Association between Nighttime Work and HbA1c Levels in South Korea
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Variables
2.3. Statistical Analysis
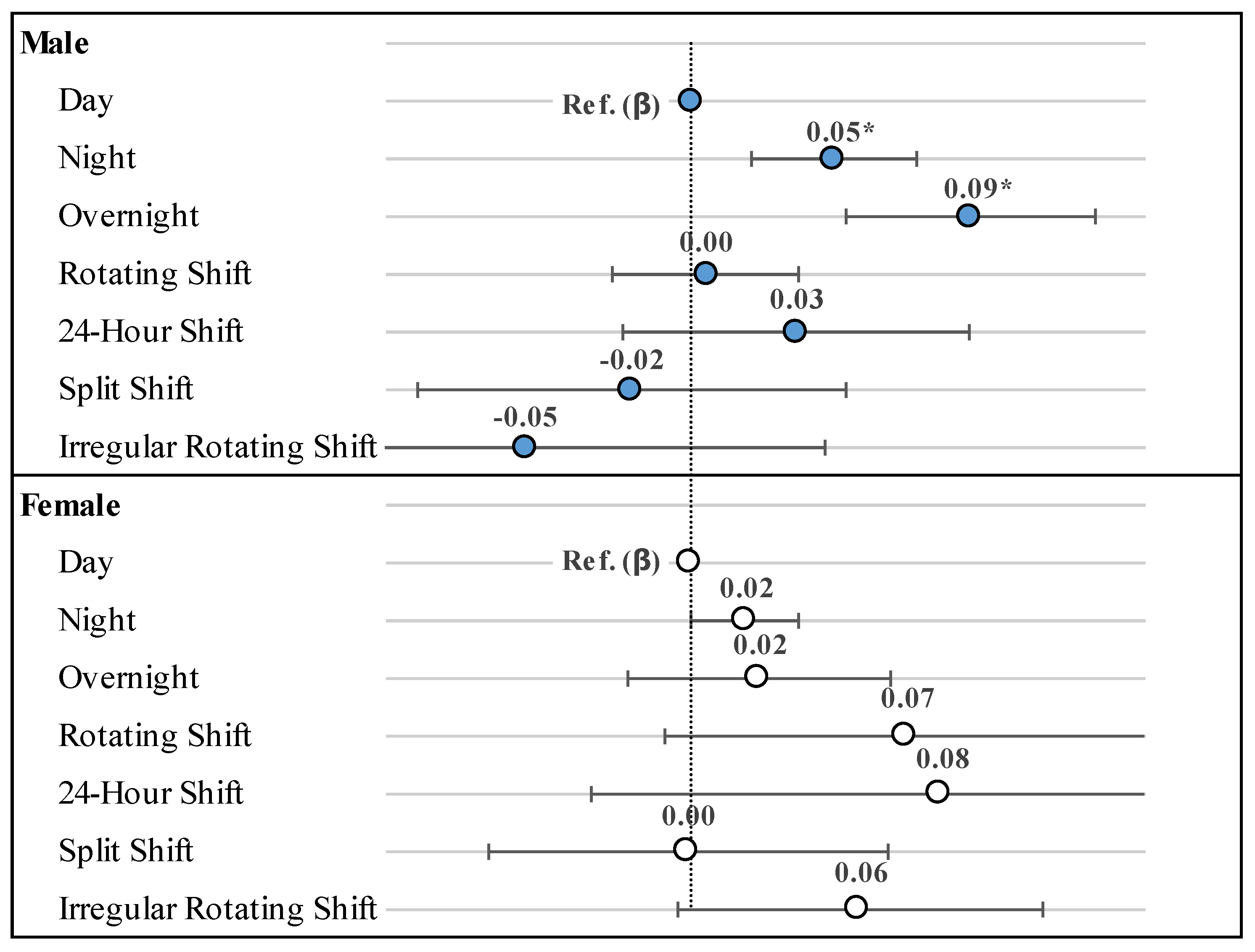
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.K. Analysis of the Relationship between Working Hour Mismatch and Worker’s Health. Health Soc. Welf. Rev. 2015, 35, 135–165. [Google Scholar]
- Sparks, K.; Cooper, C.; Fried, Y.; Shirom, A. The effects of hours of work on health: A meta-analytic review. In Managerial, Occupational and Organizational Stress Research; Routledge: Oxfordshire, UK, 2018; pp. 451–468. [Google Scholar]
- Caruso, C.C.; Hitchcock, E.M.; Dick, R.B.; Russo, J.M.; Schmitt, J.M. Overtime and Extended Work Shifts; Recent Findings on Illnesses, Injuries, and Health Behaviors; U.S. Department of Health and Human Services, NIOSH Publication: Washington, DC, USA, 2004; p. 143. [Google Scholar]
- Spurgeon, A.; Harrington, J.M.; Cooper, C.L. Health and safety problems associated with long working hours: A review of the current position. Occup. Environ. Med. 1997, 54, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Tucker, P.; Folkard, S. Working Time, Health and Safety: A Research Synthesis Paper; Conditions of Work and Employment Series 31; ILO: Geneva, Switzerland, 2012. [Google Scholar]
- Won, J.C.; Lee, J.H.; Kim, J.H.; Kang, E.S.; Won, K.C.; Kim, D.J.; Lee, M.K. Diabetes Fact Sheet in Korea, 2016: An Appraisal of Current Status. Diabetes Metab. J. 2018, 42, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Seuring, T.; Archangelidi, O.; Suhrcke, M. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. Pharmacoeconomics 2015, 33, 811–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Diabetes 1: Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- Forouhi, N.G.; Luan, J.; Hennings, S.; Wareham, N.J. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: The Ely study 1990–2000. Diabet. Med. 2007, 24, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Davidson, M.B.; DeFronzo, R.A.; Heine, R.J.; Henry, R.R.; Pratley, R.; Zinman, B. Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care 2007, 30, 753–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [Green Version]
- The DREAM (Diabetes Reduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [CrossRef] [Green Version]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjöström, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Uusitupa, M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Cho, N.H. Diabetes epidemiology in Korean. Diabetes 2001, 25, 1–10. [Google Scholar]
- Kweon, S.; Kim, Y.; Jang, M.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.; Senan, C.; Gamier, P.; Saint-Paul, M. Papoz L: Epidemiological features of glycated haemoglobin Ale-distribution in a healthy population. Diabetologa 1989, 32, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Dukes, P.P.; Goldwasser, E. Inhibition of erythropoiesis by estrogens. Endocrinology 1961, 69, 21–25. [Google Scholar] [CrossRef]
- Peschle, C.; Magli, M.C.; Cillo, C.; Lettieri, F.; Cacciapuoti, A.; Pizzella, E.; Marone, G. Erythroid colony formation and erythropoietin activity I mice treated with estradiol benzoate. Life Sci. 1977, 21, 1303–1310. [Google Scholar] [CrossRef]
- Roenneberg, T.; Merrow, M. The circadian clock and human health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ 2016, 355, i5210. [Google Scholar] [CrossRef]
- Wang, X.S.; Armstrong, M.E.G.; Cairns, B.J.; Key, T.J.; Travis, R.C. Shift work and chronic disease: The epidemiological evidence. Occup. Med. 2011, 61, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, Y.; Yang, C.; Tong, X.; Sun, H.; Cong, Y.; Yin, X.; Li, L.; Cao, S.; Dong, X.; Gong, Y.; et al. Shift work and diabetes mellitus: A meta-analysis of observational studies. Occup. Environ. Med. 2015, 72, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Claus, M.; Schuster, M.; Oberlinner, C.; Webendörfer, S. Rotating shift work and prevalence of diabetes mellitus and prediabetes in male employees of a large german chemical company: Results of a cross-sectional study. Occup. Environ. Med. 2017, 74, A73. [Google Scholar]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, E.K.; Lee, M.Y.; Jeong, E.S.; Sung, D.J. Difference of Selected Metabolic Syndrome Makers in Nurses on Rotating Shift and Daytime Fixed Work Schedules. Korean Soc. Wellness 2018, 13, 61–72. [Google Scholar] [CrossRef]
- Hong, D.U.; Lee, D.C.; Lee, S.Y.; Kim, Y.S. A Prospective Cohort Study of Exercise and the Incidence of Type 2 Diabetes in Impaired Fasting Glucose Group. J. Prev. Med. Public Health 2008, 41, 45–50. [Google Scholar]
- Park, S.Y.; Lee, S.W.; Lee, H.S.; Byun, A.R.; Kwon, Y.E. Effects of Intermittent Leisure Time Physical Activity on Glycemic Control in Korean Adult Men with Diabetes and Prediabetes. Korean Acad. Fam. Med. 2019, 9, 29–35. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hillard, P.J.A. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Jun, K.Y. Association between Occupational Environments including Working Hours and Sleep Duration among Korean Adults. Master’s Thesis, Hanyang University Graduate School, Seoul, Korea, 2015. [Google Scholar]
- Ha, Y.M. The Effect of Sleep Quality and Quantity on Type II Diabetes Mellitus. Master’s Thesis, Chung-Ang University Graduate School of Health and Nursing, Anseong, Korea, 2015. [Google Scholar]
- Knutson, K.L.; Ryden, A.M.; Mander, B.A.; Cauter, E.V. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch. Intern. Med. 2006, 166, 1768–1774. [Google Scholar] [CrossRef] [Green Version]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 2007, 56, 1729–1734. [Google Scholar] [CrossRef] [Green Version]
- You, H.S.; Lee, J.H.; Choi, W.J.; Lee, J.W. Relationship between Skipping Meals and Impaired Fasting Glucose in Non-Diabetic Korean Adults. Korean J. Fam. Pract. 2017, 7, 824–829. [Google Scholar] [CrossRef]
- Zhang, X.; Gregg, E.W.; Williamson, D.F.; Barker, L.E.; Thomas, W.; Bullard, K.M.; Imperatore, G.; Williams, D.E.; Albright, A.L. A1C level and future risk of diabetes: A systematic review. Diabetes Care 2010, 33, 1665–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Kim, T.H.; Lim, S.; Park, K.S.; Jang, H.C.; Cho, N.H. Hemoglobin A1c as a diagnostic tool for diabetes screening and new-onset diabetes prediction: A 6-year community-based prospective study. Diabetes Care 2011, 34, 944–949. [Google Scholar] [CrossRef] [PubMed]
| Variables | HbA1c (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | ||||||||||||
| n | % | n | % | Mean ± SD | p-Value | n | % | Mean ± SD | p-Value | |||||
| Total | 9591 | 100.0 | 4773 | 49.8 | 5.60 | ± | 0.39 | 4818 | 50.2 | 5.55 | ± | 0.34 | ||
| Work schedule | 0.0071 | 0.2566 | ||||||||||||
| Day | 8180 | 85.3 | 4103 | 86.0 | 5.59 | ± | 0.43 | 4077 | 84.6 | 5.55 | ± | 0.42 | ||
| Night and overnight | 883 | 9.2 | 302 | 6.3 | 5.64 | ± | 0.46 | 581 | 12.1 | 5.56 | ± | 0.42 | ||
| Other | 528 | 5.5 | 368 | 7.7 | 5.64 | ± | 0.49 | 160 | 3.3 | 5.60 | ± | 0.44 | ||
| Age | <0.0001 | <0.0001 | ||||||||||||
| 30–39 | 2107 | 22.0 | 1135 | 23.8 | 5.43 | ± | 0.31 | 972 | 20.2 | 5.31 | ± | 0.30 | ||
| 40–49 | 2623 | 27.3 | 1253 | 26.3 | 5.53 | ± | 0.42 | 1370 | 28.4 | 5.42 | ± | 0.34 | ||
| 50–59 | 2441 | 25.5 | 1138 | 23.8 | 5.65 | ± | 0.41 | 1303 | 27.0 | 5.64 | ± | 0.37 | ||
| 60–69 | 1615 | 16.8 | 821 | 17.2 | 5.73 | ± | 0.47 | 794 | 16.5 | 5.78 | ± | 0.45 | ||
| ≥70 | 805 | 8.4 | 426 | 8.9 | 5.83 | ± | 0.58 | 379 | 7.9 | 5.90 | ± | 0.51 | ||
| Educational level | 0.8381 | 0.8638 | ||||||||||||
| ≤Elementary | 1404 | 14.6 | 515 | 10.8 | 5.74 | ± | 0.49 | 889 | 18.5 | 5.79 | ± | 0.50 | ||
| Middle | 919 | 9.6 | 446 | 9.3 | 5.72 | ± | 0.48 | 473 | 9.8 | 5.68 | ± | 0.41 | ||
| High | 2982 | 31.1 | 1418 | 29.7 | 5.62 | ± | 0.46 | 1564 | 32.5 | 5.54 | ± | 0.40 | ||
| ≥College | 4286 | 44.7 | 2394 | 50.2 | 5.53 | ± | 0.39 | 1892 | 39.3 | 5.43 | ± | 0.34 | ||
| Occupational status | 0.0719 | 0.0607 | ||||||||||||
| White | 3936 | 41.0 | 1952 | 40.9 | 5.52 | ± | 0.39 | 1984 | 41.2 | 5.43 | ± | 0.35 | ||
| Pink | 1886 | 19.7 | 565 | 11.8 | 5.61 | ± | 0.44 | 1321 | 27.4 | 5.60 | ± | 0.45 | ||
| Blue | 3769 | 39.3 | 2256 | 47.3 | 5.66 | ± | 0.47 | 1513 | 31.4 | 5.68 | ± | 0.44 | ||
| Household income | 0.451 | 0.3665 | ||||||||||||
| Low | 1022 | 10.7 | 386 | 8.1 | 5.72 | ± | 0.58 | 636 | 13.2 | 5.74 | ± | 0.50 | ||
| Middle | 5143 | 53.6 | 2,621 | 54.9 | 5.60 | ± | 0.42 | 2522 | 52.3 | 5.56 | ± | 0.42 | ||
| High | 3426 | 35.7 | 1,766 | 37.0 | 5.56 | ± | 0.43 | 1660 | 34.5 | 5.48 | ± | 0.37 | ||
| Household composition | 0.5193 | 0.3746 | ||||||||||||
| One-person household | 936 | 9.8 | 412 | 8.6 | 5.56 | ± | 0.47 | 524 | 10.9 | 5.70 | ± | 0.53 | ||
| One-generation household | 2280 | 23.8 | 1238 | 25.9 | 5.68 | ± | 0.49 | 1042 | 21.6 | 5.66 | ± | 0.44 | ||
| ≥Two-generation household | 6375 | 66.5 | 3123 | 65.4 | 5.57 | ± | 0.41 | 3252 | 67.5 | 5.50 | ± | 0.38 | ||
| Physical activity | 0.7231 | 0.9600 | ||||||||||||
| Active | 4086 | 42.6 | 2161 | 45.3 | 5.57 | ± | 0.43 | 1,925 | 40.0 | 5.54 | ± | 0.41 | ||
| Inactive | 5505 | 57.4 | 2612 | 54.7 | 5.62 | ± | 0.45 | 2,893 | 60.0 | 5.56 | ± | 0.43 | ||
| Smoking status | <0.0001 | 0.4196 | ||||||||||||
| Current smoker | 1864 | 19.4 | 1648 | 34.5 | 5.61 | ± | 0.45 | 216 | 4.5 | 5.47 | ± | 0.37 | ||
| Former smoker | 2381 | 24.8 | 2100 | 44.0 | 5.62 | ± | 0.45 | 281 | 5.8 | 5.43 | ± | 0.45 | ||
| Non-smoker | 5346 | 55.7 | 1025 | 21.5 | 5.54 | ± | 0.40 | 4,321 | 89.7 | 5.57 | ± | 0.42 | ||
| Drinking status | <0.0001 | <0.0001 | ||||||||||||
| 2–4 times/week | 2471 | 25.8 | 1824 | 38.2 | 5.57 | ± | 0.42 | 647 | 13.4 | 5.41 | ± | 0.38 | ||
| 2–4 times/month | 2289 | 23.9 | 1264 | 26.5 | 5.58 | ± | 0.45 | 1025 | 21.3 | 5.47 | ± | 0.40 | ||
| Never or occasionally | 4831 | 50.4 | 1685 | 35.3 | 5.64 | ± | 0.44 | 3146 | 65.3 | 5.61 | ± | 0.43 | ||
| Hours of sleep | 0.0426 | 0.2398 | ||||||||||||
| <7 h | 3779 | 39.4 | 1932 | 40.5 | 5.62 | ± | 0.46 | 1847 | 38.3 | 5.59 | ± | 0.41 | ||
| ≥7 h | 5812 | 60.6 | 2841 | 59.5 | 5.58 | ± | 0.42 | 2971 | 61.7 | 5.53 | ± | 0.43 | ||
| Eating habits | 0.0975 | <0.0001 | ||||||||||||
| Eating three meals regularly | 5438 | 56.7 | 2773 | 58.1 | 5.64 | ± | 0.46 | 2665 | 55.3 | 5.63 | ± | 0.43 | ||
| Skip meal (s) | 4153 | 43.3 | 2000 | 41.9 | 5.54 | ± | 0.41 | 2153 | 44.7 | 5.46 | ± | 0.39 | ||
| Total energy intake (kcal) a | 0.4732 | 0.8141 | ||||||||||||
| Quintile 1 | 1919 | 20.0 | 956 | 20.0 | 5.64 | ± | 0.48 | 963 | 20.0 | 5.56 | ± | 0.46 | ||
| Quintile 2 | 1918 | 20.0 | 954 | 20.0 | 5.62 | ± | 0.49 | 964 | 20.0 | 5.57 | ± | 0.40 | ||
| Quintile 3 | 1919 | 20.0 | 955 | 20.0 | 5.58 | ± | 0.41 | 964 | 20.0 | 5.56 | ± | 0.43 | ||
| Quintile 4 | 1918 | 20.0 | 954 | 20.0 | 5.60 | ± | 0.44 | 964 | 20.0 | 5.55 | ± | 0.43 | ||
| Quintile 5 | 1917 | 20.0 | 954 | 20.0 | 5.54 | ± | 0.35 | 963 | 20.0 | 5.53 | ± | 0.39 | ||
| BMI (kg/m2) b | <0.0001 | <0.0001 | ||||||||||||
| Obese (≥25) | 3340 | 34.8 | 2015 | 42.2 | 5.66 | ± | 0.44 | 1325 | 27.5 | 5.71 | ± | 0.46 | ||
| Normal (18.5~24.9) | 5964 | 62.2 | 2669 | 55.9 | 5.55 | ± | 0.43 | 3295 | 68.4 | 5.51 | ± | 0.39 | ||
| Underweight (<18.5) | 287 | 3.0 | 89 | 1.9 | 5.51 | ± | 0.35 | 198 | 4.1 | 5.33 | ± | 0.34 | ||
| Hypertension (mm Hg) | 0.0017 | <0.0001 | ||||||||||||
| Hypertension (≥140) | 2758 | 28.8 | 1582 | 33.1 | 5.71 | ± | 0.47 | 1176 | 24.4 | 5.78 | ± | 0.48 | ||
| Prehypertension (120~139) | 2605 | 27.2 | 1520 | 31.8 | 5.58 | ± | 0.43 | 1085 | 22.5 | 5.59 | ± | 0.39 | ||
| Normal (<120) | 4228 | 44.1 | 1671 | 35.0 | 5.51 | ± | 0.39 | 2557 | 53.1 | 5.44 | ± | 0.36 | ||
| Fasting glucose level (mg/dL) | <0.0001 | <0.0001 | ||||||||||||
| Impaired fasting glucose (100~125) | 3206 | 33.4 | 1930 | 40.4 | 5.79 | ± | 0.49 | 1276 | 26.5 | 5.85 | ± | 0.48 | ||
| Normal (<100) | 6385 | 66.6 | 2843 | 59.6 | 5.47 | ± | 0.35 | 3542 | 73.5 | 5.45 | ± | 0.34 | ||
| Year | <0.0001 | <0.0001 | ||||||||||||
| 2016 | 2258 | 23.5 | 1144 | 24.0 | 5.57 | ± | 0.45 | 1114 | 23.1 | 5.50 | ± | 0.40 | ||
| 2017 | 2382 | 24.8 | 1181 | 24.7 | 5.55 | ± | 0.40 | 1201 | 24.9 | 5.53 | ± | 0.41 | ||
| 2018 | 2492 | 26.0 | 1220 | 25.6 | 5.58 | ± | 0.41 | 1272 | 26.4 | 5.55 | ± | 0.43 | ||
| 2019 | 2459 | 25.6 | 1228 | 25.7 | 5.68 | ± | 0.49 | 1231 | 25.6 | 5.64 | ± | 0.43 | ||
| Variable | HbA1c (%) | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| β | SE | p-Value | β | SE | p-Value | |
| Work schedule | ||||||
| Day | Ref. | Ref. | ||||
| Night and overnight | 0.061 | 0.023 | 0.0085 | 0.019 | 0.017 | 0.2687 |
| Other | 0.008 | 0.025 | 0.7557 | 0.049 | 0.032 | 0.1273 |
| Age | ||||||
| 30–39 | Ref. | Ref. | ||||
| 40–49 | 0.056 | 0.015 | 0.0002 | 0.062 | 0.013 | <0.0001 |
| 50–59 | 0.135 | 0.017 | <0.0001 | 0.200 | 0.017 | <0.0001 |
| 60–69 | 0.207 | 0.022 | <0.0001 | 0.272 | 0.024 | <0.0001 |
| ≥70 | 0.296 | 0.039 | <0.0001 | 0.326 | 0.038 | <0.0001 |
| Educational level | ||||||
| ≤Elementary | −0.017 | 0.028 | 0.5471 | 0.009 | 0.026 | 0.7357 |
| Middle | −0.020 | 0.026 | 0.4381 | 0.014 | 0.024 | 0.5605 |
| High | −0.012 | 0.015 | 0.4321 | −0.003 | 0.013 | 0.8335 |
| ≥College | Ref. | Ref. | ||||
| Occupational status | ||||||
| White | Ref. | Ref. | ||||
| Pink | 0.040 | 0.020 | 0.0460 | 0.014 | 0.014 | 0.3233 |
| Blue | 0.036 | 0.015 | 0.0166 | −0.010 | 0.017 | 0.5349 |
| Household income | ||||||
| Low | 0.005 | 0.031 | 0.8590 | −0.001 | 0.022 | 0.9491 |
| Middle | 0.013 | 0.013 | 0.3162 | 0.013 | 0.011 | 0.2480 |
| High | Ref. | Ref. | ||||
| Household composition | ||||||
| One-person household | −0.040 | 0.021 | 0.0522 | 0.032 | 0.021 | 0.1370 |
| One-generation household | −0.002 | 0.016 | 0.9219 | 0.010 | 0.015 | 0.4869 |
| ≥Two-generation household | Ref. | Ref. | ||||
| Physical activity | ||||||
| Active | Ref. | Ref. | ||||
| Inactive | 0.006 | 0.012 | 0.5834 | −0.002 | 0.011 | 0.8164 |
| Smoking status | ||||||
| Current smoker | 0.080 | 0.016 | < 0.0001 | −0.010 | 0.023 | 0.6642 |
| Former smoker | 0.015 | 0.015 | 0.3137 | −0.027 | 0.023 | 0.2503 |
| Non-smoker | Ref. | Ref. | ||||
| Drinking status | ||||||
| 2–4 times/week | −0.113 | 0.014 | < 0.0001 | −0.118 | 0.016 | <0.0001 |
| 2–4 times/month | −0.046 | 0.015 | 0.0020 | −0.035 | 0.013 | 0.0077 |
| Never or occasionally | Ref. | Ref. | ||||
| Hours of sleep | ||||||
| <7 h | 0.021 | 0.012 | 0.0727 | 0.023 | 0.011 | 0.0393 |
| ≥7 h | Ref. | Ref. | ||||
| Eating habits | ||||||
| Eating three meals regularly | Ref. | Ref. | ||||
| Skipping meals | −0.020 | 0.013 | 0.1198 | −0.045 | 0.011 | <0.0001 |
| Total energy intake (kcal) a | ||||||
| Quintile 1 | 0.004 | 0.018 | 0.8165 | −0.014 | 0.018 | 0.4402 |
| Quintile 2 | 0.022 | 0.019 | 0.2519 | 0.000 | 0.016 | 0.9795 |
| Quintile 3 | Ref. | Ref. | ||||
| Quintile 4 | 0.018 | 0.018 | 0.3109 | −0.007 | 0.016 | 0.6509 |
| Quintile 5 | 0.000 | 0.017 | 0.9834 | 0.007 | 0.016 | 0.6596 |
| BMI (kg/m2) b | ||||||
| Obese (≥25) | 0.071 | 0.012 | <0.0001 | 0.080 | 0.013 | <0.0001 |
| Normal (18.5~24.9) | Ref. | Ref. | ||||
| Underweight (<18.5) | −0.031 | 0.031 | 0.3094 | −0.067 | 0.025 | 0.0079 |
| Hypertension (mm Hg) | ||||||
| Hypertension (≥140) | 0.064 | 0.016 | <0.0001 | 0.079 | 0.018 | <0.0001 |
| Prehypertension (120~139) | 0.022 | 0.014 | 0.1189 | 0.030 | 0.014 | 0.0272 |
| Normal (<120) | Ref. | Ref. | ||||
| Fasting glucose level (mg/dL) | ||||||
| Impaired fasting glucose (100~125) | 0.267 | 0.013 | <0.0001 | 0.297 | 0.015 | <0.0001 |
| Normal (<100) | Ref. | Ref. | ||||
| Year | ||||||
| 2016 | Ref. | Ref. | ||||
| 2017 | −0.021 | 0.016 | 0.1844 | 0.033 | 0.015 | 0.0321 |
| 2018 | −0.003 | 0.017 | 0.8475 | 0.048 | 0.015 | 0.0018 |
| 2019 | 0.095 | 0.018 | <0.0001 | 0.120 | 0.015 | <0.0001 |
| Variable | HbA1c (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Male (n = 4773) | Female (n = 4818) | |||||||||||
| Night and Overnight (n = 302) | Other (n = 368) | Night and Overnight (n = 581) | Other (n = 160) | ||||||||||
| Β | β | SE | p-Value | β | SE | p-Value | β | SE | p-Value | β | SE | p-Value | |
| Physical activity | |||||||||||||
| Active | Ref. | 0.023 | 0.034 | 0.4995 | 0.014 | 0.035 | 0.6842 | −0.011 | 0.027 | 0.6791 | −0.015 | 0.038 | 0.7016 |
| Inactive | Ref. | 0.094 | 0.032 | 0.0031 | −0.002 | 0.037 | 0.9605 | 0.037 | 0.022 | 0.0899 | 0.092 | 0.048 | 0.0561 |
| Hours of sleep | |||||||||||||
| <7 h | Ref. | 0.108 | 0.033 | 0.0009 | −0.049 | 0.038 | 0.2011 | 0.024 | 0.026 | 0.3685 | 0.053 | 0.048 | 0.2747 |
| ≥7 h | Ref. | 0.034 | 0.032 | 0.2894 | 0.065 | 0.032 | 0.0434 | 0.014 | 0.022 | 0.5105 | 0.042 | 0.041 | 0.3095 |
| Eating habits | |||||||||||||
| Eating three meals regularly | Ref. | 0.053 | 0.033 | 0.1095 | −0.008 | 0.035 | 0.8227 | −0.006 | 0.025 | 0.8017 | 0.056 | 0.050 | 0.2661 |
| Skipping meals | Ref. | 0.064 | 0.031 | 0.0401 | 0.028 | 0.036 | 0.4494 | 0.035 | 0.022 | 0.1206 | 0.055 | 0.041 | 0.1852 |
| Total energy intake (kcal) a | |||||||||||||
| Quintile 1 | Ref. | 0.108 | 0.057 | 0.0579 | 0.067 | 0.077 | 0.3870 | 0.025 | 0.038 | 0.5129 | 0.132 | 0.110 | 0.2336 |
| Quintile 2 | Ref. | 0.059 | 0.056 | 0.2922 | −0.042 | 0.045 | 0.3539 | 0.008 | 0.038 | 0.8297 | 0.079 | 0.074 | 0.2863 |
| Quintile 3 | Ref. | 0.057 | 0.041 | 0.1679 | −0.075 | 0.052 | 0.1555 | 0.010 | 0.044 | 0.8155 | 0.036 | 0.052 | 0.4823 |
| Quintile 4 | Ref. | −0.021 | 0.050 | 0.6658 | 0.021 | 0.057 | 0.7097 | 0.021 | 0.036 | 0.5720 | −0.011 | 0.057 | 0.8546 |
| Quintile 5 | Ref. | 0.045 | 0.046 | 0.3293 | 0.070 | 0.053 | 0.1913 | 0.066 | 0.030 | 0.0298 | 0.070 | 0.047 | 0.1352 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-S.; Joo, J.H.; Park, E.-C. Association between Nighttime Work and HbA1c Levels in South Korea. Healthcare 2022, 10, 1977. https://doi.org/10.3390/healthcare10101977
Lee Y-S, Joo JH, Park E-C. Association between Nighttime Work and HbA1c Levels in South Korea. Healthcare. 2022; 10(10):1977. https://doi.org/10.3390/healthcare10101977
Chicago/Turabian StyleLee, Yeon-Suk, Jae Hong Joo, and Eun-Cheol Park. 2022. "Association between Nighttime Work and HbA1c Levels in South Korea" Healthcare 10, no. 10: 1977. https://doi.org/10.3390/healthcare10101977
APA StyleLee, Y.-S., Joo, J. H., & Park, E.-C. (2022). Association between Nighttime Work and HbA1c Levels in South Korea. Healthcare, 10(10), 1977. https://doi.org/10.3390/healthcare10101977








