Spinal Cord Stimulation in Chronic Low Back Pain Syndrome: Mechanisms of Modulation, Technical Features and Clinical Application
Abstract
1. Introduction
2. Mechanisms of Pain Relief during Spinal Cord Stimulation
2.1. Spinal Segmental Mechanisms
2.2. Suprasegmental Mechanisms
3. Technical Features
3.1. Stimulation Waveform
3.2. Arrays and Electrodes
3.3. Pulse Generator
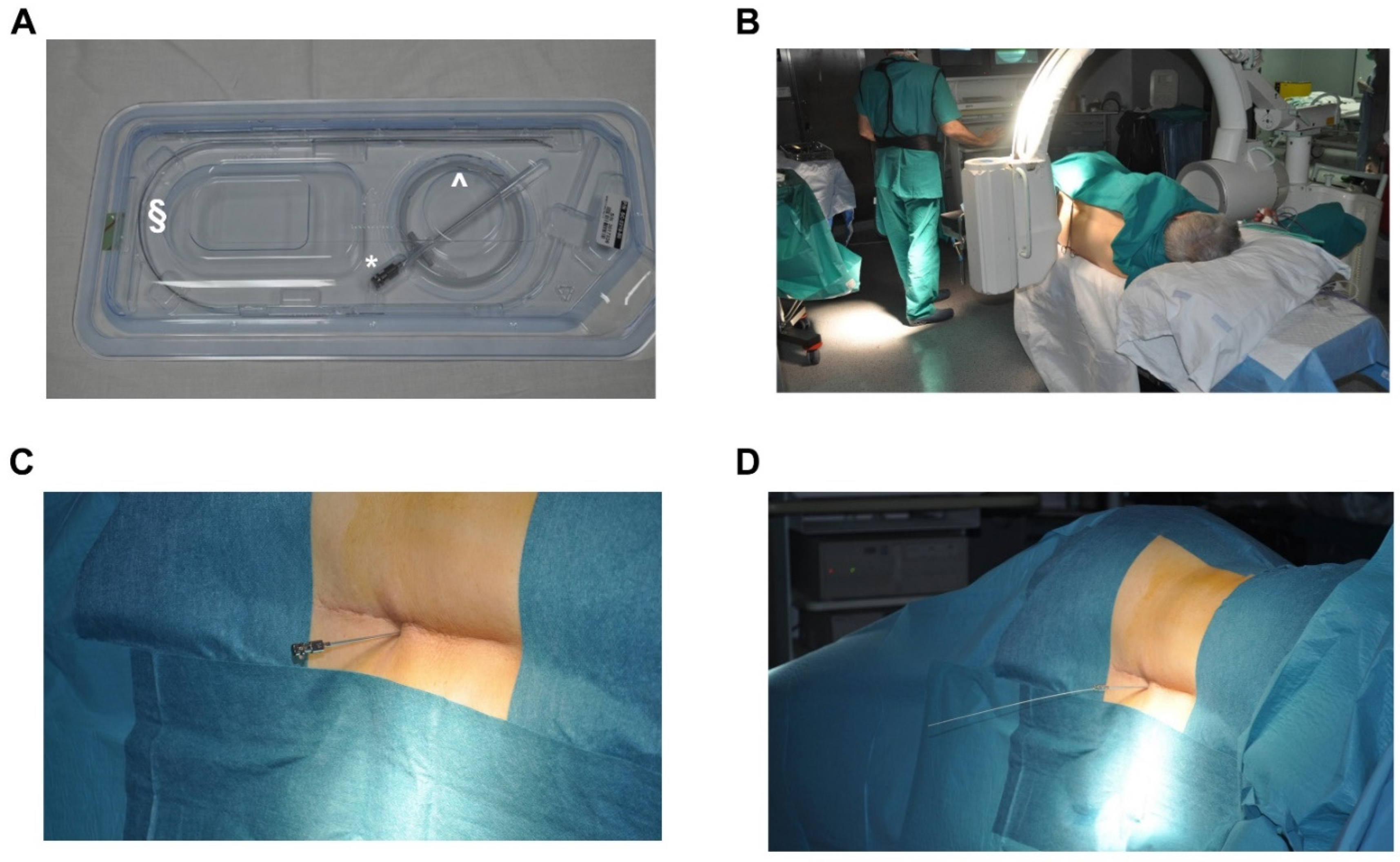
3.4. Procedure of Implantation
4. Clinical Considerations in Chronic Low Back Pain
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geertzen, J.H.B.; Van Wilgen, C.P.; Schrier, E.; Dijkstra, P.U. Chronic pain in rehabil. medicine. Disabil. Rehabil. 2006, 28, 363–367. [Google Scholar] [CrossRef]
- Rozenberg, S. Chronic low back pain: Definition and treatment. Rev. Prat. 2008, 58, 265–272. [Google Scholar]
- Schug, S.A.; Lavand’Homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D.; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic postsurgical or posttraumatic pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.; Hazzard, M.A.; Huang, K.T.; Ugiliweneza, B.; Patil, C.G.; Boakye, M.; Lad, S.P. Outcomes of Percutaneous and Paddle Lead Implantation for Spinal Cord Stimulation: A Comparative Analysis of Complications, Reoperation Rates, and Health-Care Costs. Neuromodul. Technol. Neural Interface 2013, 16, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for chronic pain. Lancet 2021, 397, 2111–2124. [Google Scholar] [CrossRef]
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Lempka, S.F.; Patil, P.G. Innovations in spinal cord stimulation for pain. Curr. Opin. Biomed. Eng. 2018, 8, 51–60. [Google Scholar] [CrossRef]
- Granville, M.; Berti, A.; Jacobson, R.E. Use of Spinal Cord Stimulation in Elderly Patients with Multi-Factorial Chronic Lumbar and Non-Radicular Lower Extremity Pain. Cureus 2017, 9, e1855. [Google Scholar] [CrossRef]
- Kemler, M.A.; Barendse, G.A.; van Kleef, M.; de Vet, H.C.; Rijks, C.P.; Furnée, C.A.; Wildenberg, F.A.V.D. Spinal Cord Stimulation in Patients with Chronic Reflex Sympathetic Dystrophy. N. Engl. J. Med. 2000, 343, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008, 63, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Galafassi, G.Z.; de Aguiar, P.H.S.P.; Simm, R.F.; Franceschini, P.R.; Filho, M.P.; Pagura, J.R.; de Aguiar, P.H.P. Neuromodulation for Medically Refractory Neuropathic Pain: Spinal Cord Stimulation, Deep Brain Stimulation, Motor Cortex Stimulation, and Posterior Insula Stimulation. World Neurosurg. 2020, 146, 246–260. [Google Scholar] [CrossRef]
- Duarte, R.V.; Nevitt, S.; McNicol, E.; Taylor, R.S.; Buchser, E.; North, R.B.; Eldabe, S. Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain 2019, 161, 24–35. [Google Scholar] [CrossRef]
- Chakravarthy, K.; Kent, A.R.; Raza, A.; Xing, F.; Kinfe, T.M.; Mph, A.R. Burst Spinal Cord Stimulation: Review of Preclinical Studies and Comments on Clinical Outcomes. Neuromodul. Technol. Neural Interface 2018, 21, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.A.; Franken, G. Spinal cord stimulation in chronic neuropathic pain: Mechanisms of action, new locations, new paradigms. Pain 2020, 161, S104–S113. [Google Scholar] [CrossRef]
- Guan, Y. Spinal Cord Stimulation: Neurophysiological and Neurochemical Mechanisms of Action. Curr. Pain Headache Rep. 2012, 16, 217–225. [Google Scholar] [CrossRef]
- Sivanesan, E.; Maher, D.P.; Raja, S.N.; Linderoth, B.; Guan, Y. Supraspinal Mechanisms of Spinal Cord Stimulation for Modulation of Pain. Anesthesiology 2019, 130, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Saadé, N.E.; Tabet, M.S.; Banna, N.R.; Atweh, S.F.; Jabbur, S.J. Inhibition of nociceptive evoked activity in spinal neurons through a dorsal column-brainstem-spinal loop. Brain Res. 1985, 339, 115–118. [Google Scholar] [CrossRef]
- Bantli, H.; Bloedel, J.R.; Thienprasit, P. Supraspinal interactions resulting from experimental dorsal column stimulation. J. Neurosurg. 1975, 42, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ansah, O.; Meyerson, B.; Pertovaara, A.; Linderoth, B. Exploration of supraspinal mechanisms in effects of spinal cord stimulation: Role of the locus coeruleus. Neuroscience 2013, 253, 426–434. [Google Scholar] [CrossRef]
- Saadé, E.N.; Atweh, S.F.; Privat, A.; Jabbur, S.J. Inhibitory effects from various types of dorsal column and raphe magnus stimulations on nociceptive withdrawal flexion reflexes. Brain Res. 1999, 846, 72–86. [Google Scholar] [CrossRef]
- Song, Z.; Ansah, O.; Meyerson, B.; Pertovaara, A.; Linderoth, B. The rostroventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain. Neuroscience 2013, 247, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.-O.; Linderoth, B.; O’Connor, W.T.; Franck, J.; Falkenberg, T.; Ungerstedt, U.; Brodin, E. Repeated spinal cord stimulation decreases the extracellular level of γ-aminobutyric acid in the periaqueductal gray matter of freely moving rats. Brain Res. 1995, 699, 231–241. [Google Scholar] [CrossRef]
- Linderoth, B.; Gazelius, B.; Franck, J.; Brodin, E. Dorsal Column Stimulation Induces Release of Serotonin and Substance P in the Cat Dorsal Horn. Neurosurgery 1992, 31, 289–297. [Google Scholar] [CrossRef]
- Schechtmann, G.; Song, Z.; Ultenius, C.; Meyerson, B.A.; Linderoth, B. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain 2008, 139, 136–145. [Google Scholar] [CrossRef]
- Song, Z.; Meyerson, B.A.; Linderoth, B. Muscarinic receptor activation potentiates the effect of spinal cord stimulation on pain-related behavior in rats with mononeuropathy. Neurosci. Lett. 2008, 436, 7–12. [Google Scholar] [CrossRef]
- Stiller, C.-O.; Cui, J.-G.; O’Connor, W.T.; Brodin, E.; Meyerson, B.A.; Linderoth, B. Release of γ-Aminobutyric Acid in the Dorsal Horn and Suppression of Tactile Allodynia by Spinal Cord Stimulation in Mononeuropathic Rats. Neurosurgery 1996, 39, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Yoshimura, M.; Nishi, S.; Shimoji, K. Synaptic responses of substantia gelatinosa neurones to dorsal column stimulation in rat spinal cord in vitro. J. Physiol. 1994, 478, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.-G.; O’Connor, W.; Ungerstedt, U.; Linderoth, B.; Meyerson, B.A. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain 1997, 73, 87–95. [Google Scholar] [CrossRef]
- Lind, G.; Schechtmann, G.; Winter, J.; Meyerson, B.A.; Linderoth, B. Baclofen-enhanced spinal cord stimulation and intrathecal baclofen alone for neuropathic pain: Long-term outcome of a pilot study. Eur. J. Pain 2008, 12, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Wu, S.-X.; Liu, X.-Y.; Wang, W.; Li, Y.-Q. Effects of c-fos antisense oligodeoxynucleotide on 5-HT-induced upregulation of preprodynorphin, preproenkephalin, and glutamic acid decarboxylase mRNA expression in cultured rat spinal dorsal horn neurons. Biochem. Biophys. Res. Commun. 2003, 309, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Meyerson, B.A.; Linderoth, B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain 2011, 152, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth. Analg. 1967, 46, 489–491. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; McNamee, J.P.; Wu, L.; Piantadosi, S. Artificial neural networks: Application to electrical stimulation of the human nervous system. Neurosurg. Focus 1997, 2, E3. [Google Scholar] [CrossRef]
- Brill, S.; Defrin, R.; Aryeh, I.G.; Zusman, A.M.; Benyamini, Y. Short- and long-term effects of conventional spinal cord stimulation on chronic pain and health perceptions: A longitudinal controlled trial. Eur. J. Pain 2022, 26, 1849–1862. [Google Scholar] [CrossRef]
- De Ridder, D.; Plazier, M.; Kamerling, N.; Menovsky, T.; Vanneste, S. Burst Spinal Cord Stimulation for Limb and Back Pain. World Neurosurg. 2013, 80, 642–649.e1. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S. Burst and Tonic Spinal Cord Stimulation: Different and Common Brain Mechanisms. Neuromodulation 2016, 19, 47. [Google Scholar] [CrossRef]
- Deer, T.; Slavin, K.V.; Amirdelfan, K.; North, R.B.; Burton, A.W.; Yearwood, T.L.; Tavel, E.; Staats, P.; Falowski, S.; Pope, J.; et al. Success Using Neuromodulation with BURST (SUNBURST) Study: Results from a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodul. Technol. Neural Interface 2018, 21, 56–66. [Google Scholar] [CrossRef]
- Ross, E.; Abejón, D. Improving Patient Experience with Spinal Cord Stimulation: Implications of Position-Related Changes in Neurostimulation. Neuromodul. Technol. Neural Interface 2014, 17, 36–41. [Google Scholar] [CrossRef]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Brown, L.L.; Yearwood, T.L.; et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef]
- Tiede, J.; Brown, L.; Gekht, G.; Vallejo, R.; Yearwood, T.; Morgan, D. Novel Spinal Cord Stimulation Parameters in Patients with Predominant Back Pain. Neuromodul. Technol. Neural Interface 2013, 16, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.J.; Tavakkolizadeh, M.; Love-Jones, S.; Patel, N.K.; Gu, J.W.; Bains, A.; Doan, Q.; Moffitt, M. Effects of Rate on Analgesia in Kilohertz Frequency Spinal Cord Stimulation: Results of the PROCO Randomized Controlled Trial. Neuromodul. Technol. Neural Interface 2018, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Amirdelfan, K.; Gliner, B.E.; Kapural, L.; Sitzman, B.T.; Vallejo, R.; Yu, C.; Caraway, D.; Rotte, A.; Province-Azalde, R.; Krames, E. A proposed definition of remission from chronic pain, based on retrospective evaluation of 24-month outcomes with spinal cord stimulation. Postgrad. Med. 2019, 131, 278–286. [Google Scholar] [CrossRef]
- Morales, A.; Yong, R.J.; Kaye, A.D.; Urman, R.D. Spinal Cord Stimulation: Comparing Traditional Low-frequency Tonic Waveforms to Novel High Frequency and Burst Stimulation for the Treatment of Chronic Low Back Pain. Curr. Pain Headache Rep. 2019, 23, 25. [Google Scholar] [CrossRef]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Yearwood, T.L.; Bundschu, R.; et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain. Neurosurgery 2016, 79, 667–677. [Google Scholar] [CrossRef]
- Al-Kaisy, A.; Van Buyten, J.-P.; Smet, I.; Palmisani, S.; Pang, D.; Smith, T. Sustained Effectiveness of 10 kHz High-Frequency Spinal Cord Stimulation for Patients with Chronic, Low Back Pain: 24-Month Results of a Prospective Multicenter Study. Pain Med. 2014, 15, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Holsheimer, J.; Wesselink, W.A. Effect of Anode-Cathode Configuration on Paresthesia Coverage in Spinal Cord Stimulation. Neurosurgery 1997, 41, 654–660. [Google Scholar] [CrossRef]
- North, R.B.; Ewend, M.G.; Lawton, M.T.; Piantadosi, S. Spinal cord stimulation for chronic, intractable pain: Superiority of “multi-channel” devices. Pain 1991, 44, 119–130. [Google Scholar] [CrossRef]
- Kumar, K.; Malik, S.; Demeria, D. Treatment of Chronic Pain with Spinal Cord Stimulation versus Alternative Therapies: Cost-effectiveness Analysis. Neurosurgery 2002, 51, 106–116. [Google Scholar] [CrossRef]
- He, J.; Barolat, G.; Holsheimer, J.; Struijk, J. Perception threshold and electrode position for spinal cord stimulation. Pain 1994, 59, 55–63. [Google Scholar] [CrossRef][Green Version]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The Appropriate Use of Neurostimulation of the Spinal Cord and Peripheral Nervous System for the Treatment of Chronic Pain and Ischemic Diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodul. Technol. Neural Interface 2014, 17, 515–550. [Google Scholar] [CrossRef] [PubMed]
- Flouty, O.; Oya, H.; Kawasaki, H.; Wilson, S.; Reddy, C.G.; Jeffery, N.D.; Brennan, T.J.; Gibson-Corley, K.N.; Utz, M.; Gillies, G.T.; et al. A new device concept for directly modulating spinal cord pathways: Initialin vivoexperimental results. Physiol. Meas. 2012, 33, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, M.; Gandar, J.; Greiner, N.; Moraud, E.M.; Wenger, N.; Shkorbatova, P.; Musienko, P.E.; Minev, I.; Lacour, S.P.; Courtine, G. Advantages of soft subdural implants for the delivery of electrochemical neuromodulation therapies to the spinal cord. J. Neural Eng. 2018, 15, 026024. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sandoval, A.; Pal, A.; Mishra, A.M.; Sherman, S.; Parikh, A.R.; Joshi-Imre, A.; Arreaga-Salas, D.; Gutierrez-Heredia, G.; Martinez, A.C.D.; Nathan, J.A.; et al. Chronic softening spinal cord stimulation arrays. J. Neural Eng. 2018, 15, 045002. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, A.T.; Leveque, J.-C.; Rubin, L.; Bulsara, K.; Gorecki, J.P. Laminectomy versus Percutaneous Electrode Placement for Spinal Cord Stimulation. Neurosurgery 2000, 46, 399–406. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.H.; Petrucci, L.; Dorsi, M.J. Spinal Cord Stimulation Electrode Design: A Prospective, Randomized, Controlled Trial Comparing Percutaneous with Laminectomy Electrodes: Part II—Clinical Outcomes. Neurosurgery 2005, 57, 990–996. [Google Scholar] [CrossRef]
- Ackroyd, R.; Bush, D.J.; Graves, J.; McVey, J.; Horton, S. Survey of assessment criteria prior to implantation of spinal cord stimulators in United Kingdom pain management centres. Eur. J. Pain 2005, 9, 57–60. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.H.; Olin, J.C.; Sieracki, J.M. Spinal cord stimulation electrode design: Prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes—Part I: Technical outcomes. Neurosurgery 2002, 51, 389–390. [Google Scholar]
- Gybels, J.; Erdine, S.; Maeyaert, J.; Meyerson, B.; Winkelmüller, W.; Augustinsson, L.; Bonezzi, C.; Brasseur, L.; Dejongste, M.; Kupers, R.; et al. Neuromodulation of pain: A consensus statement prepared in Brussels 16–18 January 1998 by the following task force of the European Federation of IASP Chapters (EFIC). Eur. J. Pain 1998, 2, 203–209. [Google Scholar] [CrossRef]
- Quack, F.; Wille, C.; Schu, S.; Vesper, J.; Kinfe, T.M. Paddle Versus Cylindrical Leads for Percutaneous Implantation in Spinal Cord Stimulation for Failed Back Surgery Syndrome: A Single-Center Trial. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2014, 75, 467–473. [Google Scholar] [CrossRef]
- Kumar, K.; Buchser, E.; Linderoth, B.; Meglio, M.; Van Buyten, J.-P. Avoiding Complications from Spinal Cord Stimulation: Practical Recommendations from an International Panel of Experts. Neuromodul. Technol. Neural Interface 2007, 10, 24–33. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Kidd, D.H.; Olin, J.; Sieracki, J.M.; Farrokhi, F.; Petrucci, L.; Cutchis, P.N. Spinal Cord Stimulation for Axial Low Back Pain. Spine 2005, 30, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Arle, J.E.; Carlson, K.W.; Mei, L.; Shils, J.L. Modeling Effects of Scar on Patterns of Dorsal Column Stimulation. Neuromodul. Technol. Neural Interface 2014, 17, 320–333. [Google Scholar] [CrossRef]
- Lee, D.; Gillespie, E.; Bradley, K. Dorsal Column Steerability with Dual Parallel Leads using Dedicated Power Sources: A Computational Model. J. Vis. Exp. 2011, 48, e2443. [Google Scholar] [CrossRef]
- Hornberger, J.; Kumar, K.; Verhulst, E.; Clark, M.A.; Hernandez, J. Rechargeable spinal cord stimulation versus non-rechargeable system for patients with failed back surgery syndrome: A cost-consequences analysis. Clin. J. Pain 2008, 24, 244. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Komoroski, C.; Roy, L. Evaluation of an innovative spinal cord stimulator platform for the treatment of chronic pain. Pain Manag. 2018, 8, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.J.; Hargens, L.M.; Breitenfeldt, M.D.; Doth, A.H.; Ryan, M.P.; Gunnarsson, C.; Safriel, Y. The Rate of Magnetic Resonance Imaging in Patients with Spinal Cord Stimulation. Spine 2015, 40, E531–E537. [Google Scholar] [CrossRef] [PubMed]
- Holsheimer, J.; Boer, J.A.D.; Struijk, J.J.; Rozeboom, A.R. MR assessment of the normal position of the spinal cord in the spinal canal. AJNR Am. J. Neuroradiol. 1994, 15, 951–959. [Google Scholar]
- Olin, J.C.; Kidd, D.H.; North, R.B. Postural Changes in Spinal Cord Stimulation Perceptual Thresholds. Neuromodul. Technol. Neural Interface 1998, 1, 171–175. [Google Scholar] [CrossRef]
- Schultz, D.M.; Webster, L.; Kosek, P.; Dar, U.; Tan, Y.; Sun, M. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Physician 2012, 15, 1–12. [Google Scholar] [CrossRef]
- Russo, M.; Cousins, M.J.; Brooker, C.; Taylor, N.; Boesel, T.; Sullivan, R.; Poree, L.; Shariati, N.H.; Hanson, E.; Parker, J. Effective Relief of Pain and Associated Symptoms with Closed-Loop Spinal Cord Stimulation System: Preliminary Results of the Avalon Study. Neuromodul. Technol. Neural Interface 2018, 21, 38–47. [Google Scholar] [CrossRef]
- Zhao, Z.; Ahmadi, A.; Hoover, C.; Grado, L.; Peterson, N.; Wang, X.; Freeman, D.; Murray, T.; Lamperski, A.; Darrow, D.; et al. Optimization of Spinal Cord Stimulation Using Bayesian Preference Learning and Its Validation. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Darrow, D.P.; Balser, D.Y.; Freeman, D.; Pelrine, E.; Krassioukov, A.; Phillips, A.; Netoff, T.; Parr, A.; Samadani, U. Effect of epidural spinal cord stimulation after chronic spinal cord injury on volitional movement and cardiovascular function: Study protocol for the phase II open label controlled E-STAND trial. BMJ Open 2022, 12, e059126. [Google Scholar] [CrossRef]
- Francio, V.T.; Polston, K.; Murphy, M.; Hagedorn, J.; Sayed, D. Management of Chronic and Neuropathic Pain with 10 kHz Spinal Cord Stimulation Technology: Summary of Findings from Preclinical and Clinical Studies. Biomedicines 2021, 9, 644. [Google Scholar] [CrossRef]
- Zhu, J.; Falco, F.; Onyewu, C.O.; Joesphson, Y.; Vesga, R.; Jari, R. Alternative approach to needle placement in spinal cord stimulator trial/implantation. Pain Physician 2011, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Medical Advisory Secretariat. Spinal cord stimulation for neuropathic pain: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2005, 5, 1–78. [Google Scholar]
- Kinfe, T.M.; Schu, S.; Quack, F.J.; Wille, C.; Vesper, J. Percutaneous Implanted Paddle Lead for Spinal Cord Stimulation: Technical Considerations and Long-Term Follow-Up. Neuromodul. Technol. Neural Interface 2012, 15, 402–407. [Google Scholar] [CrossRef]
- Last, A.R.; Hulbert, K. Chronic low back pain: Evaluation and management. Am. Fam. Physician. 2009, 79, 1067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reis, F.; Macedo, E.; Junior, M.C.F.; Amstalden, E.I.; Appenzeller, S. Retroperitoneal Ewing’s sarcoma/embryonal tumor: A rare differential diagnosis of back pain. Radiol. Bras. 2017, 50, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Weinstein, J.N. Low Back Pain. N. Engl. J. Med. 2001, 344, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.P.; Junior, N.A.S.; Appenzeller, S.; Reis, F. Radicular compression syndrome after exercise in a young patient: Not everything is a herniated disk! Radiol. Bras. 2018, 51, 408–409. [Google Scholar] [CrossRef]
- Nagel, S.J.; Lempka, S.F.; Machado, A.G. Percutaneous Spinal Cord Stimulation for Chronic Pain. Neurosurg. Clin. N. Am. 2014, 25, 723–733. [Google Scholar] [CrossRef]
- Sitzman, B.T.; Provenzano, D.A. Best Practices in Spinal Cord Stimulation. Spine 2017, 42, S67–S71. [Google Scholar] [CrossRef] [PubMed]
- Fama, C.A.; Chen, N.; Prusik, J.; Kumar, V.; Wilock, M.; Roth, S.; Pilitsis, J.G. The Use of Preoperative Psychological Evaluations to Predict Spinal Cord Stimulation Success: Our Experience and a Review of the Literature. Neuromodul. Technol. Neural Interface 2016, 19, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Grider, J.S.; Manchikanti, L.; Carayannopoulos, A.; Sharma, M.L.; Balog, C.C.; Harned, M.E.; Grami, V.; Justiz, R.; Nouri, K.H.; Hayek, S.M.; et al. Effectiveness of Spinal Cord Stimulation in Chronic Spinal Pain: A Systematic Review. Pain Physician 2016, 19, E33–E54. [Google Scholar] [CrossRef]
- Taylor, R.S. Spinal Cord Stimulation in Complex Regional Pain Syndrome and Refractory Neuropathic Back and Leg Pain/Failed Back Surgery Syndrome: Results of a Systematic Review and Meta-Analysis. J. Pain Symptom. Manag. 2006, 31, S13–S19. [Google Scholar] [CrossRef]
- Eldabe, S.; Thomson, S.; Duarte, R.; Brookes, M.; deBelder, M.; Raphael, J.; Davies, E.; Taylor, R. The Effectiveness and Cost-Effectiveness of Spinal Cord Stimulation for Refractory Angina (RASCAL Study): A Pilot Randomized Controlled Trial. Neuromodulation 2016, 19, 60. [Google Scholar] [CrossRef]
- Amann, W.; Berg, P.; Gersbach, P.; Gamain, J.; Raphael, J.; Ubbink, D. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: Results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS). Eur. J. Vasc. Endovasc. Surg. 2003, 26, 280–286. [Google Scholar] [CrossRef]
- Peng, P.W.H.; Fedoroff, I.; Jacques, L.; Kumar, K. Survey of the practice of spinal cord stimulators and intrathecal analgesic delivery implants for management of pain in Canada. Pain Res. Manag. 2007, 12, 281–285. [Google Scholar] [CrossRef]
- Cameron, T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: A 20-year literature review. J. Neurosurg. Spine 2004, 100, 254–267. [Google Scholar] [CrossRef]
- Kumar, K.; Nath, R.; Wyant, G.M. Treatment of chronic pain by epidural spinal cord stimulation: A 10-year experience. J. Neurosurg. 1991, 75, 402–407. [Google Scholar] [CrossRef]
- Gupta, M.; Abd-Elsayed, A.; Hughes, M.; Rotte, A. A Retrospective Review of Lead Migration Rate in Patients Permanently Implanted with Percutaneous Leads and a 10 kHz SCS Device. Pain Res. Manag. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Van Buyten, J.P.; Van Zundert, J.; Vueghs, P.; Vanduffel, L. Efficacy of spinal cord stimulation: 10 years of experience in a pain centre in Belgium. Eur. J. Pain 2001, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Hunter, G.; Demeria, D. Spinal Cord Stimulation in Treatment of Chronic Benign Pain: Challenges in Treatment Planning and Present Status, a 22-Year Experience. Neurosurgery 2006, 58, 481–496. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.H.; Zahurak, M.; James, C.S.; Long, D.M. Spinal Cord Stimulation for Chronic, Intractable Pain. Neurosurgery 1993, 32, 384–395. [Google Scholar] [CrossRef]
- Antonovich, D.D.; Gama, W.; Ritter, A.; Wolf, B.J.; Nobles, R.H.; Selassie, M.A.; Hillegass, M.G. Reoperation Rates of Percutaneous and Paddle Leads in Spinal Cord Stimulator Systems: A Single-Center Retrospective Analysis. Pain Med. 2020, 22, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Taccola, G.; Barber, S.; Horner, P.J.; Bazo, H.A.C.; Sayenko, D. Complications of epidural spinal stimulation: Lessons from the past and alternatives for the future. Spinal Cord 2020, 58, 1049–1059. [Google Scholar] [CrossRef]
- Al Tamimi, M.; Aoun, S.G.; Gluf, W. Spinal Cord Compression Secondary to Epidural Fibrosis Associated with Percutaneously Placed Spinal Cord Stimulation Electrodes: Case Report and Review of the Literature. World Neurosurg. 2017, 104, 1051.e1–1051.e5. [Google Scholar] [CrossRef]
- Guzzi, G.; Volpentesta, G.; Chirchiglia, D.; Della Torre, A.; Lavano, F.; Lavano, A. Cervical spinal cord compression from delayed epidural scar tissue formation around plate lead for SCS. J. Neurosurg. Sci. 2019, 63, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Dam-Hieu, P.; Magro, E.; Seizeur, R.; Simon, A.; Quinio, B. Cervical cord compression due to delayed scarring around epidural electrodes used in spinal cord stimulation. J. Neurosurg. Spine 2010, 12, 409–412. [Google Scholar] [CrossRef]
- Duarte, R.V.; McNicol, E.; Colloca, L.; Taylor, R.S.; North, R.B.; Eldabe, S. Randomized Placebo-/Sham-Controlled Trials of Spinal Cord Stimulation: A Systematic Review and Methodological Appraisal. Neuromodulation 2020, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Lamer, T.J.; Moeschler, S.M.; Gazelka, H.M.; Hooten, W.M.; Bendel, M.A.; Murad, M.H. Spinal Stimulation for the Treatment of Intractable Spine and Limb Pain. Mayo Clin. Proc. 2019, 94, 1475–1487. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzzi, G.; Della Torre, A.; La Torre, D.; Volpentesta, G.; Stroscio, C.A.; Lavano, A.; Longhini, F. Spinal Cord Stimulation in Chronic Low Back Pain Syndrome: Mechanisms of Modulation, Technical Features and Clinical Application. Healthcare 2022, 10, 1953. https://doi.org/10.3390/healthcare10101953
Guzzi G, Della Torre A, La Torre D, Volpentesta G, Stroscio CA, Lavano A, Longhini F. Spinal Cord Stimulation in Chronic Low Back Pain Syndrome: Mechanisms of Modulation, Technical Features and Clinical Application. Healthcare. 2022; 10(10):1953. https://doi.org/10.3390/healthcare10101953
Chicago/Turabian StyleGuzzi, Giusy, Attilio Della Torre, Domenico La Torre, Giorgio Volpentesta, Carmelino Angelo Stroscio, Angelo Lavano, and Federico Longhini. 2022. "Spinal Cord Stimulation in Chronic Low Back Pain Syndrome: Mechanisms of Modulation, Technical Features and Clinical Application" Healthcare 10, no. 10: 1953. https://doi.org/10.3390/healthcare10101953
APA StyleGuzzi, G., Della Torre, A., La Torre, D., Volpentesta, G., Stroscio, C. A., Lavano, A., & Longhini, F. (2022). Spinal Cord Stimulation in Chronic Low Back Pain Syndrome: Mechanisms of Modulation, Technical Features and Clinical Application. Healthcare, 10(10), 1953. https://doi.org/10.3390/healthcare10101953







