Clinical Anatomy and Medical Malpractice—A Narrative Review with Methodological Implications
Abstract
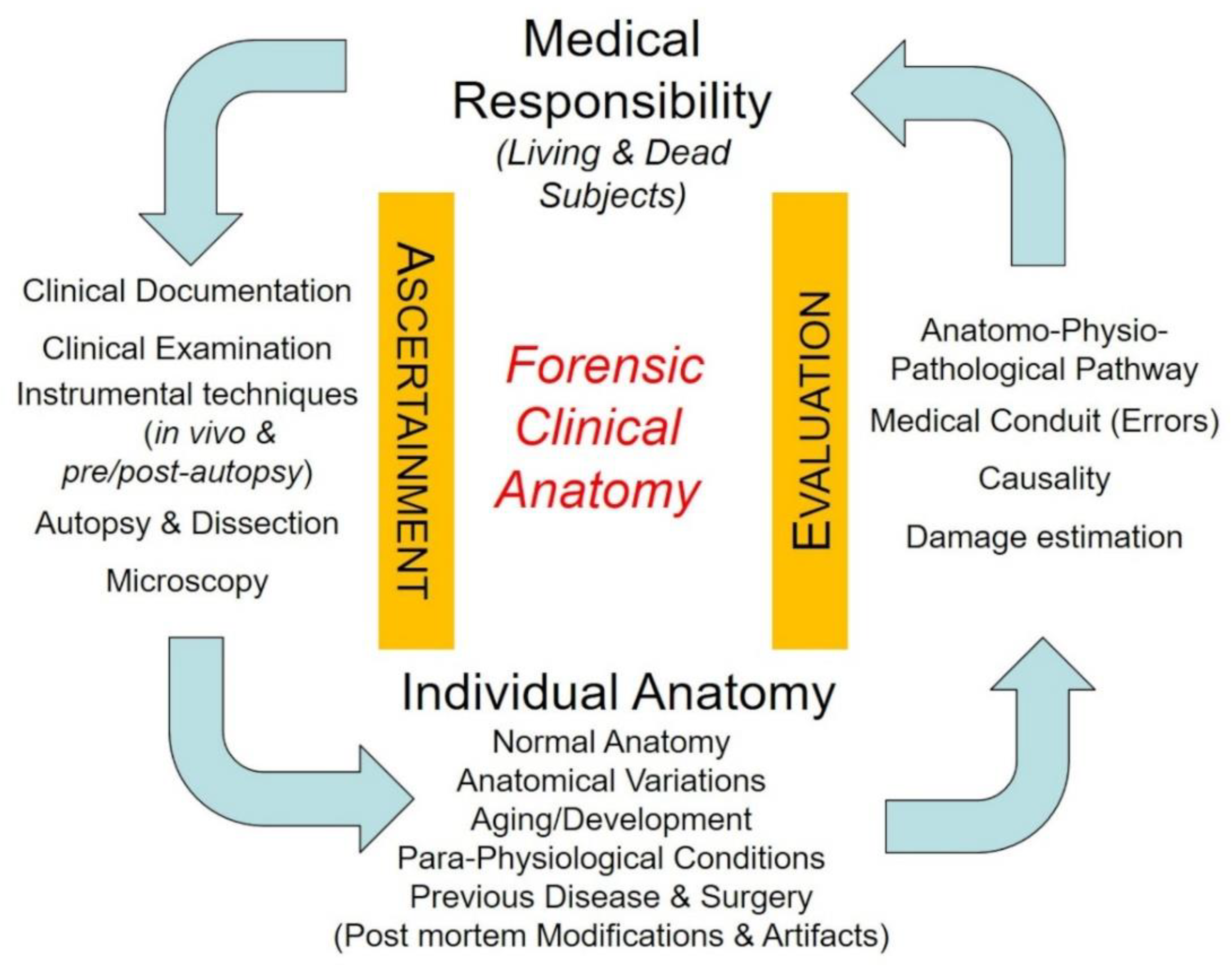
:1. Introduction
2. Individual Anatomy in Clinical/Surgical and Forensic Settings
3. Clinical Anatomy in Medical Malpractice Analysis—Methodological Issues
- (1)
- how aspects of individual anatomy (of relevance from a forensic point of view) may arise from the application of methods of ascertainment, and how they may be furtherly ascertained through specific anatomical methodology;
- (2)
- how data about individual anatomy, once fully ascertained and consistently discussed in the light of pertinent scientific knowledge, may help in the correct application of the criteria of evaluation and in final judgment about the identification of profiles of medical responsibility/liability.
3.1. The Relevance of the Individual Anatomy in the Application of Methods of Ascertainment on Living Persons
3.2. The Relevance of the Individual Anatomy in the Application of Methods of Ascertainment on Dead Persons
3.2.1. Examination of Clinical Documentation
3.2.2. Pre-Autopsy Examinations
3.2.3. Autopsy
3.2.4. Post-Autopsy Imaging Techniques
3.2.5. Microscopic, Ultrastructural and Molecular Analyses
3.3. Evaluation Criteria and Individual Anatomy
3.3.1. Reconstruction of the Physio–Pathological Pathway
3.3.2. Identification-Evaluation of Errors
- -
- are the relevant anatomical data (anatomical measurements or parameters, anatomical variations or modifications) described in the literature? And in which type of studies (case reports, case series, reviews, meta-analysis, …)?
- -
- what is the reported prevalence (also with reference to different methods of ascertainment)?
- -
- is it possible to identify the above anatomical data in the living person? And how (physical examination, imaging or other instrumental analyses, surgical intervention)?
- -
- Are there instrumental or surgical procedures that are specifically required (rules of professional medical conduit) to clarify the individual anatomy, on the basis of the evaluation of risk-benefit ratios (complication risks, economic costs, …) of diagnostic procedures?
- -
- If detailing of individual anatomy is not possible (absence of instrumental or surgical procedures), are there specific operative protocols to preserve anatomical structures, even in the presence of congenital or acquired variability?
- -
- General “Anatomical” errors could be the following:
- -
- omitted consideration of the anatomical data (may they be normal, variant, or modified) due to ignorance, error being more severe with increasing prevalence (and consequent foreseeability) of the anatomical pattern;
- -
- omitted procedures able to identify the relevant anatomical data;
- -
- inadequate management of an ascertained or supposed anatomical situation in terms of prognosis and therapy;
- -
- omitted operative protocols aimed at preserving anatomical structures, particularly in the possibility of frequent anatomical variations or modifications.
3.3.3. Discussion of Causal Value
- -
- if individual anatomy had been correctly identified, considered, and managed, the damage would have been prevented? And with what probability?
3.3.4. Damage Estimation
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porzionato, A.; Macchi, V.; Stecco, C.; Loukas, M.; Tubbs, R.S.; De Caro, R. Forensic clinical anatomy: A new field of study with application to medico-legal issues. Clin. Anat. 2017, 30, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Macchi, V.; Loukas, M.; De Caro, R. Forensic clinical anatomy—Definitions, Methods and Fields. In P5 Medicine and Justice. Innovation, Unitariness and Evidence; Ferrara, S.D., Ed.; Springer: Cham, Switzerland, 2017; pp. 377–394. [Google Scholar]
- Ferrara, S.D. Medical malpractice and legal medicine. Int. J. Legel Med. 2013, 127, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Seabury, S.; Lakdawalla, D.; Chandra, A. Malpractice risk according to physician specialty. N. Engl. J. Med. 2011, 365, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Studdert, D.M.; Bismark, M.M.; Mello, M.M.; Singh, H.; Spittal, M.J. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N. Engl. J. Med. 2016, 374, 354–362. [Google Scholar] [CrossRef]
- Ferrara, S.D.; Bajanowski, T.; Cecchi, R.; Boscolo-Berto, R.; Viel, G. Bio-medicolegal scientific research in Europe: A comprehensive bibliometric overview. Int. J. Legel Med. 2011, 125, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Berto, R.; Viel, G.; Cecchi, R.; Terranova, C.; Vogliardi, S.; Bajanowski, T.; Ferrara, S.D. Journals publishing bio-medicolegal research in Europe. Int. J. Legel Med. 2012, 126, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Macchi, V.; De Caro, R. Forensic clinical anatomy of the spleen. Forensic Sci. Int. 2019, 304, 109772. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Elsamadicy, A.A.; Sergesketter, A.R.; Frakes, M.D.; Lad, S.P. Review of Neurosurgery Medical Professional Liability Claims in the United States. Neurosurgery 2018, 83, 997–1006. [Google Scholar] [CrossRef]
- Thomas, R.; Gupta, R.; Griessenauer, C.J.; Moore, J.M.; Adeeb, N.; Motiei-Langroudi, R.; Guidal, B.; Agarwal, N.; Alterman, R.L.; Friedlander, R.M.; et al. Medical Malpractice in Neurosurgery: A Comprehensive Analysis. World Neurosurg. 2018, 110, e552–e559. [Google Scholar] [CrossRef]
- Pękala, P.A.; Henry, B.M.; Pękala, J.R.; Hsieh, W.C.; Vikse, J.; Sanna, B.; Walocha, J.A.; Tubbs, R.S.; Tomaszewski, K.A. Prevalence of foramen arcuale and its clinical significance: A meta-analysis of 55,985 subjects. J. Neurosurg. Spine 2017, 27, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Johnson, P.C.; Shoja, M.M.; Loukas, M.; Oakes, W.J. Foramen arcuale: Anatomical study and review of the literature. J. Neurosurg. Spine 2007, 6, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Moreira, H.; Magalhães, T.; Dinis-Oliveira, R.; Taveira-Gomes, A. Forensic evaluation of medical liability cases in general surgery. Med. Sci. Law 2014, 54, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Talpur, K.A.; Laghari, A.A.; Yousfani, S.A.; Malik, A.M.; Memon, A.I.; Khan, S.A. Anatomical variations and congenital anomalies of extra hepatic biliary system encountered during laparoscopic cholecystectomy. J. Pak. Med. Assoc. 2010, 60, 89–93. [Google Scholar] [PubMed]
- Couinaud, C. Le Foie, Etudes Anatomiques Et Chirurgicales; Masson et Cie: Paris, France, 1957; pp. 232–240. [Google Scholar]
- Atri, M.; Bret, P.M.; Fraser-Hill, M.A. Intrahepatic portal venous variations: Prevalence with ultrasound. Radiology 1992, 184, 523–526. [Google Scholar] [CrossRef]
- Macchi, V.; Porzionato, A.; Morra, A.; Zanon, G.F.; De Caro, R. Pattern of branching of the left portal vein: An anatomo-radiological study. Surg. Radiol. Anat. 2015, 37, 463–471. [Google Scholar] [CrossRef]
- Cucchetti, A.; Peri, E.; Cescon, M.; Zanello, M.; Ercolani, G.; Zanfi, C.; Bertuzzo, V.; Di Gioia, P.; Pinna, A.D. Anatomic variations of intrahepatic bile ducts in a European series and meta-analysis of the literature. J. Gastrointest. Surg. 2011, 15, 623–630. [Google Scholar] [CrossRef]
- Zengerink, I.; Reijman, M.; Mathijssen, N.M.; Eikens-Jansen, M.P.; Bos, P.K. Hip Arthroplasty Malpractice Claims in the Netherlands: Closed Claim Study 2000–2012. J. Arthroplasty 2016, 31, 1890–1893. [Google Scholar] [CrossRef]
- Navarro, R.A.; Schmalzried, T.P.; Amstutz, H.C.; Dorey, F.J. Surgical approach and nerve palsy in total hip arthroplasty. J. Arthroplast. 1995, 10, 1–5. [Google Scholar] [CrossRef]
- Tomaszewski, K.A.; Graves, M.J.; Henry, B.M.; Popieluszko, P.; Roy, J.; Pękala, P.A.; Hsieh, W.C.; Vikse, J.; Walocha, J.A. Surgical anatomy of the sciatic nerve: A meta-analysis. J. Orthop. Res. 2016, 34, 1820–1827. [Google Scholar] [CrossRef] [Green Version]
- Jacob, A.K.; Mantilla, C.B.; Sviggum, H.P.; Schroeder, D.R.; Pagnano, M.W.; Hebl, J.R. Perioperative nerve injury after total hip arthroplasty: Regional anesthesia risk during a 20-year cohort study. Anesthesiology 2011, 115, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Unwin, A.; Scott, J. Nerve palsy after hip replacement: Medico-legal implications. Int. Orthop. 1999, 23, 133–137. [Google Scholar] [CrossRef]
- Sultan, S. Longo procedure (Stapled hemorrhoidopexy): Indications, results. J. Visc. Surg. 2015, 152, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.Z.; English, W.; Hotouras, A.; Bryant, C.; Taylor, F.; Andreani, S.; Wexner, S.D.; Banerjee, S. A systematic review of the literature assessing the outcomes of stapled haemorrhoidopexy versus open haemorrhoidectomy. Tech. Coloproctol. 2021, 25, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, C.Y.V.; Sreevathsa, M.R.; Chowdari, G.A.; Rao, K. Association of Muscle Fibers with Histopathology in Doughnut Specimens Following Stapled Hemorrhoidopexy and Their Impacts on Postoperative Outcomes. Surg. J. 2022, 8, e199–e207. [Google Scholar] [CrossRef]
- Alipour, S. Physical Breast Examination in Pregnancy and Lactation. Adv. Exp. Med. Biol. 2020, 1252, 9–16. [Google Scholar]
- D’Ascenzi, F.; Anselmi, F.; Piu, P.; Fiorentini, C.; Carbone, S.F.; Volterrani, L.; Focardi, M.; Bonifazi, M.; Mondillo, S. Cardiac Magnetic Resonance Normal Reference Values of Biventricular Size and Function in Male Athlete’s Heart. JACC Cardiovasc. Imag. 2019, 12, 1755–1765. [Google Scholar] [CrossRef]
- Caso, P.; D’Andrea, A.; Caso, I.; Severino, S.; Calabrò, P.; Allocca, F.; Mininni, N.; Calabrò, R. The athlete’s heart and hypertrophic cardiomyopathy: Two conditions which may be misdiagnosed and coexistent. Which parameters should be analysed to distinguish one disease from the other? J. Cardiovasc. Med. 2006, 7, 257–266. [Google Scholar] [CrossRef]
- Al-Khodairy, A.W.; Bovay, P.; Gobelet, C. Sciatica in the female patient: Anatomical considerations, aetiology and review of the literature. Eur. Spine J. 2007, 16, 721–731. [Google Scholar] [CrossRef]
- Schmalzried, T.P.; Amstutz, H.C.; Dorey, F.J. Nerve palsy associated with total hip replacement. Risk factors and prognosis. J. Bone Jt. Surg. Am. 1991, 73, 1074–1080. [Google Scholar] [CrossRef]
- DeHart, M.M.; Riley, L.H., Jr. Nerve injuries in total hip arthroplasty. J. Am. Acad. Orthop. Surg. 1999, 7, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hasija, R.; Kelly, J.J.; Shah, N.V.; Newman, J.M.; Chan, J.J.; Robinson, J.; Maheshwari, A.V. Nerve injuries associated with total hip arthroplasty. J. Clin. Orthop. Trauma 2018, 9, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Macchi, V.; Fenato, F.; Parenti, A.; De Caro, R. Femoral nerve injury after gynecologic laparoscopy. J. Minim. Invasive Gynecol. 2008, 15, 105–107. [Google Scholar] [CrossRef]
- Sauvageau, A.; Racette, S. Postmortem changes mistaken for traumatic lesions: A highly prevalent reason for coroner’s autopsy request. Am. J. Forensic Med. Pathol. 2008, 29, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Rae, G.; Husain, M.; McGoey, R.; Swartz, W. Postmortem Aortic Dissection: An Artifact of the Embalming Process. J. Forensic Sci. 2016, 61 (Suppl. 1), S246–S249. [Google Scholar] [CrossRef]
- Carraro, M.; Tosatto, S.C.E.; Rizzuto, R. The Origin of Personalized Medicine and the Systems Biology Revolution. In P5 Medicine and Justice. Innovation, Unitariness and Evidence; Ferrara, S.D., Ed.; Springer: Cham, Switzerland, 2017; pp. 23–36. [Google Scholar]
- Tarfusser, C.J. Scientific Evidence and Proof. Towards a Personalized Justice. In P5 Medicine and Justice. Innovation, Unitariness and Evidence; Ferrara, S.D., Ed.; Springer: Cham, Switzerland, 2017; pp. 1–12. [Google Scholar]
- Tremblay, J.; Hamet, P. Role of genomics on the path to personalized medicine. Metabolism 2013, 62 (Suppl. 1), S2–S5. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Petkar, N.; Georgalas, C.; Bhattacharyya, A. High-rising epiglottis in children: Should it cause concern? J. Am. Board Fam. Med. 2007, 20, 495–496. [Google Scholar] [CrossRef]
- Yen, K.; Krauskopf, A. Clinical Forensic Imaging. In P5 Medicine and Justice. Innovation, Unitariness and Evidence; Ferrara, S.D., Ed.; Springer: Cham, Switzerland, 2017; pp. 533–543. [Google Scholar]
- Brokaw, J.; Craig, S.; DeNeal, S.; Morris, K.; Halle, J. Radial nerve innervation of the first dorsal interosseous muscle: A functional study. Clin. Anat. 2010, 23, 227–233. [Google Scholar] [CrossRef]
- Snoeck, T.; Balestra, C.; Calberson, F.; Pouders, C.; Provyn, S. The innervation of the axillary arch determined by surface stimulodetection electromyography. J. Anat. 2012, 221, 275–278. [Google Scholar] [CrossRef]
- Porzionato, A.; Macchi, V.; Massaro, L.; Morra, A.; Sarasin, G.; Rambaldo, A.; De Caro, R. Double origin of the superior cerebellar artery associated with homolateral haemorrhagic infarction of cerebellum. Folia Neuropathol. 2016, 54, 410–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, S.G.; Bolliger, S.A.; Ampanozi, G.; Oesterhelweg, L.; Thali, M.J.; Flach, P.M. Postmortem CT angiography: Capabilities and limitations in traumatic and natural causes of death. Radiographics 2014, 34, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Flach, P.M.; Gascho, D.; Schweitzer, W.; Ruder, T.D.; Berger, N.; Ross, S.G.; Thali, M.J.; Ampanozi, G. Imaging in forensic radiology: An illustrated guide for postmortem computed tomography technique and protocols. Forensic Sci. Med. Pathol. 2014, 10, 583–606. [Google Scholar] [CrossRef] [PubMed]
- Flach, P.M.; Thali, M.J.; Germerott, T. Times have changed! Forensic radiology—A new challenge for radiology and forensic pathology. AJR Am. J. Roentgenol. 2014, 202, W325–W334. [Google Scholar] [CrossRef]
- Ruder, T.D.; Thali, M.J.; Hatch, G.M. Essentials of forensic post-mortem MR imaging in adults. Br. J. Radiol. 2014, 87, 20130567. [Google Scholar] [CrossRef]
- Grabherr, S.; Grimm, J.; Dominguez, A.; Vanhaebost, J.; Mangin, P. Advances in post-mortem CT-angiography. Br. J. Radiol. 2014, 87, 20130488. [Google Scholar] [CrossRef]
- Grabherr, S.; Grimm, J.; Baumann, P.; Mangin, P. Application of contrast media in post-mortem imaging (CT and MRI). Radiol. Med. 2015, 120, 824–834. [Google Scholar] [CrossRef]
- Bolliger, S.A.; Thali, M.J. Imaging and virtual autopsy: Looking back and forward. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140253. [Google Scholar] [CrossRef]
- Dedouit, F.; Campana, L.; Uldin, T.; Grabherr, S. Post-Mortem Forensic Imaging. In P5 Medicine and Justice. Innovation, Unitariness and Evidence; Ferrara, S.D., Ed.; Springer: Cham, Switzerland, 2017; pp. 545–560. [Google Scholar]
- Arthurs, O.J.; Hutchinson, J.C.; Sebire, N.J. Current issues in postmortem imaging of perinatal and forensic childhood deaths. Forensic Sci. Med. Pathol. 2017, 13, 58–66. [Google Scholar] [CrossRef]
- Jackowski, C.; Sonnenschein, M.; Thali, M.J.; Aghayev, E.; von Allmen, G.; Yen, K.; Dirnhofer, R.; Vock, P. Virtopsy: Postmortem minimally invasive angiography using cross section techniques—Implementation and preliminary results. J. Forensic Sci. 2005, 50, 1175–1186. [Google Scholar] [CrossRef]
- Saunders, S.L.; Morgan, B.; Raj, V.; Robinson, C.E.; Rutty, G.N. Targeted post-mortem computed tomography cardiac angiography: Proof of concept. Int. J. Legel Med. 2011, 125, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.S.; Benamore, R.E.; Peebles, C.; Roobottom, C.; Traill, Z.C. Technical report: Diagnosis of coronary artery disease using minimally invasive autopsy: Evaluation of a novel method of post-mortem coronary CT angiography. Clin. Radiol. 2011, 66, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, S.; Doenz, F.; Steger, B.; Dirnhofer, R.; Dominguez, A.; Sollberger, B.; Gygax, E.; Rizzo, E.; Chevallier, C.; Meuli, R.; et al. Multi-phase post-mortem CT angiography: Development of a standardized protocol. Int. J. Legel Med. 2011, 125, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Zerlauth, J.B.; Doenz, F.; Dominguez, A.; Palmiere, C.; Uské, A.; Meuli, R.; Grabherr, S. Surgical interventions with fatal outcome: Utility of multi-phase postmortem CT angiography. Forensic Sci. Int. 2013, 225, 32–41. [Google Scholar] [CrossRef]
- Vilariño Villaverde, R.; Bruguier, C.; Zerlauth, J.B.; De Froidmont, S.; Grabherr, S. Tearing of the left iliac vessels in lumbar surgery revealed by multiphase post-mortem CT-angiography (MPMCTA). Leg. Med. 2016, 20, 44–48. [Google Scholar] [CrossRef]
- Di Nunno, N.; Luigi, V.; Viola, L.; Francesco, V. Epidemiological case survey of medical malpractice in some medical and surgical specialties. Forensic Sci. Int. 2005, 149, 139–142. [Google Scholar] [CrossRef]
- Chevallier, C.; Doenz, F.; Vaucher, P.; Palmiere, C.; Dominguez, A.; Binaghi, S.; Mangin, P.; Grabherr, S. Postmortem computed tomography angiography vs. conventional autopsy: Advantages and inconveniences of each method. Int. J. Legel Med. 2013, 127, 981–989. [Google Scholar] [CrossRef]
- Grabherr, S.; Heinemann, A.; Vogel, H.; Rutty, G.; Morgan, B.; Woźniak, K.; Dedouit, F.; Fischer, F.; Lochner, S.; Wittig, H.; et al. Postmortem CT Angiography Compared with Autopsy: A Forensic Multicenter Study. Radiology 2018, 288, 270–276. [Google Scholar] [CrossRef]
- Medcalf, J.E.; Johnson, C.P.; Taktak, A.; Grabherr, S. Variations in the anatomy of the vertebral artery cervical loop segment—A potential predisposing factor for traumatic basal subarachnoid hemorrhage? Forensic Sci. Med. Pathol. 2016, 12, 444–450. [Google Scholar] [CrossRef]
- San Millán Ruíz, D.; Fasel, J.H.; Rüfenacht, D.A.; Gailloud, P. The sphenoparietal sinus of breschet: Does it exist? An anatomic study. AJNR Am. J. Neuroradiol. 2004, 25, 112–120. [Google Scholar]
- van Eijk, R.P.; van der Zwan, A.; Bleys, R.L.; Regli, L.; Esposito, G. Novel Application of Postmortem CT Angiography for Evaluation of the Intracranial Vascular Anatomy in Cadaver Heads. AJR Am. J. Roentgenol. 2015, 205, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Sabatasso, S.; Doenz, F.; Bass, A.; Michaud, K.; Mangin, P.; Grabherr, S. Application of multi-phase postmortem CT-angiography in the investigation of vascular pathology and modified vascular anatomy: A special case of “vascular patchwork”. Arch. Med. Sadowej. Kryminol. 2015, 65, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Sarda-Quarello, L.; Bartoli, C.; Laurent, P.E.; Torrents, J.; Piercecchi-Marti, M.D.; Sigaudy, S.; Ariey-Bonnet, D.; Gorincour, G. Whole body perinatal postmortem CT angiography. Diagn. Interv. Imaging. 2016, 97, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Ruder, T.D.; Hatch, G.M.; Ebert, L.C.; Flach, P.M.; Ross, S.; Ampanozi, G.; Thali, M.J. Whole body postmortem magnetic resonance angiography. J. Forensic Sci. 2012, 57, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Rutty, G.N.; Morgan, B. Future Evidence in Forensic Imaging. In P5 Medicine and Justice. Innovation, Unitariness and Evidence; Ferrara, S.D., Ed.; Springer: Cham, Switzerland, 2017; pp. 577–585. [Google Scholar]
- Rutty, G.N.; Swift, B. Accuracy of magnetic resonance imaging in determining cause of sudden death in adults: Comparison with conventional autopsy. Histopathology 2004, 44, 187–189. [Google Scholar] [CrossRef]
- Sessa, F.; Esposito, M.; Messina, G.; Di Mizio, G.; Di Nunno, N.; Salerno, M. Sudden Death in Adults: A Practical Flow Chart for Pathologist Guidance. Healthcare 2021, 9, 870. [Google Scholar] [CrossRef]
- Salerno, M.; Sessa, F.; Piscopo, A.; Montana, A.; Torrisi, M.; Patanè, F.; Murabito, P.; Volti, G.L.; Pomara, C. No Autopsies on COVID-19 Deaths: A Missed Opportunity and the Lockdown of Science. J. Clin. Med. 2020, 9, 1472. [Google Scholar] [CrossRef]
- Sessa, F.; Bertozzi, G.; Cipolloni, L.; Baldari, B.; Cantatore, S.; D’Errico, S.; Di Mizio, G.; Asmundo, A.; Castorina, S.; Salerno, M.; et al. Clinical-Forensic Autopsy Findings to Defeat COVID-19 Disease: A Literature Review. J. Clin. Med. 2020, 9, 2026. [Google Scholar] [CrossRef]
- Sessa, F.; Salerno, M.; Pomara, C. Autopsy Tool in Unknown Diseases: The Experience with Coronaviruses (SARS-CoV, MERS-CoV, SARS-CoV-2). Medicina 2021, 57, 309. [Google Scholar] [CrossRef]
- World Health Organization. Infection Prevention and Control for the Safe Management of a Dead Body in the Context of COVID-19. Interim Guidance: 24 March 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/331538/WHO-COVID-19-lPC_DBMgmt-2020.1-eng.pdf (accessed on 25 September 2022).
- Italian Ministry of Health. Emergency Indications Related to the COVID-19 Epidemic Concerning the Funeral Sector, Cemetery, and Cremation. Circular of General Direction of Health Prevention. 2020. Available online: http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2020&codLeg=73965&parte=1%20&serie=null (accessed on 25 September 2022).
- Karami, P.; Naghavi, M.; Feyzi, A.; Aghamohammadi, M.; Novin, M.S.; Mobaien, A.; Qorbanisani, M.; Karami, A.; Norooznezhad, A.H. Mortality of a pregnant patient diagnosed with COVID-19: A case report with clinical, radiological, and histopathological findings. Travel Med. Infect. Dis. 2020, 101665. [Google Scholar] [CrossRef]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.-Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, B. Harmonization of medico-legal autopsy rules. Int. J. Legel Med. 1999, 113, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mangin, P.; Bonbled, F.; Väli, M.; Luna, A.; Bajanowski, T.; Hougen, H.P.; Ludes, B.; Ferrara, S.D.; Cusack, D.; Keller, E.; et al. European Council of Legal Medicine (ECLM) accreditation of forensic pathology services in Europe. Int. J. Legel Med. 2015, 129, 395–403. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.R.; DeJoseph, M.E.; Gill, J.R. An approach to iatrogenic deaths. Forensic Sci. Med. Pathol. 2016, 12, 68–80. [Google Scholar] [CrossRef]
- Advenier, A.S.; De La Grandmaison, G.L.; Cavard, S.; Pyatigorskaya, N.; Malicier, D.; Charlier, P. Laryngeal anomalies: Pitfalls in adult forensic autopsies. Med. Sci. Law 2014, 54, 1–7. [Google Scholar] [CrossRef]
- Hindsø, L.; Jakobsen, L.S.; Jacobsen, C.; Lynnerup, N.; Banner, J. Epicardial adipose tissue volume estimation by postmortem computed tomography of eviscerated hearts. Forensic Sci. Med. Pathol. 2017, 13, 468–472. [Google Scholar] [CrossRef]
- Soerdjbalie-Maikoe, V.; van Rijn, R.R. Embryology, normal anatomy, and imaging techniques of the hyoid and larynx with respect to forensic purposes: A review article. Forensic Sci. Med. Pathol. 2008, 4, 132–139. [Google Scholar] [CrossRef]
- Porzionato, A.; Macchi, V.; Mazzarolo, C.; Morra, A.; Sanudo, J.R.; De Caro, R. Symmetrical apophyses on the posterior margins of the thyroid cartilage: A previously undescribed anatomical variation with potential forensic implications. Am. J. Forensic Med. Pathol. 2019, 40, 84–88. [Google Scholar] [CrossRef]
- Cecchetto, G. Micro-Imaging in Forensic Medicine. In P5 Medicine and Justice. Innovation, Unitariness and Evidence; Ferrara, S.D., Ed.; Springer: Cham, Switzerland, 2017; pp. 561–575. [Google Scholar]
- Happel, C.M.; Klose, C.; Witton, G.; Angrisani, G.L.; Wienecke, S.; Groos, S.; Bach, F.W.; Bormann, D.; Männer, J.; Yelbuz, T.M. Non-destructive, high-resolution 3-dimensional visualization of a cardiac defect in the chick embryo resembling complex heart defect in humans using micro-computed tomography: Double outlet right ventricle with left juxtaposition of atrial appendages. Circulation 2010, 122, e561–e564. [Google Scholar]
- Langheinrich, A.C.; Wienhard, J.; Vormann, S.; Hau, B.; Bohle, R.M.; Zygmunt, M. Analysis of the fetal placental vascular tree by X-ray micro-computed tomography. Placenta 2004, 25, 95–100. [Google Scholar] [CrossRef]
- Wehrli, F.W.; Song, H.K.; Saha, P.K.; Wright, A.C. Quantitative MRI for the assessment of bone structure and function. NMR Biomed. 2006, 19, 731–764. [Google Scholar] [CrossRef] [PubMed]
- Ladinsky, G.A.; Wehrli, F.W. Noninvasive assessment of bone microarchitecture by MRI. Curr. Osteoporos. Rep. 2006, 4, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.; Pasquier, A.; Petiet, A.; Wiggins, C.; Kraska, A.; Joseph-Mathurin, N.; Aujard, F.; Mestre-Francés, N.; Dhenain, M. Micro-MRI study of cerebral aging: Ex vivo detection of hippocampal subfield reorganization, microhemorrhages and amyloid plaques in mouse lemur primates. PLoS ONE 2013, 8, e56593. [Google Scholar] [CrossRef] [Green Version]
- De Caro, R.; Parenti, A.; Montisci, M.; Guidolin, D.; Macchi, V. Solitary tract nuclei in acute heart failure. Stroke 2000, 31, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Macchi, V.; Guidolin, D.; Parenti, A.; Ferrara, S.D.; De Caro, R. Histopathology of carotid body in heroin addiction. Possible chemosensitive impairment. Histopathology 2005, 46, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Longatti, P.; Porzionato, A.; Basaldella, L.; Fiorindi, A.; De Caro, P.; Feletti, A. The human area postrema: Clear-cut silhouette and variations shown in vivo. J. Neurosurg. 2015, 122, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Guidolin, D.; Emmi, A.; Boscolo-Berto, R.; Sarasin, G.; Rambaldo, A.; Macchi, V.; De Caro, R. High-quality Digital 3D Reconstruction of Microscopic Findings in Forensic Pathology: The Terminal Pathway of a Heart Stab Wound. J. Forensic Sci. 2020, 65, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, S.D.; Baccino, E.; Bajanowski, T.; Boscolo-Berto, R.; Castellano, M.; De Angel, R.; Pauliukevičius, A.; Ricci, P.; Vanezis, P.; Vieira, D.N.; et al. Malpractice and medical liability. European Guidelines on Methods of Ascertainment and Criteria of Evaluation. Int. J. Legel Med. 2013, 127, 545–557. [Google Scholar] [CrossRef]
- Ferrara, S.D.; Boscolo-Berto, R.; Viel, G. Malpractice and Medical Liability. European State of the Art and Guidelines; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Papantchev, V.; Stoinova, V.; Aleksandrov, A.; Todorova-Papantcheva, D.; Hristov, S.; Petkov, D.; Nachev, G.; Ovtscharoff, W. The role of Willis circle variations during unilateral selective cerebral perfusion: A study of 500 circles. Eur. J. Cardiothor. Surg. 2013, 44, 743–753. [Google Scholar] [CrossRef]
- Oliva, A.; Grassi, V.M.; Campuzano, O.; Brion, M.; Arena, V.; Partemi, S.; Coll, M.; Pascali, V.L.; Brugada, J.; Carracedo, A.; et al. Medico-legal perspectives on sudden cardiac death in young athletes. Int. J. Legel Med. 2017, 131, 393–409. [Google Scholar] [CrossRef]
- Zhuo, P.; Gao, D.; Xia, Q.; Ran, D.; Xia, W. Sciatic nerve injury in children after gluteal intramuscular injection: Case reports on medical malpractice. Med. Sci. Law 2019, 59, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Montisci, M.; Manani, G. Brachial plexus injury following subclavian vein catheterization: A case report. J. Clin. Anesth. 2003, 15, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Halalmeh, D.R.; Sandio, A.; Tubbs, R.S.; Moisi, M.D. Anatomical Variations That Can Lead to Spine Surgery at the Wrong Level: Part I, Cervical Spine. Cureus 2020, 12, e8667. [Google Scholar] [CrossRef]
- Shah, M.; Halalmeh, D.R.; Sandio, A.; Tubbs, R.S.; Moisi, M.D. Anatomical Variations That Can Lead to Spine Surgery at The Wrong Level: Part II Thoracic Spine. Cureus 2020, 12, e8684. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Halalmeh, D.R.; Sandio, A.; Tubbs, R.S.; Moisi, M.D. Anatomical Variations That Can Lead to Spine Surgery at the Wrong Level: Part III Lumbosacral Spine. Cureus 2020, 12, e9433. [Google Scholar] [CrossRef]
- Cesmebasi, A.; Spinner, R.J. An anatomic-based approach to the iatrogenic spinal accessory nerve injury in the posterior cervical triangle: How to avoid and treat it. Clin. Anat. 2015, 28, 761–766. [Google Scholar] [CrossRef]
- Porzionato, A.; Macchi, V.; Stecco, C.; Mazzi, A.; Rambaldo, A.; Sarasin, G.; Parenti, A.R.; Scipioni, A.; De Caro, R. Quality management of Body Donation Program at the University of Padova. Anat. Sci. Educ. 2012, 5, 264–272. [Google Scholar] [CrossRef]
- Pirri, C.; Stecco, C.; Porzionato, A.; Boscolo-Berto, R.; Fortelny, R.H.; Macchi, V.; Konschake, M.; Merigliano, S.; De Caro, R. Forensic Implications of Anatomical Education and Surgical Training With Cadavers. Front. Surg. 2021, 8, 641581. [Google Scholar] [CrossRef]



| Potential Errors |
Examples [References] |
Graduation of Medical Conduit Inadequacy |
Causality Questions with Reference to Individual Anatomy (Exclusion/Possibility/ Probability/Certainty) |
|---|---|---|---|
| Improper procedure execution | - Wrong site of gluteal intramuscular injection with lesion of the sciatic nerve [102] - Improper subclavian vein cannulation with injury of the brachial plexus [103] - Wrong site of trocar insertion with injury of the femoral nerve [35] | - Possibility of unpredictable anatomical variations/ modifications? | - Correct execution would have prevented the iatrogenic lesion? |
| Neglected identification of anatomical variation/modification at preoperative imaging | - Improper anatomical definition of a vascular anatomical variation (double superior cerebellar artery instead of the double origin of superior cerebellar artery [46]) | - Difficulty of radiological analysis? | - Correct identification would have prevented iatrogenic lesion? |
| Neglected preoperative imaging | - Un-identified foramen arcuale due to omitted pre-surgical Computed Tomography [12] | - Protocol indications? | - Preoperative imaging would have permitted identification of anatomical variation/modification? - Following correct identification would have prevented iatrogenic lesion? |
| Improper anatomical localization due to variations/modifications and surgery at wrong site | - Wrong spinal enumeration and surgery at the wrong site due to anatomical variations (transitional vertebrae, rib variants, hemivertebrae, fused vertebrae) or modifications (tumors, infections, previous surgery) [104,105,106] | - Difficulty of radiological/ surgical anatomical analysis? | - Correct enumeration would have prevented damage due to wrong site surgery? |
| Omitted/delayed diagnosis/ prognosis/therapy of lesions of anatomical structures | - Undervalue of post-operative symptoms/signs and omitted/delayed/erroneous instrumental examinations for nerve injury (brachial plexus [103], femoral nerve [35], sciatic nerve [102]) | - Difficulty of clinical/surgical/ radiological analysis? | - What would it be the damage if correct diagnosis/prognosis/therapy? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porzionato, A.; Macchi, V.; Stecco, C.; Boscolo-Berto, R.; Loukas, M.; Tubbs, R.S.; De Caro, R. Clinical Anatomy and Medical Malpractice—A Narrative Review with Methodological Implications. Healthcare 2022, 10, 1915. https://doi.org/10.3390/healthcare10101915
Porzionato A, Macchi V, Stecco C, Boscolo-Berto R, Loukas M, Tubbs RS, De Caro R. Clinical Anatomy and Medical Malpractice—A Narrative Review with Methodological Implications. Healthcare. 2022; 10(10):1915. https://doi.org/10.3390/healthcare10101915
Chicago/Turabian StylePorzionato, Andrea, Veronica Macchi, Carla Stecco, Rafael Boscolo-Berto, Marios Loukas, Ronald Shane Tubbs, and Raffaele De Caro. 2022. "Clinical Anatomy and Medical Malpractice—A Narrative Review with Methodological Implications" Healthcare 10, no. 10: 1915. https://doi.org/10.3390/healthcare10101915
APA StylePorzionato, A., Macchi, V., Stecco, C., Boscolo-Berto, R., Loukas, M., Tubbs, R. S., & De Caro, R. (2022). Clinical Anatomy and Medical Malpractice—A Narrative Review with Methodological Implications. Healthcare, 10(10), 1915. https://doi.org/10.3390/healthcare10101915










