Barriers and Facilitators to Intradialytic Parenteral Nutrition Implementation Targeting Protein Energy Wasting in Malaysian Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Phase 1: Cross-Sectional Survey
2.2.1. Sampling
2.2.2. Questionnaire Development
- Section A elicited four questions targeting sampled hospitals and HD units relating to demographic characteristics of the service provider, number of patients receiving HD treatment, access to nephrologists, and IDPN prescriptions in outpatient HD units.
- Section B elicited seven questions targeting the type of patients receiving IDPN, who initiates IDPN, and the preferred type of IDPN bag.
- Section C yielded eight questions focusing on IDPN prescription and administration.
- Section D included three questions on monitoring and evaluation of IDPN treatment of patients.
- Section E included three questions on the pharmacist’s role and tasks in IDPN delivery.
2.3. Phase 2: Evaluating Survey Outcomes
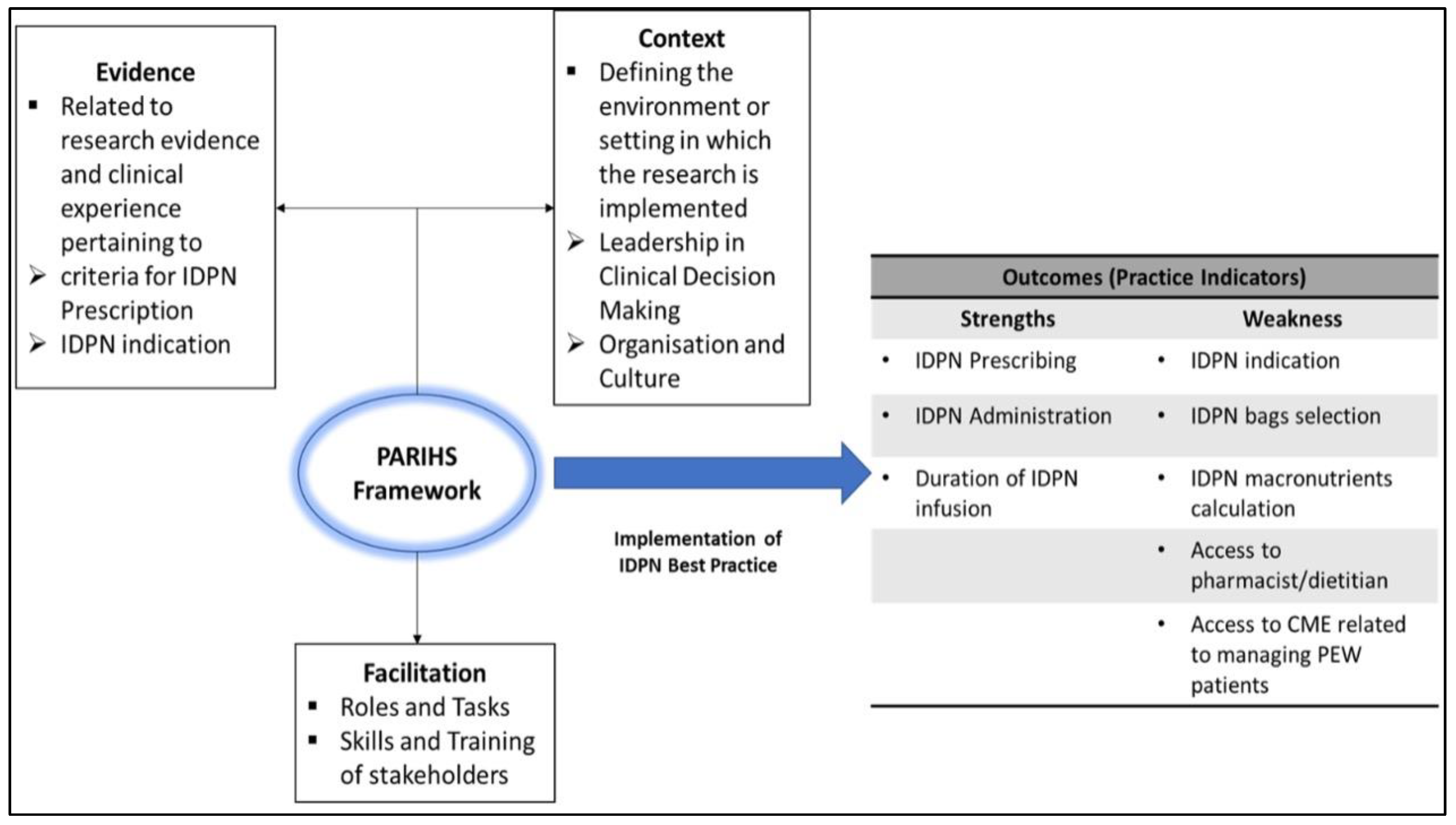
2.3.1. Evidence for Rating
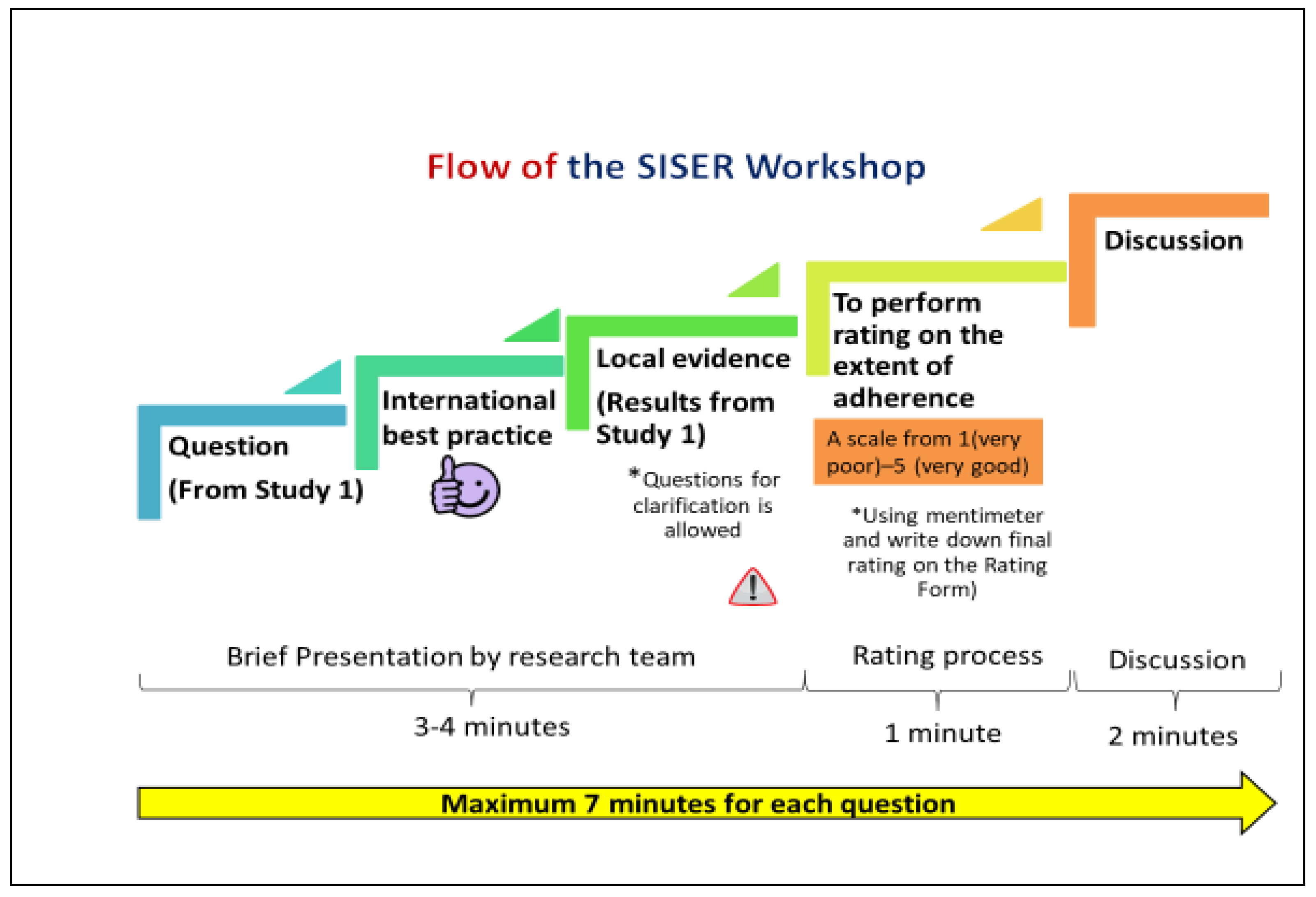
2.3.2. Sample Recruitment for the SIS-ER Workshop
2.3.3. Terms of Reference
2.3.4. Rating Process
2.4. Statistical Analysis
3. Results
3.1. Status of IDPN Delivery
3.1.1. Demographics of IDPN Practice
3.1.2. Best Practice Indicators for IDPN Prescription (Evidence)
3.1.3. Leadership in Clinical Decision Making (Context)
3.1.4. Organization and Culture (Context)
3.1.5. Roles, Tasks, and Performance in IDPN Delivery (Facilitation)
3.2. SIS-ER Proceedings
3.2.1. Facilitators and Barriers to Good Practice
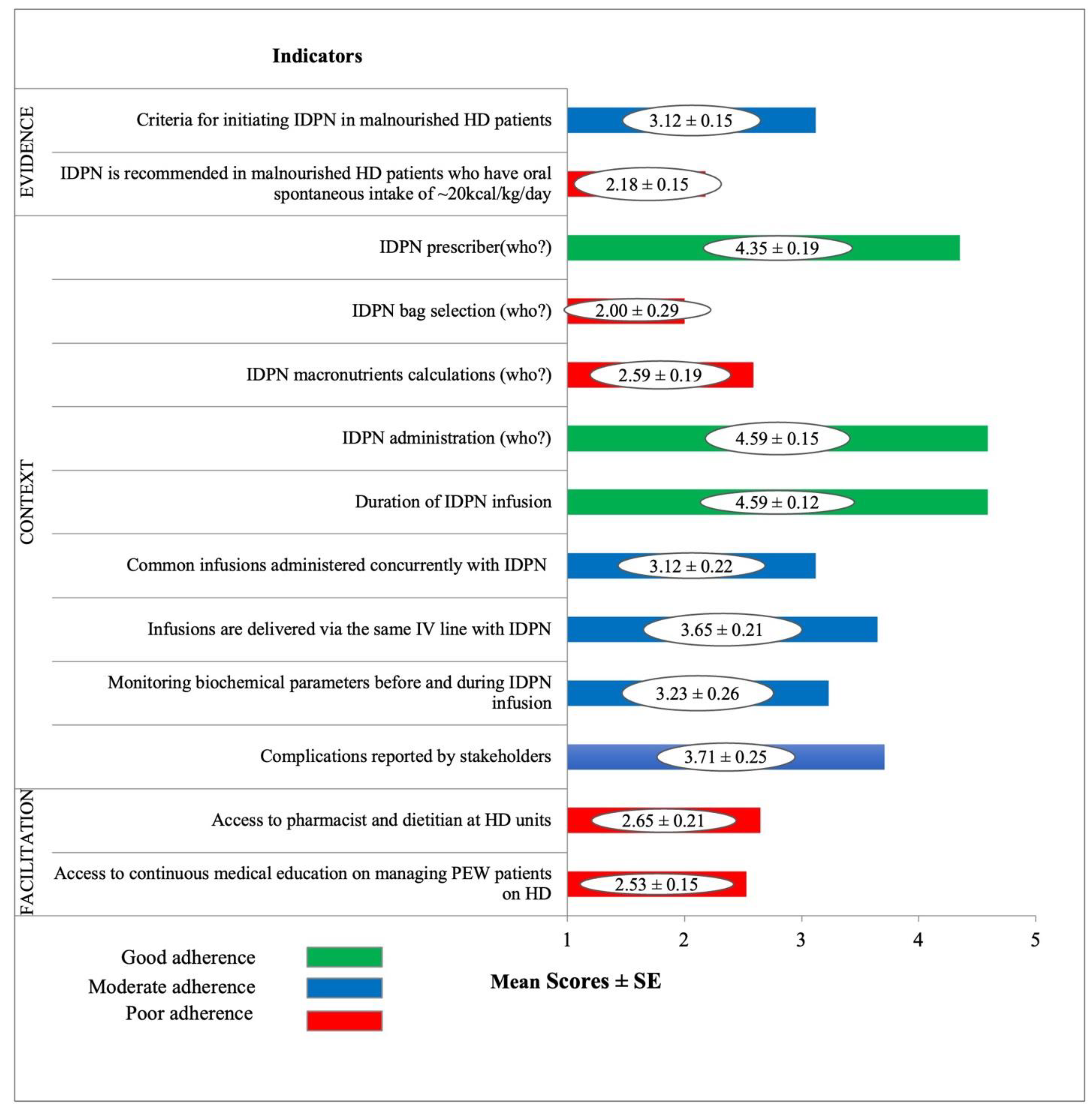
- In the Evidence domain, ‘Criteria for initiating IDPN in malnourished HD patients’ was rated as moderate adherence while IDPN is recommended in malnourished HD patients who have oral spontaneous intake of ~20 kcal/kg/day’ was rated as poor adherence.
- In the Context domain, three indicators relating to IDPN prescription, administration, and infusion duration were rated as good adherence. Moderately rated indictors related to sharing intravenous access, concurrent infusions, and monitoring of biochemical parameters and complication reporting. Bag selection and macronutrient calculation for IDPN were rated as poor adherence.
- In the Facilitation domain, ‘Access to pharmacist and dietitian at HD unit’ and ‘Access to continuous medical education on managing PEW patients on HD’ were rated as poor adherence.
3.2.2. Ratings as per Profession
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, A.Y.M.; Okpechi, I.G.; Ye, F.; Kovesdy, C.P.; Brunori, G.; Burrowes, J.D.; Campbell, K.; Damster, S.; Fouque, D.; Friedman, A.N.; et al. Assessing Global Kidney Nutrition Care. CJASN 2022, 17, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies from the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; Wee, P.T.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Sahathevan, S.; Khor, B.H.; Ng, H.M.; Gafor, A.H.A.; Daud, Z.A.M.; Mafra, D.; Karupaiah, T. Understanding Development of Malnutrition in Hemodialysis Patients: A Narrative Review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef]
- Osman, M.A.; Alrukhaimi, M.; Ashuntantang, G.E.; Bellorin-Font, E.; Gharbi, M.B.; Braam, B.; Courtney, M.; Feehally, J.; Harris, D.C.; Jha, V.; et al. Global nephrology workforce: Gaps and opportunities toward a sustainable kidney care system. Kidney Int. 2018, 8, 52–63. [Google Scholar] [CrossRef]
- Khor, B.H.; Chinna, K.; Abdul Gafor, A.H.; Morad, Z.; Ahmad, G.; Bavanandam, S.; Visvanathan, R.; Yahya, R.; Goh, B.; Bee, B.; et al. The state of nutrition care in outpatient hemodialysis settings in Malaysia: A nationwide survey. BMC Health Serv. Res. 2018, 18, 939. [Google Scholar] [CrossRef]
- Sahathevan, S.; Khor, B.H.; Singh, B.K.S.; Sabatino, A.; Fiaccadori, E.; Daud, Z.A.M.; Ali, M.S.; Narayanan, S.S.; Tallman, D.; Chinna, K.; et al. Association of ultrasound-derived metrics of the quadriceps muscle with protein energy wasting in haemodialysis patients: A multicenter cross-sectional study. Nutrients 2020, 12, 3597. [Google Scholar] [CrossRef]
- Sahathevan, S.; Karupaiah, T.; Khor, B.-H.; Singh, B.K.S.; Daud, Z.A.M.; Fiaccadori, E.; Sabatino, A.; Chinna, K.; Gafor, A.H.A.; Bavanandan, S.; et al. Muscle Status Response to Oral Nutritional Supplementation in Hemodialysis Patients with Protein Energy Wasting: A Multi-Center Randomized, Open Label-Controlled Trial. Front. Nutr. 2021, 8, 743324. [Google Scholar] [CrossRef]
- Anderson, J.; Peterson, K.; Bourne, D.; Boundy, E. Effectiveness of Intradialytic Parenteral Nutrition in Treating Protein-Energy Wasting in Hemodialysis: A Rapid Systematic Review. J. Ren. Nutr. 2019, 29, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Al Batani, R.; Abdullah, D.C.; Bahari, M.B. Evaluation of the total parenteral nutrition service at Universiti Sains Malaysia Hospital. e-Spen Eur. e-J. Clin. Nutr. Metab. 2007, 2, e111–e115. [Google Scholar] [CrossRef]
- Sadu Singh, B.K.; Abdul Gafor, A.H.; Fiaccadori, E.; Karupaiah, T. Enabling intradialytic parenteral nutrition in maintenance hemodialysis patients in Malaysia: The what, who and how scenarios of implementation? Malays. Appl. Biol. 2018, 47, 1–11. [Google Scholar]
- Kitson, A.L.; Rycroft-Malone, J.; Harvey, G.; McCormack, B.; Seers, K.; Titchen, A. Evaluating the successful implementation of evidence into practice using the PARIHS framework: Theoretical and practical challenges. Implement. Sci. 2008, 3, 1. [Google Scholar] [CrossRef]
- Nilsen, P.; Bernhardsson, S. Context matters in implementation science: A scoping review of determinant frameworks that describe contextual determinants for implementation outcomes. BMC Health Serv. Res. 2019, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Damschroder, L.; Helfrich, C.; Hagedorn, H.J. Guide for applying a revised version of the PARIHS framework for implementation. Implement. Sci. 2011, 6, 99. [Google Scholar] [CrossRef]
- Harvey, G.; Kitson, A. PARIHS revisited: From heuristic to integrated framework for the successful implementation of knowledge into practice. Implement. Sci. 2016, 11, 33. [Google Scholar] [CrossRef]
- Ali, A.B.; Chapman-Kiddell, C.; Reeves, M.M. Current practices in the delivery of parenteral nutrition in Australia. Eur. J. Clin. Nutr. 2007, 61, 554–560. [Google Scholar] [CrossRef][Green Version]
- Mafrici, B.; Wilcox, N. (Eds.) Guideline for the Use and Administration of Intradialytic Parenteral Nutrition (IDPN) in Adult Hemodialysis Patients; Nottingham University Hospitals NHS Trust: Nottingham, UK, 2018. [Google Scholar]
- BC Renal. Intradialytic Parenteral Nutrition (IDPN): Provincial Standards and Guidelines; British Columbia Provincial Renal Agency: Columbia, UK, 2021. [Google Scholar]
- Cano, N.J.M.; Aparicio, M.; Brunori, G.; Carrero, J.J.; Cianciaruso, B.; Fiaccadori, E.; Lindholm, B.; Teplan, V.; Fouque, D.; Guarnieri, G. ESPEN guidelines on parenteral nutrition: Adult Renal Failure. Clin. Nutr. 2009, 28, 401–414. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI clinical practice guideline for nutrition in CKD. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Johnson, T.P. Snowball Sampling: Introduction; Wiley & Sons: Chichester, UK, 2014. [Google Scholar]
- Ng, S.H.; Swinburn, B.; Kelly, B.; Vandevijvere, S.; Yeatman, H.; Ismail, M.N.; Karupaiah, T. Extent of implementation of food environment policies by the Malaysian Government: Gaps and priority recommendations. Public Health Nutr. 2018, 21, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; McCormack, B.; Coffey, A.; McCarthy, G. Evaluating the context within which continence care is provided in rehabilitation units for older people. Int. J. Older People Nurs. 2007, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yang, Y.; Sui, W.; Hu, Y.; Li, H.; Wang, F.; Qian, K.; Ji, J.; Tao, M. Implementation of evidence into practice for cancer-related fatigue management of hospitalized adult patients using the PARIHS framework. PLoS ONE 2017, 12, e0187257. [Google Scholar]
- Medical Services Unit. Nephrology Services Operational Policy; Medical Development Division, Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2010.
- Lambert, K.; Conley, M.M. Practice Patterns Relating to the Use of Intradialytic Parenteral Nutrition in Australian Renal Units: Results from a Survey of Renal Dietitians. J. Ren. Nutr. 2019, 30, 163–167. [Google Scholar] [CrossRef]
- Lee, C.B.; Chen, M.S.; Powell, M.J.; Chu, C.M.Y. Organisational change to health promoting hospitals: A review of the literature. Springer Sci. Rev. 2013, 1, 13–23. [Google Scholar] [CrossRef]
- Beattie, C.; Allard, J.; Raman, M. Comparison between premixed and compounded parenteral nutrition solutions in hospitalized patients requiring parenteral nutrition. Nutr. Clin. Pract. 2016, 31, 229–234. [Google Scholar] [CrossRef]
- Stevenson, J.; Tong, A.; Campbell, K.L.; Craig, J.C.; Lee, V.W. Perspectives of healthcare providers on the nutritional management of patients on hemodialysis in Australia: An interview study. Br. Med. J. 2018, 8, e020023. [Google Scholar]
- Centers for Medicare and Medicaid Services (CMS) HHS. Medicare and medic aid programmes; conditions for coverage for end-stage renal disease facilities; final rule. Fed. Regist. 2008, 73, 203. [Google Scholar]
| Domain | Related Q | Related QA-Section | Best Practice Indicators |
|---|---|---|---|
| Evidence derived from knowledge-based sources which include research, clinical and patient experience, and information from local context [14] | Q12 | SECTION C-IDPN Prescription and Delivery | Criteria for initiating IDPN in malnourished HD patients |
| Q13 | SECTION C-IDPN Prescription and Delivery | IDPN is recommended in malnourished HD patients who have oral spontaneous intake of ~20kcal/kg/day | |
| Context refers to the environment or setting in which the proposed change is to be implemented and has, further, three sub-elements which include organizational culture, leadership, and evaluation [14] | Q6 | SECTION B- IDPN Use by Prescribers and Stakeholders | IDPN prescriber |
| Q7 | SECTION B- IDPN Use by Prescribers and Stakeholders | IDPN bag selection (who?) | |
| Q15 | SECTION C-IDPN Prescription and Delivery | IDPN macronutrients calculations (who?) | |
| Q16 | SECTION C-IDPN Prescription and Delivery | IDPN administration (who?) | |
| Q17 | SECTION C-IDPN Prescription and Delivery | Duration of IDPN infusion | |
| Q18 | SECTION C-IDPN Prescription and Delivery | Common infusions administered concurrently with IDPN | |
| Q19 | SECTION C-IDPN Prescription and Delivery | Infusions are delivered via the same IV line with IDPN | |
| Q20 & 21 | SECTION D- IDPN Monitoring and Evaluation of Treatment | Monitoring biochemical parameters before and during IDPN infusion | |
| Q22 | SECTION D- IDPN Monitoring and Evaluation of Treatment | Complications reported by stakeholders | |
| Facilitation refers to the method to simplify things for others through support and assistance in changing their attitudes, habits, skills, thinking process, and working [14] | Q23 | SECTION E-Pharmacist’s Role and Tasks in IDPN Delivery | Access to pharmacist and dietitian at HD units |
| Q25 | SECTION E-Pharmacist’s Role and Tasks in IDPN Delivery | Access to continuous medical education on managing PEW patients on HD |
| (a) Survey Outcomes on IDPN Practice at Malaysian Hospitals. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hospitals with PN Service (n = 56) (n, %) | Hospitals Providing IDPN at Outpatient HD Units (n = 13) (n, %) | |||||||
| Type of Hospital | ||||||||
| Government | 45 (80.4) | 12 (92.3) | ||||||
| Private | 11 (19.6) | 1 (7.7) | ||||||
| NGO | 0 (0.0) | 0 (0.0) | ||||||
| Location of Hospitals | ||||||||
| Urban | 39 (69.6) | 12 (92.3) | ||||||
| Rural | 17 (30.4) | 1 (7.7) | ||||||
| Frequency of Outpatients on HD | ||||||||
| Less than 50 | 17 (30.4) | 4 (30.8) | ||||||
| 50–100 | 26 (46.4) | 6 (46.1) | ||||||
| More than 100 | 13 (23.2) | 3 (23.1) | ||||||
| Frequency of Nephrologist’s Access | ||||||||
| 1 | 39 (69.6) | 3 (23.1) | ||||||
| 2 or more | 17 (30.4) | 10 (76.9) | ||||||
| Best Practice Indicators for IDPN Prescription (n = 13) | ||||||||
| Criteria for Initiating IDPN | ||||||||
| Body mass index (BMI) < 23 | 7 (53.8) | |||||||
| Serum albumin < 38 g/L | 11 (84.6) | |||||||
| Weight loss of 10% over 6 months | 6 (46.2) | |||||||
| Dietary intake < 25 kcal/kg BW | 11 (84.6) | |||||||
| Pharmacist Recommendation to Initiate IDPN for Patients with at least 20 kcal/kg/day of Spontaneous Oral Intake? | ||||||||
| Yes | 6 (46.2) | |||||||
| No | 7 (53.8) | |||||||
| Leadership in Clinical Decision Making (n = 13) | ||||||||
| IDPN Prescribed By | ||||||||
| Doctor only | 10 (76.9) | |||||||
| Doctor and pharmacists | 3 (23.1) | |||||||
| IDPN Bag Selected By | ||||||||
| Doctor only | 3 (23.1) | |||||||
| Pharmacist only | 4 (30.8) | |||||||
| Doctor and pharmacist | 6 (46.1) | |||||||
| Who Calculates the IDPN Macronutrients? * | ||||||||
| Doctor | 1 (6.25) | |||||||
| Pharmacist | 6 (37.5) | |||||||
| Dietitian | 1 (6.25) | |||||||
| Standard formula used | 8 (50.0) | |||||||
| Type of IDPN Bags Supplied | ||||||||
| Compounded bags by hospital pharmacy | 1 (7.7) | |||||||
| Standard bags | 9 (69.2) | |||||||
| Combination compounded and standard bags | 3 (23.1) | |||||||
| IDPN Prescribing Protocol Availability | ||||||||
| Yes | 0 (0.0) | |||||||
| No | 13 (100.0) | |||||||
| Organization and Culture (n = 13) | ||||||||
| Staff Responsibility for IDPN Administration * | ||||||||
| Doctor | 1 (7.7) | |||||||
| Nurse | 13 (100.0) | |||||||
| Medical Assistant | 3 (23.1) | |||||||
| Dietitian | 0 (0.0) | |||||||
| Pharmacist | 0 (0.0) | |||||||
| IDPN Infusion Time | ||||||||
| 3.5 h or less | 1 (7.7) | |||||||
| 4 h | 12 (92.3) | |||||||
| Infusions Administered Concurrently with IDPN * | ||||||||
| IV saline | 1 (7.7) | |||||||
| IV antibiotics | 2 (15.4) | |||||||
| Blood products | 3 (23.1) | |||||||
| No infusions | 6 (46.2) | |||||||
| Are Infusions Given via the Same IV Line with IDPN? | ||||||||
| Yes | 2 (15.4) | |||||||
| No | 11 (84.6) | |||||||
| Roles, Tasks, and Performance of Pharmacists (n = 13) | ||||||||
| Access to Supporting Staff * | ||||||||
| Full time pharmacist only | 2 (15.4) | |||||||
| Full time dietitian only | 1 (7.7) | |||||||
| Both pharmacist and dietitian | 7 (53.8) | |||||||
| No access | 3 (23.1) | |||||||
| Is the Pharmacist Aware About PEW in Chronic Kidney Failure Patients? | ||||||||
| Yes | 10 (76.9) | |||||||
| No | 3 (23.1) | |||||||
| Access to CME on Managing PEW Patients on HD | ||||||||
| Yes | 6 (46.2) | |||||||
| No | 7 (53.8) | |||||||
| (b) Factors Affecting IDPN Practice at Outpatient HD Units. | ||||||||
| Characteristics | IDPN for all Patients (n,%) | IDPN for Outpatients (n,%) | ||||||
| n | Yes | No | p-Value a | n | Yes | No | p-Value a | |
| Urban | 39 | 24 (61.5) | 15 (38.5) | 0.009 | 24 | 12 (50.0) | 12 (50.0) | >0.05 |
| Rural | 17 | 4 (23.5) | 13 (76.5) | 4 | 1 (25.0) | 3 (75.0) | ||
| Nephrologist Availability | ||||||||
| 1 | 39 | 14 (35.9) | 25 (64.1) | 0.001 | 39 | 3 (7.7) | 36 (92.3) | <0.001 |
| 2 or more | 17 | 14 (82.4) | 3 (17.6) | 17 | 10 (58.8) | 7 (41.2) | ||
| No | Indicators | Dietitian (n = 5) | Doctor (n = 2) | Nurse (n = 4) | Pharmacist (n = 6) |
|---|---|---|---|---|---|
| 1 | Criteria for initiating IDPN in malnourished HD patients | 20% (2.80) | 0% (3.00) | 50% (3.50) | 17% (3.17) |
| 2 | IDPN is recommended in malnourished HD patients with oral spontaneous intake of ~20 kcal/kg/day | 0% (2.00) | 0% (2.00) | 0% (2.00) | 0% (2.00) |
| 3 | IDPN prescriber | 100% (4.60) | 100% (4.00) | 75% (4.00) | 100% (4.50) |
| 4 | IDPN bag selection (who?) | 40% (2.40) | 0% (1.00) | 0% (2.50) | 0% (1.67) |
| 5 | IDPN macronutrients calculation (who?) | 0% (2.40) | 0% (1.00) | 25% (2.50) | 17% (1.67) |
| 6 | IDPN administration (who?) | 100% (4.80) | 100% (5.00) | 75% (4.25) | 100% (4.50) |
| 7 | Duration of IDPN infusion | 100% (5.00) | 100% (4.00) | 100% (4.25) | 100% (4.67) |
| 8 | Common infusions administered concurrently with IDPN | 60% (3.20) | 0% (2.00) | 25% (3.00) | 67% (3.50) |
| 9 | Infusions are delivered via the same IV line with IDPN | 60% (3.60) | 100% (4.00) | 50% (3.25) | 83% (3.83) |
| 10 | Monitoring of biochemical parameters before and during IDPN infusion | 40% (3.40) | 0% (2.50) | 50% (3.75) | 33% (3.00) |
| 11 | Complications reported by stakeholders | 60% (3.80) | 100% (4.00) | 75% (3.75) | 67% (3.50) |
| 12 | Access to pharmacist and dietitian at HD units | 40% (2.60) | 0% (2.50) | 25% (2.75) | 0% (2.67) |
| 13 | Access to continuous medical education on managing PEW patients on HD | 0% (2.60) | 50% (3.00) | 0% (2.75) | 0% (2.17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, B.K.S.; Khor, B.-H.; Sahathevan, S.; Gafor, A.H.A.; Fiaccadori, E.; Chinna, K.; Ng, S.-H.; Karupaiah, T. Barriers and Facilitators to Intradialytic Parenteral Nutrition Implementation Targeting Protein Energy Wasting in Malaysian Hemodialysis Patients. Healthcare 2022, 10, 2090. https://doi.org/10.3390/healthcare10102090
Singh BKS, Khor B-H, Sahathevan S, Gafor AHA, Fiaccadori E, Chinna K, Ng S-H, Karupaiah T. Barriers and Facilitators to Intradialytic Parenteral Nutrition Implementation Targeting Protein Energy Wasting in Malaysian Hemodialysis Patients. Healthcare. 2022; 10(10):2090. https://doi.org/10.3390/healthcare10102090
Chicago/Turabian StyleSingh, Birinder Kaur Sadu, Ban-Hock Khor, Sharmela Sahathevan, Abdul Halim Abdul Gafor, Enrico Fiaccadori, Karuthan Chinna, See-Hoe Ng, and Tilakavati Karupaiah. 2022. "Barriers and Facilitators to Intradialytic Parenteral Nutrition Implementation Targeting Protein Energy Wasting in Malaysian Hemodialysis Patients" Healthcare 10, no. 10: 2090. https://doi.org/10.3390/healthcare10102090
APA StyleSingh, B. K. S., Khor, B.-H., Sahathevan, S., Gafor, A. H. A., Fiaccadori, E., Chinna, K., Ng, S.-H., & Karupaiah, T. (2022). Barriers and Facilitators to Intradialytic Parenteral Nutrition Implementation Targeting Protein Energy Wasting in Malaysian Hemodialysis Patients. Healthcare, 10(10), 2090. https://doi.org/10.3390/healthcare10102090







