Effects of Circuit Training on Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Types of Outcome Measures
2.2.1. Primary Outcomes
- Pain
- Quality of life.
2.2.2. Secondary Outcomes
- Physical function;
- Activity of daily living;
- Health-related quality of life;
- Anxiety;
- Depression;
- Stiffness;
- KOA symptom;
- High-density lipoprotein;
- Triglycerides.;
- HDL;
- Sport and recreation activities.
2.3. Data Sources
2.4. Eligibility Criteria
2.4.1. Inclusion Criteria
- Patients with KOA and with no age limit.
- Publications with no language limitation and with full text available.
- CT.
- Randomized controlled trials and controlled clinical studies.
2.4.2. Exclusion Criteria
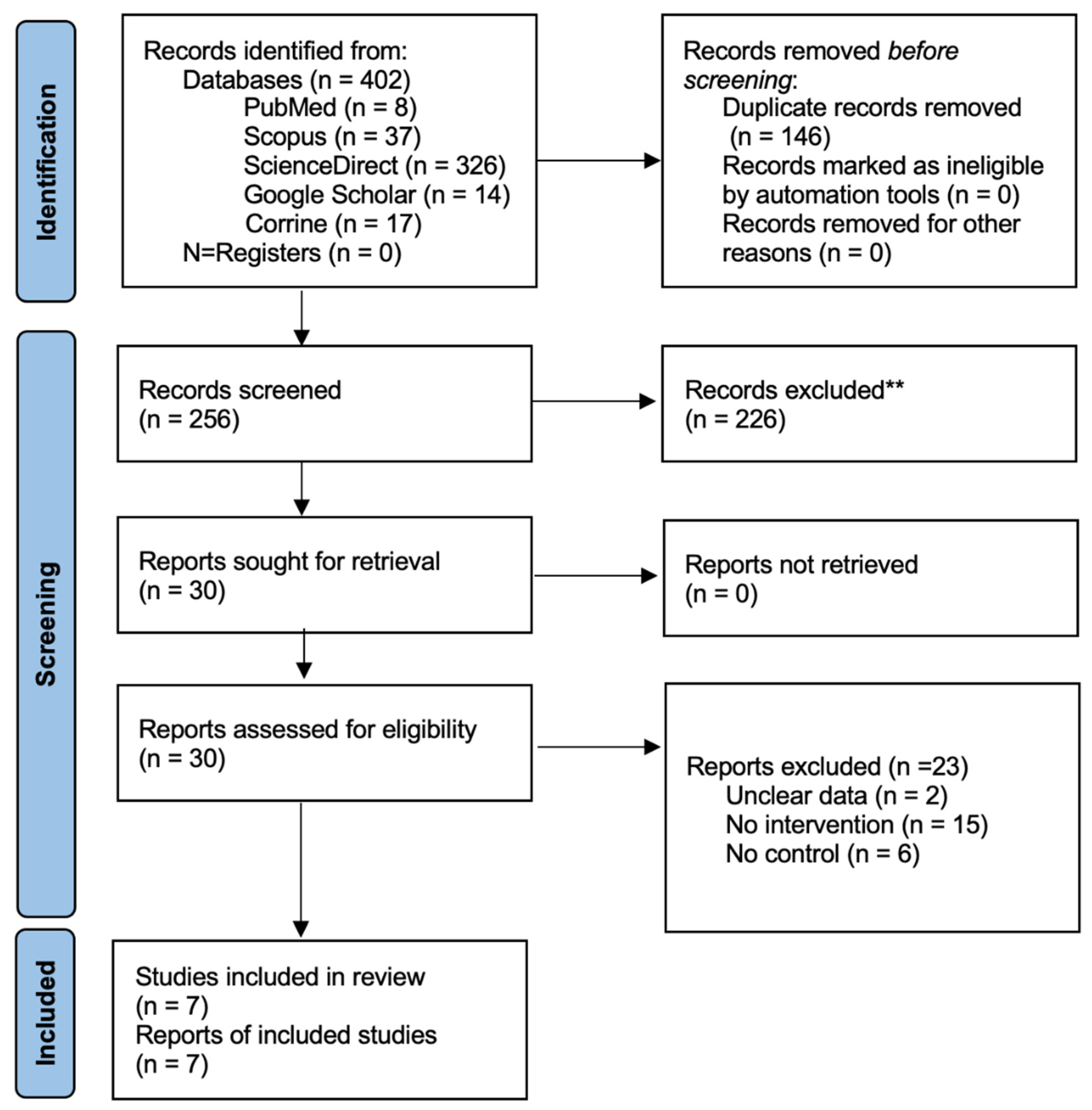
2.5. Study Selection
2.6. Data Extraction
2.7. Assessment of Risk of Bias
2.8. Analysis
2.8.1. Measurement of Treatment Effect
2.8.2. Sensitivity Analysis
2.9. Summary of Findings Table
- Key findings that were summarized (participants, comparative, and baseline data, and results) [37];
- Statistical results that have been condensed;
- A summary of the evidence’s quality, the degree of the effect, and the source of information utilized in the assumed risk.
3. Results
3.1. Included Studies
3.2. Participants Characteristics
3.3. Intervention Characteristics
3.4. Comparison
3.5. Risk of Bias in Included Studies
3.5.1. Random Sequence and Allocation Concealment
3.5.2. Blinding of Participants, Personnel, and Outcome Assessment
3.5.3. Incomplete Outcome Data
3.5.4. Selective Reporting
3.5.5. Other Potential Sources of Bias
3.6. Outcomes
3.6.1. Primary Outcomes
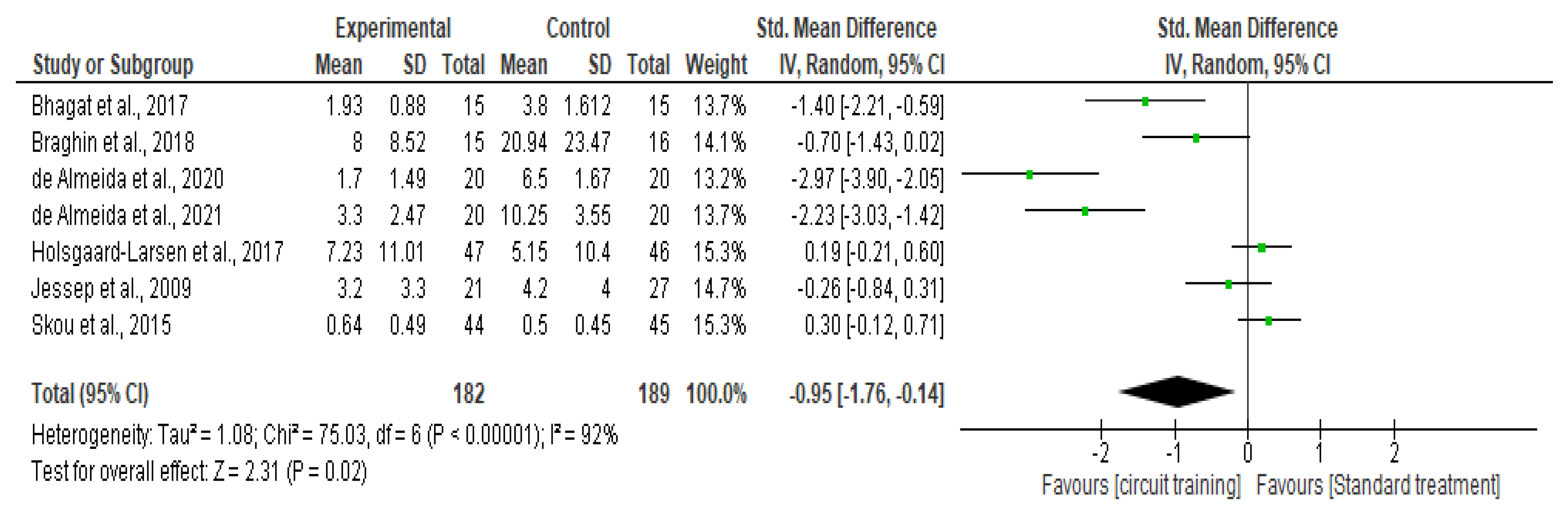
Knee Pain
Quality of Life
3.6.2. The Secondary Outcomes
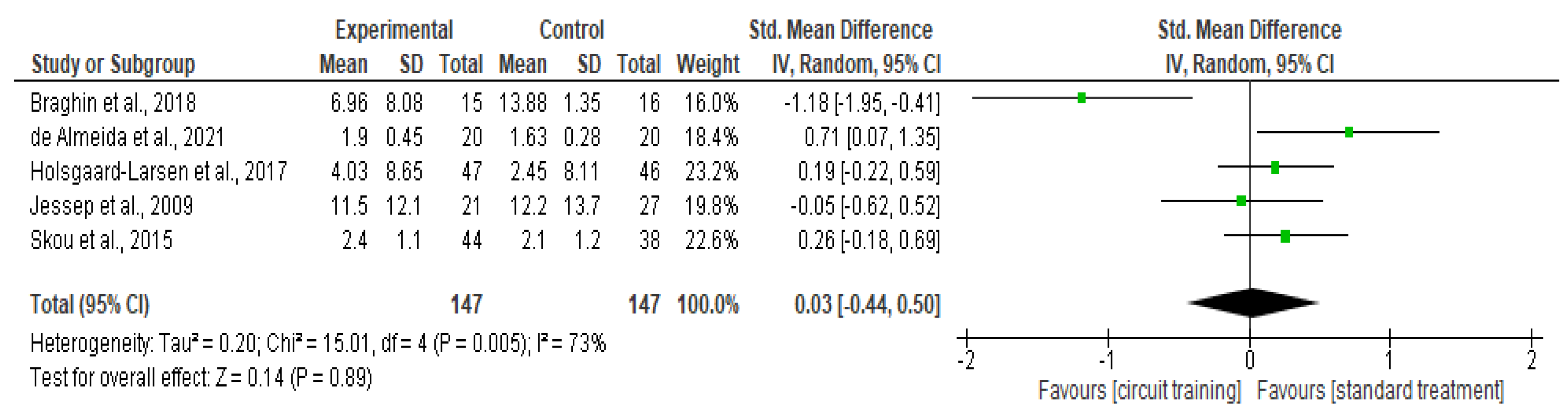
Physical Function
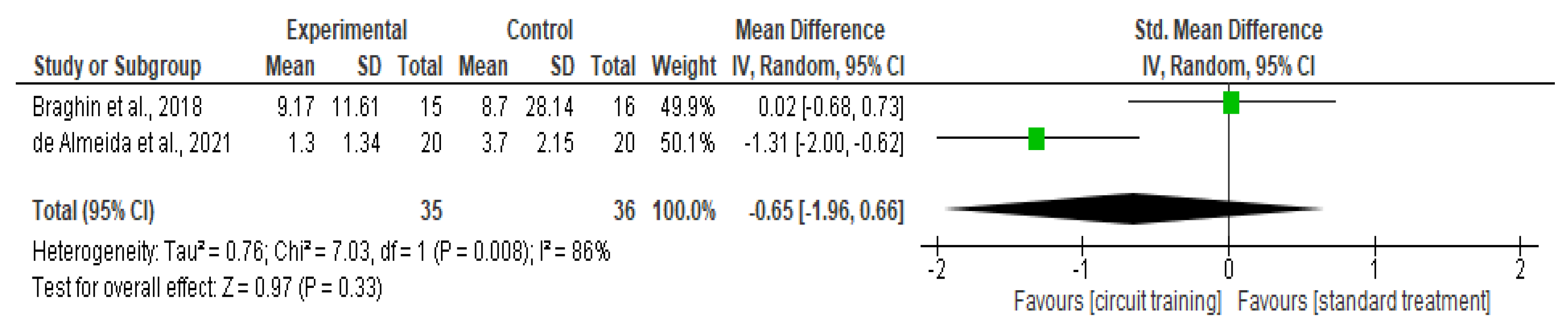
Knee Stiffness
Health-Related Quality of Life
Knee Symptom
Depression
Anxiety
Sports Recreation
The Activity of Daily Living
Triglycerides
High-Density Lipoprotein
4. Discussion
4.1. Summary of Main Results
4.2. Overall Completeness and Applicability of Evidence
4.3. Quality of the Evidence
4.4. Potential Biases in the Review Process
4.5. Agreements and Disagreements with Other Studies or Reviews
5. Conclusions
5.1. Implications for Practice
5.2. Implications for Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Afolabi, H.A.; bin Zakaria, Z.; Hashim, M.N.M.; Vinayak, C.R.; Shokri, A.B.A. Body Mass Index and predisposition of patients to knee osteoarthritis. Obes. Med. 2019, 16, 100143. [Google Scholar] [CrossRef]
- Segal, N.; Glass, N.; Niu, J.; Mcculloch, C.; Felson, D.; Guermazi, A.; Roemer, F.; Lewis, C.; Nevitt, M.; Torner, J. Does the rate of knee OA progression increase with age? Articular cartilage changes over seven years in most. Osteoarthr. Cartil. 2015, 23, A178. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [Green Version]
- National Collaborating Centre for Chronic Conditions (Great Britain); National Institute for Clinical Excellence (Great Britain). Osteoarthritis: National Clinical Guidelines for Care and Management in Adults; Royal College of Physicians: London, UK, 2008. [Google Scholar]
- Laslett, L.L.; Quinn, S.J.; Winzenberg, T.M.; Sanderson, K.; Cicuttini, F.; Jones, G. A prospective study of the impact of musculoskeletal pain and radiographic osteoarthritis on health related quality of life in community dwelling older people. BMC Musculoskelet. Disord. 2012, 13, 168. [Google Scholar] [CrossRef] [Green Version]
- Hawker, G.A.; Gignac, M.A.; Badley, E.; Davis, A.M.; French, M.R.; Li, Y.; Perruccio, A.V.; Power, J.D.; Sale, J.; Lou, W. A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care Res. 2011, 63, 1382–1390. [Google Scholar] [CrossRef]
- Lee, J.; Chang, R.W.; Ehrlich-Jones, L.; Kwoh, C.K.; Nevitt, M.; Semanik, P.A.; Sharma, L.; Sohn, M.W.; Song, J.; Dunlop, D.D. Sedentary behavior and physical function: Objective evidence from the Osteoarthritis Initiative. Arthritis Care Res. 2015, 67, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Nebel, M.B.; Sims, E.L.; Keefe, F.J.; Kraus, V.B.; Guilak, F.; Caldwell, D.S.; Pells, J.J.; Queen, R.; Schmitt, D. The relationship of self-reported pain and functional impairment to gait mechanics in overweight and obese persons with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2009, 90, 1874–1879. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.; van der Leeden, M.; Knol, D.; van der Esch, M.; Roorda, L.; Verschueren, S.; van Dieën, J.; Lems, W.F.; Dekker, J. Association of postural control with muscle strength, proprioception, self-reported knee instability and activity limitations in patients with knee osteoarthritis. J. Rehabil. Med. 2013, 45, 192–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afolabi, H.A.; Zakariya, Z.B.; Shokri, A.B.A.; Hasim, M.N.B.M.; Vinayak, R.; Afolabi-Owolabi, O.T.; Elesho, R.F. The relationship between obesity and other medical comorbidities. Obes. Med. 2020, 17, 100164. [Google Scholar] [CrossRef]
- Kawano, M.M.; Araújo, I.L.A.; Castro, M.C.; Matos, M.A. Assessment of quality of life in patients with knee osteoarthritis. Acta Ortop. Bras. 2015, 23, 307–310. [Google Scholar] [CrossRef]
- Vitaloni, M.; Botto-van Bemden, A.; Sciortino Contreras, R.M.; Scotton, D.; Bibas, M.; Quintero, M.; Monfort, J.; Carné, X.; de Abajo, F.; Oswald, E.; et al. Global management of patients with knee osteoarthritis begins with quality of life assessment: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 493–512. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Ferrans, C.E.; Halyard, M.Y.; Revicki, D.A.; Symonds, T.L.; Varricchio, C.G.; Kotzeva, A.; Valderas, J.M.; Alonso, J.L.; Clinical Significance Consensus Meeting Group. Exploration of the value of health-related quality-of-life information from clinical research and into clinical practice. Mayo Clin. Proc. 2007, 82, 1229–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, D.; Datta, P.P.; Majumdar, K. Relationship of activity of daily living with quality of life. Br. Biomed. Bull. 2014, 4, 757–764. [Google Scholar]
- Beavers, D.P.; Beavers, K.M.; Loeser, R.F.; Walton, N.R.; Lyles, M.F.; Nicklas, B.J.; Shapses, S.A.; Newman, J.J.; Messier, S.P. The independent and combined effects of intensive weight loss and exercise training on bone mineral density in overweight and obese older adults with osteoarthritis. Osteoarthr. Cartil. 2014, 22, 726–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhl, C.; Christensen, R.; Roos, E.M.; Zhang, W.; Lund, H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: A systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014, 66, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Reid, K.F.; Phillips, E.M.; Krivickas, L.S.; Hughes, V.A.; Roubenoff, R.; Fielding, R.A. Muscle fiber size and function in elderly humans: A longitudinal study. J. Appl. Physiol. 2008, 105, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Beavers, K.M.; Beavers, D.P.; Newman, J.J.; Anderson, A.M.; Loeser, R.F., Jr.; Nicklas, B.J.; Lyles, M.F.; Miller, G.D.; Mihalko, S.L.; Messier, S.P. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthr. Cartil. 2015, 23, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Olson, T.P.; Dengel, D.; Leon, A.; Schmitz, K. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int. J. Obes. 2007, 31, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Hinman, R.S. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. J. Sci. Med. Sport 2011, 14, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Lefèvre-Colau, M.-M.; Poiraudeau, S.; Rannou, F. Rehabilitation (exercise and strength training) and osteoarthritis: A critical narrative review. Ann. Phys. Rehabil. Med. 2016, 59, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Al-mhanna, S.B.; Afolabi, H.A.; Sheikh, A.M.; Mohamed, A.A.; Ahmed, A.Y.; Abdulle, M.M.; Mohamed, S.I.; Mohamed, M.H.; Mohamed, A.H. The Effectiveness of Mirror Therapy on Lower Extremity Motor Function among Stroke Patients: A Review. Altamash J. Dent. Med. 2022, 1, 1–8. [Google Scholar]
- Bocalini, D.S.; Lima, L.S.; de Andrade, S.; Madureira, A.; Rica, R.L.; Dos Santos, R.N.; Serra, A.J.; Silva, J.A., Jr.; Rodriguez, D.; Figueira, A., Jr. Effects of circuit-based exercise programs on the body composition of elderly obese women. Clin. Interv. Aging 2012, 7, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.B.; Pearcey, G.E.; Cahill, F.; McCarthy, H.; Stratton, S.B.; Noftall, J.C.; Buckle, S.; Basset, F.A.; Sun, G.; Button, D.C. The effect of a short-term high-intensity circuit training program on work capacity, body composition, and blood profiles in sedentary obese men: A pilot study. BioMed Res. Int. 2014, 2014, 191797. [Google Scholar] [CrossRef] [PubMed]
- Romero-Arenas, S.; Blazevich, A.J.; Martínez-Pascual, M.; Pérez-Gómez, J.; Luque, A.J.; López-Román, F.J.; Alcaraz, P.E. Effects of high-resistance circuit training in an elderly population. Exp. Gerontol. 2013, 48, 334–340. [Google Scholar] [CrossRef]
- Balachandran, A.; Krawczyk, S.N.; Potiaumpai, M.; Signorile, J.F. High-speed circuit training vs hypertrophy training to improve physical function in sarcopenic obese adults: A randomized controlled trial. Exp. Gerontol. 2014, 60, 64–71. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kang, C.-K.; Park, H.; Lee, M.-G. Effects of vitamin D supplementation and circuit training on indices of obesity and insulin resistance in T2D and vitamin D deficient elderly women. J. Exerc. Nutr. Biochem. 2014, 18, 249. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.G.; Noh, H.M.; Kim, S.Y. Weight loss effects of circuit training interventions: A systematic review and meta-analysis. Obes. Rev. 2019, 20, 1642–1650. [Google Scholar] [CrossRef]
- De Almeida, A.C.; Pedroso, M.G.; Aily, J.B.; Gonçalves, G.H.; Pastre, C.M.; Mattiello, S.M. Influence of a periodized circuit training protocol on intermuscular adipose tissue of patients with knee osteoarthritis: Protocol for a randomized controlled trial. BMC Musculoskelet. Disord. 2018, 19, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Almeida, A.C.; Aily, J.B.; Pedroso, M.G.; Gonçalves, G.H.; Pastre, C.M.; Mattiello, S.M. Reductions of cardiovascular and metabolic risk factors after a 14-week periodized training model in patients with knee osteoarthritis: A randomized controlled trial. Clin. Rheumatol. 2021, 40, 303–314. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S.; Scholten, R. Maintaining reviews: Updates, amendments and feedback. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 31. [Google Scholar]
- Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1. 0 [Updated March 2011]. The Cochrane Collaboration. 2011. Available online: www.cochrane-handbook.org (accessed on 20 January 2021).
- GRADEpro GDT. Computer Program; Version [July, 2016]; McMaster University: Hamilton, ON, USA, 2014; Available online: www.gradepro.org (accessed on 20 January 2021).
- Schünemann, H.J.; Oxman, A.D.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Guyatt, G.H. Presenting results and ‘Summary of findings’ tables. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 5. [Google Scholar]
- Bhagat, P.; Jagtap, V.; Devi, P. Effect of circuit training in osteoarthritis of knee. Asian J. Pharm. Clin. Res. 2017, 10, 333–335. [Google Scholar] [CrossRef] [Green Version]
- Braghin, R.D.M.B.; Libardi, E.C.; Junqueira, C.; Nogueira–Barbosa, M.H.; de Abreu, D.C.C. Exercise on balance and function for knee osteoarthritis: A randomized controlled trial. J. Bodyw. Mov. Ther. 2018, 22, 76–82. [Google Scholar] [CrossRef]
- De Almeida, A.C.; Aily, J.B.; Pedroso, M.G.; Gonçalves, G.H.; de Carvalho Felinto, J.; Ferrari, R.J.; Pastre, C.M.; Mattiello, S.M. A periodized training attenuates thigh intermuscular fat and improves muscle quality in patients with knee osteoarthritis: Results from a randomized controlled trial. Clin. Rheumatol. 2020, 39, 1265–1275. [Google Scholar] [CrossRef]
- Holsgaard-Larsen, A.; Clausen, B.; Søndergaard, J.; Christensen, R.; Andriacchi, T.; Roos, E. The effect of instruction in analgesic use compared with neuromuscular exercise on knee-joint load in patients with knee osteoarthritis: A randomized, single-blind, controlled trial. Osteoarthr. Cartil. 2017, 25, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessep, S.A.; Walsh, N.E.; Ratcliffe, J.; Hurley, M.V. Long-term clinical benefits and costs of an integrated rehabilitation programme compared with outpatient physiotherapy for chronic knee pain. Physiotherapy 2009, 95, 94–102. [Google Scholar] [CrossRef]
- Skou, S.T.; Rasmussen, S.; Laursen, M.B.; Rathleff, M.S.; Arendt-Nielsen, L.; Simonsen, O.; Roos, E.M. The efficacy of 12 weeks non-surgical treatment for patients not eligible for total knee replacement: A randomized controlled trial with 1-year follow-up. Osteoarthr. Cartil. 2015, 23, 1465–1475. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, Y.-Q.; Liu, Y.; Pei, S.-L.; Yang, H.-H.; Wu, J.-J.; Luo, C.-K. Effects of group psychological intervention combined with pulmonary rehabilitation exercises on anxiety and sleep disorders in patients with mild coronavirus disease 2019 (COVID-19) infections in a Fangcang hospital. Psychol. Health Med. 2022, 27, 333–342. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Chen, S.; Zhang, Y.; Zhang, Z.; Liu, C.; Lu, M.; Liu, F.; Li, S.; He, Z.; et al. The effects of resistance exercise in patients with knee osteoarthritis: A systematic review and meta-analysis. Clin. Rehabil. 2016, 30, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Castelein, B.; Wittoek, R.; Calders, P.; Van Ginckel, A. Diet-induced weight loss alone or combined with exercise in overweight or obese people with knee osteoarthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2019, 48, 765–777. [Google Scholar] [CrossRef]
- Xie, S.-H.; Wang, Q.; Wang, L.-Q.; Wang, L.; Song, K.-P.; He, C.-Q. Effect of internet-based rehabilitation programs on improvement of pain and physical function in patients with knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Med. Internet Res. 2021, 23, e21542. [Google Scholar] [CrossRef] [PubMed]


| Reference | Status of the Patient at Intervention | Sample Size | Age/Population | Control | Intervention | Duration | Outcome Measures | Pro Instrument Measure |
|---|---|---|---|---|---|---|---|---|
| 1. [42] | Grades- Reported comorbidities | 48 EX = 21 CO = 27 BMI = - | From 53 to 81 years old UK | Usual care | CT included 10 EX, for two sessions/W for five W. The EX was for 60 (Min). Supervised | 12 month |
|
|
| 2. [38] | Grades I and II OA | 30 EX = 15 CO = 15 BMI = - | From 50 to 60 years old India | Usual care | CT includes warm-up for 5 Min, walking, balance training, straight lunges, and one leg balance for 20 min/session, for four W, 3 times/W, 3 and sets. | 4 W |
|
|
| 3. [39] | Grades I, II, and III OA | 31 EX = 15 CO = 16 BMI = 30.21 ± 4.63 | From 45 to 75 years old Brazil | Usual care | CT includes warm-up for (10 min), strengthening exercises, aerobic exercise on a stationary bicycle (20 min), starting at 65–70% of maximum heart rate (MHR), stretching for (5 min), sitting and standing from a low chair. and walking while changing direction. The exercise was for 60 (min) supervised | 8 W |
|
|
| 4. [40] | Grades II and III | 40 EX = 20 CO = 20 BMI = 26 ± 3.08 | From 40 to 65 years old Brazil | Usual care | CT includes a total of 42 exercise sessions and is conducted in three sessions/W. Each session consists of warm-up for 5 (min), CT, and cool-down for (5 min). During the CT, the exercises were classified as light 20 min, moderate 30 min, and intense 40 min. There was a maximum of 30 s of rest between each stage. Supervised | 14 W |
|
|
| 5. [33] | Grades II and III | 40 EX = 20 CO = 20 BMI =< 30 kg/m2 | From 40 to 65 years old Brazil | Usual care | The CT included lower, upper body, and trunk exercises with intensity levels (light, moderate, and intense). The CT for (W 2, 3, and 5) was light exercise moderate exercises were in (W 6, 8, and 9) and intense exercises were in the (W 11, 12, and 14). Between each stage, there was a maximum of 30 s rest. The CT was for 3/W supervised | 14 W |
|
|
| 6. [43] | Grades I, II, III, and IV Reported comorbidities | 82 EX = 44 CO = 38 BMI = 30.6 ± 5.6 | 64.8 ± 8.7 years old Denmark | Usual care | CT for lower and upper extremities, consists of warm-up and cooldown periods. CT includes four exercise circles; in between the exercise was postural function: postural orientation, muscle strength, and functional exercises. The intensity was increased if the exercise quality could be maintained. The exercise was two/W with each session lasting 60 min. Supervised | 12 W |
|
|
| 7. [41] | Grades I, II, and III | 93 EX = 47 CO = 46 BMI = 27 ± 4 kg/m2 | From 40 to 70 years old Denmark | Usual care | CT consisted of five-stage: warming up (10 min of aerobic activity, functional exercise, proprioceptive (comprised three exercises), endurance strengthening exercise, and cooling down. The exercise was two/W (each session 60 min) for 8 W. Each exercise included three to four difficulty levels to ensure the progression. Supervised | 8 W |
|
|
| Outcome | Certainty Assessment | No. of Patients | Effect | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Circuit Training | Standard Treatment | Absolute (95% CI) | ||
| Pain level | 7 | RCT | Serious a | Very serious b | Not serious | Serious d | None | 182 | 182 | SMD 0.30 higher (0.37 higher to 0.56 higher) | ⊕◯◯◯ Very low |
| Quality of life | 3 | RCT | Not serious | Very serious b | Not serious | Serious d | None | 106 | 99 | SMD 0.25 lower (1.18 lower to 0.68 higher) | ⊕◯◯◯ Very low |
| Physical function | 5 | RCT | Serious a | Serious c | Not serious | Serious d | None | 147 | 147 | SMD 0.03 higher (0.44 lower to 0.5 higher) | ⊕◯◯◯ Very low |
| Health-related quality of life | 2 | RCT | Not serious | Not serious | Not serious | Serious d | None | 65 | 65 | SMD 0.36 higher (0.01 higher to 0.71 higher) | ⊕⊕⊕◯ Moderate |
| HDL | 1 | RCT | Serious a | Serious c | Not serious | Serious d | None | 20 | 20 | SMD 0.13 higher (0.49 lower to 0.75 higher) | ⊕◯◯◯ Very low |
| Triglyceride | 1 | RCT | Serious a | Serious c | Not serious | Serious d | None | 20 | 20 | SMD 0.13 higher (0.49 lower to 0.75 higher) | ⊕◯◯◯ Very low |
| Depression | 1 | RCT | Not serious | Serious c | Not serious | Serious d | None | 21 | 27 | SMD 0.12 higher (0.45 lower to 0.69 higher) | ⊕⊕◯◯ Low |
| Anxiety | 1 | RCT | Not serious | Serious c | Not serious | Serious d | None | 21 | 27 | SMD 0.12 higher (0.45 lower to 0.69 higher) | ⊕⊕◯◯ Low |
| Sports recreation | 2 | RCT | Not serious | Not serious | Not serious | Serious d | None | 91 | 84 | SMD 0.07 higher (0.23 lower to 0.37 higher) | ⊕⊕⊕◯ Moderate |
| Knee stiffness | 2 | RCT | Serious a | Serious c | Not serious | Serious d | None | 35 | 36 | SMD 0.65 lower (1.96 lower to 0.66 higher) | ⊕◯◯◯ Very low |
| The activity of daily living | 3 | RCT | Not serious | Very serious b | Not serious | Serious d | None | 112 | 111 | SMD 0.81 higher (0.85 lower to 2.48 higher) | ⊕◯◯◯ Very low |
| Knee osteoarthritis symptom | 2 | RCT | Not serious | Not serious | Not serious | Serious d | None | 91 | 84 | SMD 0.26 higher (0.05 lower to 0.58 higher) | ⊕⊕⊕◯ Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Mhanna, S.B.; Mohamed, M.; Mohd Noor, N.; Aldhahi, M.I.; Afolabi, H.A.; Mutalub, Y.B.; Irekeola, A.A.; Bello, K.E.; Wan Ghazali, W.S. Effects of Circuit Training on Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 2041. https://doi.org/10.3390/healthcare10102041
AL-Mhanna SB, Mohamed M, Mohd Noor N, Aldhahi MI, Afolabi HA, Mutalub YB, Irekeola AA, Bello KE, Wan Ghazali WS. Effects of Circuit Training on Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Healthcare. 2022; 10(10):2041. https://doi.org/10.3390/healthcare10102041
Chicago/Turabian StyleAL-Mhanna, Sameer Badri, Mahaneem Mohamed, Norhayati Mohd Noor, Monira I. Aldhahi, Hafeez Abiola Afolabi, Yahkub Babatunde Mutalub, Ahmad Adebayo Irekeola, Kizito Eneye Bello, and Wan Syaheedah Wan Ghazali. 2022. "Effects of Circuit Training on Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis" Healthcare 10, no. 10: 2041. https://doi.org/10.3390/healthcare10102041
APA StyleAL-Mhanna, S. B., Mohamed, M., Mohd Noor, N., Aldhahi, M. I., Afolabi, H. A., Mutalub, Y. B., Irekeola, A. A., Bello, K. E., & Wan Ghazali, W. S. (2022). Effects of Circuit Training on Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Healthcare, 10(10), 2041. https://doi.org/10.3390/healthcare10102041