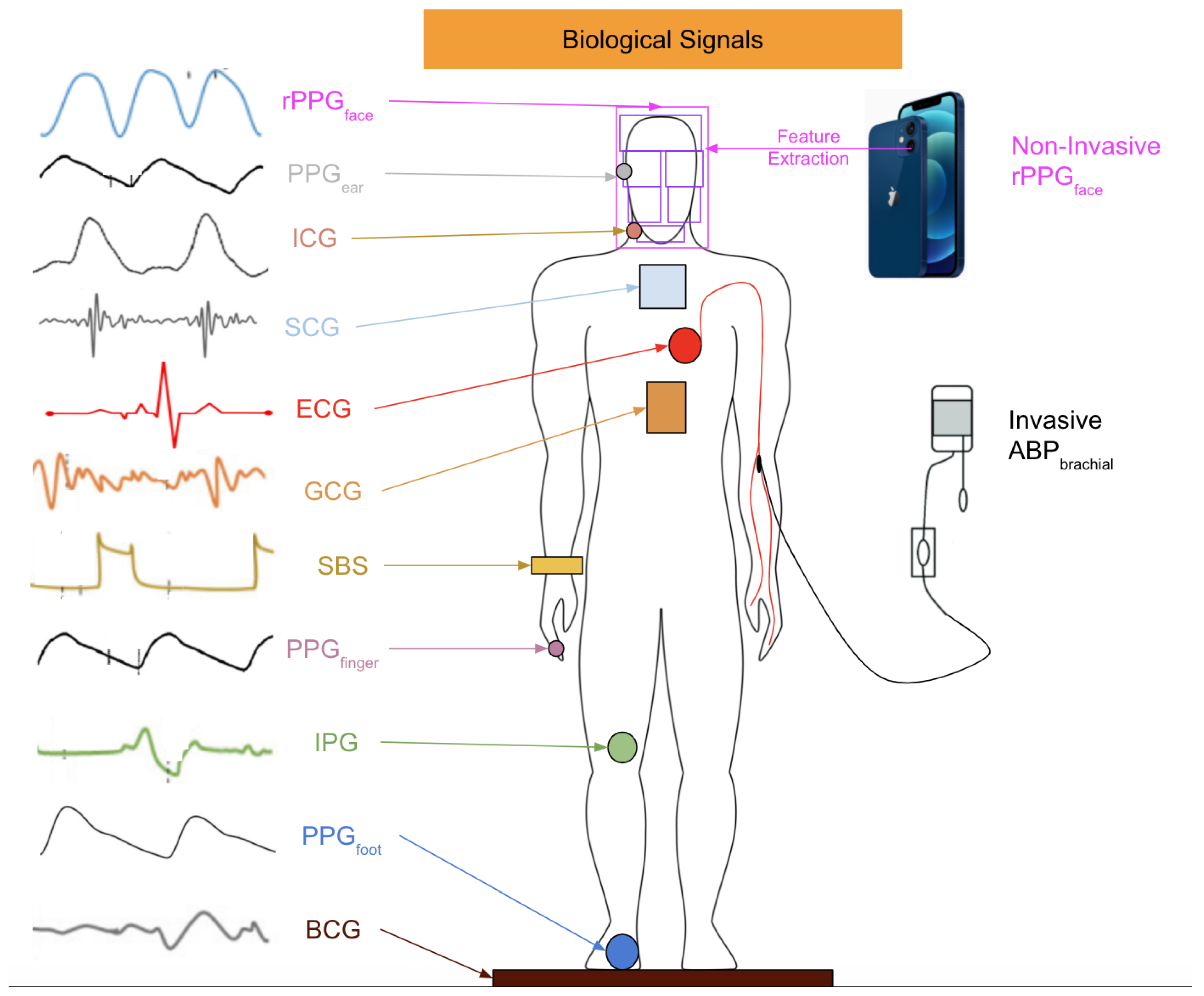





| CV Stages | SBP (mmHg) | DBP (mmHg) | Additional Biological Information |
|---|---|---|---|
| Hypotension | <90 | <60 | Low BP leading to oxygen deprivation in organs, resulting in tissue necrosis. May induce shock and cardiac arrest [9]. Symptoms: dizziness, tiredness, back pain, heart palpitations, etc. Usually a common side effect of drug therapies (e.g., beta blockers and diuretics) [10]. |
| Normotension (NT) | 90–120 | 60–80 | BP may fluctuate based on poor lifestyle habits. Examples: lack of exercise, fatty diet, anxiety, insomnia, alcoholism, aging, etc. [11]. |
| Prehypertension (PHT) | 121–139 | 81–89 | BP is higher than normal but not within range of stage 1 hypertension, also known as high-normal BP. Known as the upper range of healthy BP that determines the future risk of clinically overt hypertension [3], it is further divided into its own 1st and 2nd stages to further define hypertensive risk parameters [12]. |
| Hypertension I (HT-I) | 140–159 | 90–99 | BP is high enough to be a risk factor. Occurs when the heart is overly stressed. Treatment may not be required, but drug therapy will significantly reduce BP. SBP/DBP range refers to daytime BP as sleep naturally lowers SBP and DBP [13]. |
| Hypertension II (HT-II) | 160–179 | 100–109 | BP is very high, and CVD is very likely. Lack of treatment may likely result in end organ failure and permanent damage. Commonly found in elderly people. SBP control primarily determines risk of CVD and death [4]. |
| Hypertensive Crisis or Urgency | >180 | >110 | BP is fatally high, and premature death is likely [5]. Symptoms include chest pain, numbness, weakness in limbs, blurred vision, breathing difficulty, and other symptoms associated with stroke or myocardial infarction [14]. Immediate treatment in ICU is recommended for rapid reduction in BP. Acute cardiac, renal, and neural damage may occur if treatment is too late [6]. |
| Cumulative Frequency of Error | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg |
|---|---|---|---|
| Grade A | |||
| Grade B | |||
| Grade C |
| Dataset | Ref. | Year | Number of Subjects | Number of Subjects (Male) | Number of Subjects (Female) | Age | Race | Remarks |
|---|---|---|---|---|---|---|---|---|
| MIMIC I | [124] | 2000 | 90 | - | - | - | - | ICU patients |
| MIMIC III | [240] | 2016 | 53,423 | 27,983 () | 25,440 () | median | - | Includes MIMIC II |
| Figshare dataset | [139] | 2018 | 219 | 21–86, 61 subjects | - | hypertension | ||
| UQVS | [113] | 2012 | 32 | - | - | - | - | - |
| Dataset without a name | [241] | 2019 | 22 | 9 | 13 | 18–78 | - | Weight: 50–94 kg, height: 160–195 cm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, P.-K.; Cheung, K.-L.; Sangsiri, N.; Shek, W.J.; Wong, K.-L.; Chin, J.-W.; Chan, T.-T.; So, R.H.-Y. Blood Pressure Measurement: From Cuff-Based to Contactless Monitoring. Healthcare 2022, 10, 2113. https://doi.org/10.3390/healthcare10102113
Man P-K, Cheung K-L, Sangsiri N, Shek WJ, Wong K-L, Chin J-W, Chan T-T, So RH-Y. Blood Pressure Measurement: From Cuff-Based to Contactless Monitoring. Healthcare. 2022; 10(10):2113. https://doi.org/10.3390/healthcare10102113
Chicago/Turabian StyleMan, Ping-Kwan, Kit-Leong Cheung, Nawapon Sangsiri, Wilfred Jin Shek, Kwan-Long Wong, Jing-Wei Chin, Tsz-Tai Chan, and Richard Hau-Yue So. 2022. "Blood Pressure Measurement: From Cuff-Based to Contactless Monitoring" Healthcare 10, no. 10: 2113. https://doi.org/10.3390/healthcare10102113
APA StyleMan, P.-K., Cheung, K.-L., Sangsiri, N., Shek, W. J., Wong, K.-L., Chin, J.-W., Chan, T.-T., & So, R. H.-Y. (2022). Blood Pressure Measurement: From Cuff-Based to Contactless Monitoring. Healthcare, 10(10), 2113. https://doi.org/10.3390/healthcare10102113








