Screening of Coronary Artery Origin by Echocardiography: Definition of Normal (and Abnormal) Take-Off by Standard Echocardiographic Views in a Healthy Pediatric Population
Abstract
:1. Background
2. Methods
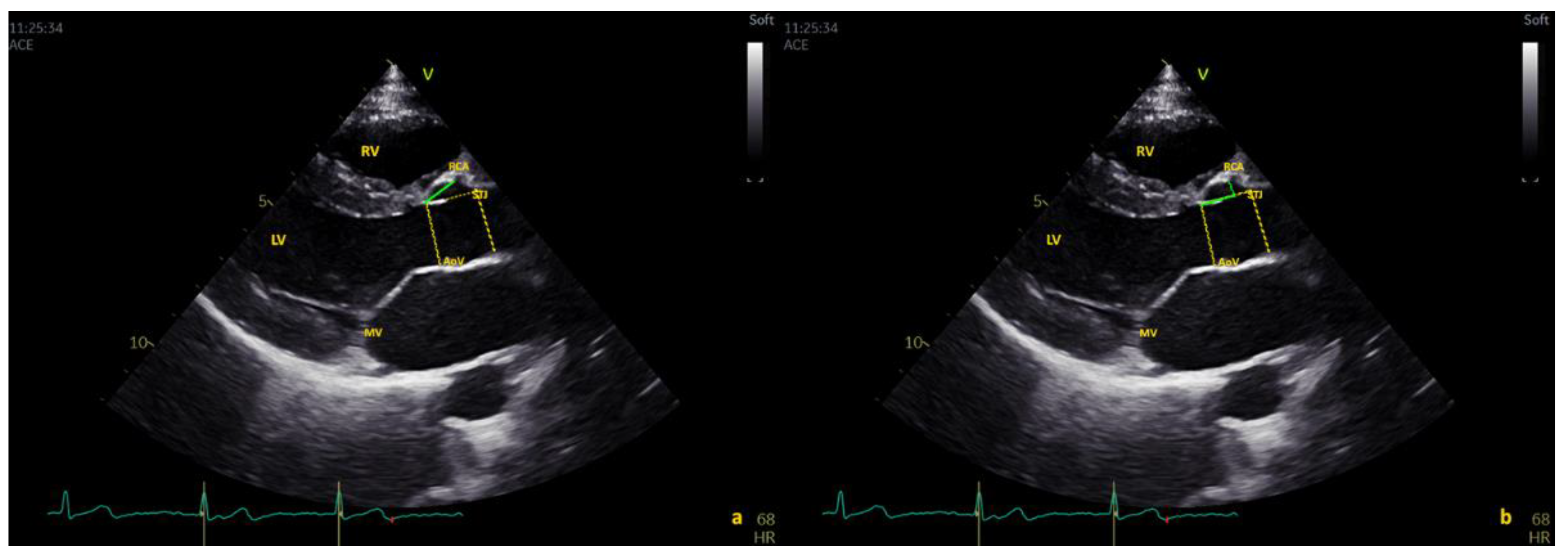
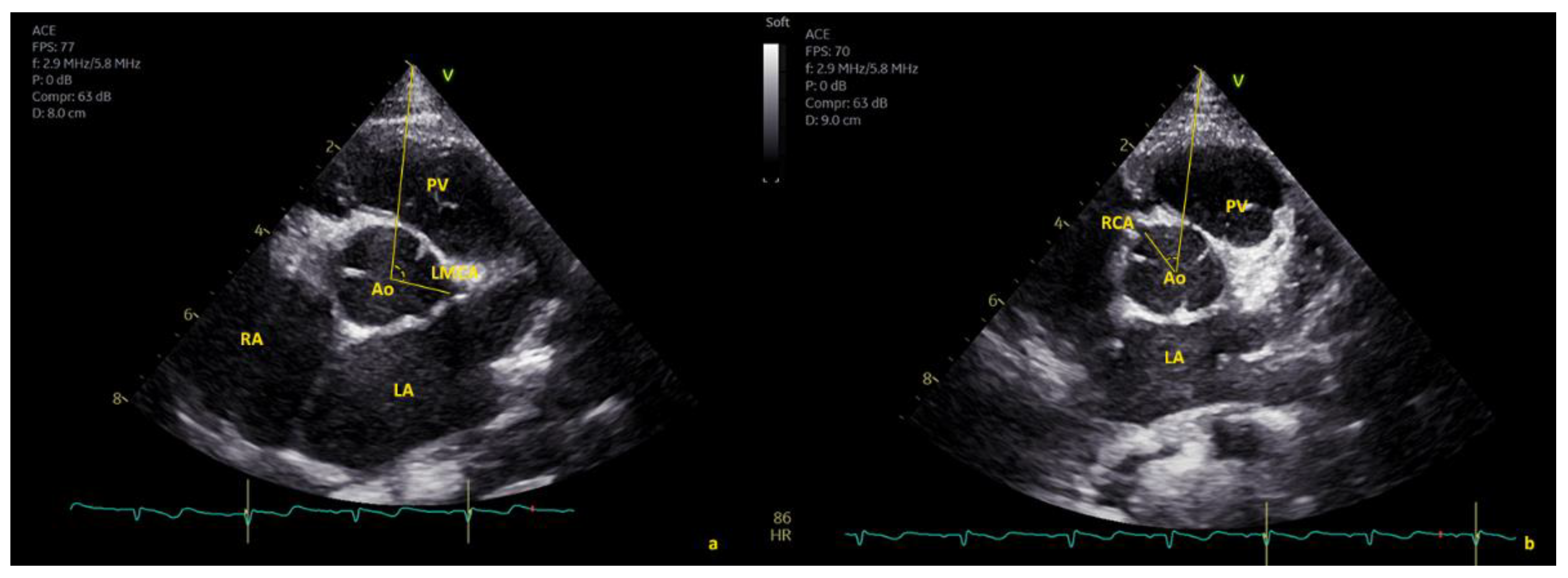
2.1. Echocardiographic Protocol
- -
- “Above the STJ” when the origin was >100% of the distance from the AO annulus to the STJ;
- -
- “At the level of STJ” when the origin was within 5% of the distance from the AO annulus to the STJ; and
- -
- “Below the STJ” when the value was <5% of the distance from the AO annulus to the STJ.
2.2. Statistical Methods
3. Results
3.1. Feasibility
3.2. Percentage of Major and Minor Anomalies
3.3. Coronary Artery Origin: Distance from the Aortic Annulus
3.3.1. Nomograms
3.3.2. Comparison of Measurement Methods
3.4. Coronary Arteries: Distance from STJ
3.4.1. Right Coronary Artery (RCA)
3.4.2. Left Main Coronary Artery (LMCA)
3.5. Correlation between Angle of Coronary Emergence in SAX and High Take-Off
3.5.1. Right Coronary Artery (RCA): High Take-Off and Reduced Angle in SAX
3.5.2. Left Main Coronary Artery (LMCA): High Take-Off and Increased Angle in SAX
3.6. Reproducibility
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerling, S.; Loose, O.; Zant, R.; Michel, H.; Melter, M.; Gündisch, C.; Krutsch, V.; Krutsch, W. Echocardiographic diagnosis of congenital coronary artery abnormalities in a continuous series of adolescent football players. Eur. J. Prev. Cardiol. 2019, 26, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Lorber, R.; Srivastava, S.; Wilder, T.J.; McIntyre, S.; DeCampli, W.M.; Williams, W.G.; Frommelt, P.C.; Parness, I.A.; Blackstone, E.H.; Jacobs, M.L.; et al. Anomalous Aortic Origin of Coronary Arteries in the Young: Echocardiographic Evaluation with Surgical Correlation. JACC Cardiovasc. Imaging 2015, 8, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, W.J.; Dean, P.N.; Schneider, D.S.; Conaway, M.R.; Kramer, C.M.; Battle, R.W. Coronary Artery Evaluation by Screening Echocardiogram in Intercollegiate Athletes. Med. Sci. Sports Exerc. 2017, 49, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Attili, A.; Hensley, A.K.; Jones, F.D.; Grabham, J.; DiSessa, T.G. Echocardiography and coronary CT angiography imaging of variations in coronary anatomy and coronary abnormalities in athletic children: Detection of coronary abnormalities that create a risk for sudden death. Echocardiography 2013, 30, 225–233. [Google Scholar] [CrossRef]
- Pelliccia, A.; Spataro, A.; Maron, B.J. Prospective echocardiographic screening for coronary artery anomalies in 1360 elite competitive athletes. Am. J. Cardiol. 1993, 72, 978–979. [Google Scholar] [CrossRef]
- Zeppilli, P.; dello Russo, A.; Santini, C.; Palmieri, V.; Natale, L.; Giordano, A.; Frustaci, A. In vivo detection of coronary artery anomalies in asymptomatic athletes by echocardiographic screening. Chest 1998, 114, 89–93. [Google Scholar] [CrossRef]
- Labombarda, F.; Coutance, G.; Pellissier, A.; Mery-Alexandre, C.; Roule, V.; Maragnes, P.; Milliez, P.; Saloux, E. Major congenital coronary artery anomalies in a paediatric and adult population: A prospective echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 761–768. [Google Scholar] [CrossRef]
- Thankavel, P.P.; Lemler, M.S.; Ramaciotti, C. Utility and importance of new echocardiographic screening methods in diagnosis of anomalous coronary origins in the pediatric population: Assessment of quality improvement. Pediatr. Cardiol. 2015, 36, 120–125. [Google Scholar] [CrossRef]
- Lytrivi, I.D.; Wong, A.H.; Ko, H.H.; Chandra, S.; Nielsen, J.C.; Srivastava, S.; Lai, W.W.; Parness, I.A. Echocardiographic diagnosis of clinically silent congenital coronary artery anomalies. Int. J. Cardiol. 2008, 126, 386–393. [Google Scholar] [CrossRef]
- Frommelt, P.; Lopez, L.; Dimas, V.; Eidem, B.; Han, K.; Helen, K.; Lorber, R.; Nii, M.; Printz, B.; Srivastava, S.; et al. Recommendations for Multimodality Assessment of Congenital Coronary Anomalies: A Guide from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 259–294. [Google Scholar] [CrossRef] [Green Version]
- Cantinotti, M.; Giordano, R.; Assanta, N.; Koestenberger, M.; Franchi, E.; Marchese, P.; Clemente, A.; Kutty, S.; D’Ascenzi, F. Echocardiographic Screening of Anomalous Origin of Coronary Arteries in Athletes with a Focus on High Take-Off. Healthcare 2021, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Haas, T.S.; Ahluwalia, A.; Murphy, C.J.; Garberich, R.F. Demographics and Epidemiology of Sudden Deaths in Young Competitive Athletes: From the United States National Registry. Am. J. Med. 2016, 129, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.F.; Siebert, D.M.; Kucera, K.L.; Thomas, L.C.; Maleszewski, J.J.; Lopez-Anderson, M.; Drezner, J.A. Etiology of Sudden Cardiac Arrest and Death in US Competitive Athletes: A 2-Year Prospective Surveillance Study. Clin. J. Sport Med. 2018, 30, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Papadakis, M.; Robertus, J.L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Sheppard, M.N. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115. [Google Scholar] [CrossRef]
- Alkhulaifi, A.M.; Chooriyil, N.; Alkuwari, M.; Ghareep, A.N.; Carr, C. Coronary artery anomalies: Unusually high incidence of anomalies with a malignant course in an Asian population. SAGE Open Med. 2017, 5, 2050312117741823. [Google Scholar] [CrossRef]
- Yamanaka, O.; Hobbs, R.E. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet. Cardiovasc. Diagn. 1990, 21, 28–40. [Google Scholar] [CrossRef]
- Loukas, M.; Andall, R.G.; Khan, A.Z.; Patel, K.; Muresian, H.; Spicer, D.E.; Tubbs, R.S. The clinical anatomy of high take-off coronary arteries. Clin. Anat. 2016, 29, 408–419. [Google Scholar] [CrossRef]
- Marchese, P.; Scalese, M.; Giordano, R.; Assanta, N.; Franchi, E.; Koestenberger, M.; Ravaglioli, A.; Kutty, S.; Cantinotti, M. Pediatric ranges of normality for 2D speckle-tracking echocardiography atrial strain: Differences between p- and r-gating and among new (Atrial Designed) and conventional (Ventricular Specific) software’s. Echocardiography 2021, 38, 2025–2031. [Google Scholar] [CrossRef]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef]
- Wyman, R.A.; Chiu, R.Y.; Rahko, P.S. The 5-minute screening echocardiogram for athletes. J. Am. Soc. Echocardiogr. 2008, 21, 786–788. [Google Scholar] [CrossRef]
- Molossi, S.; Martínez-Bravo, L.E.; Mery, C.M. Anomalous aortic origin of a coronary artery. Methodist Debakey Cardiovasc. J. 2019, 15, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Gervasi, S.; Bianco, M.; Cogliani, R.; Pescolieri, B.; Cuccaro, F.; Zeppilli, P. Anomalous origin of coronary arteries from the “wrong” sinus in athletes: Diagnosis and management strategies. Int. J. Cardiol. 2018, 252, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Mavi, A.; Ayalp, R.; Sercelik, A.; Pestemalci, T.; Batyraliev, T.; Gumusburun, E. Frequency in the anomalous origin of the left main coronary artery with angiography in a Turkish population. Acta Med. Okayama 2004, 58, 17–22. [Google Scholar] [PubMed]
- Vlodaver, Z.; Neufeld, H.N.; Edwards, J.E. Pathology of coronary disease. Semin. Roentgenol. 1972, 7, 376–394. [Google Scholar] [CrossRef]
- Rosenthal, R.L.; Carrothers, I.A.; Schussler, J.M. Benign or malignant anomaly? Very high takeoff of the left main coronary artery above the left coronary sinus. Tex. Heart Inst. J. 2012, 39, 538–541. [Google Scholar]
- Doan, T.T.; Molossi, S.; Sachdeva, S.; Wilkinson, J.C.; Loar, R.W.; Weigand, J.D.; Schlingmann, T.R.; Reaves-O’Neal, D.L.; Pednekar, A.S.; Masand, P.; et al. Dobutamine stress cardiac MRI is safe and feasible in pediatric patients with anomalous aortic origin of a coronary artery (AAOCA). Int. J. Cardiol. 2021, 334, 42–48. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Mondillo, S.; Finocchiaro, G.; Caselli, S.; Garza, M.S.D.L.; Schmied, C.; Adami, P.E.; Galderisi, M.; Adler, Y.; et al. The use of cardiac imaging in the evaluation of athletes in the clinical practice: A survey by the Sports Cardiology and Exercise Section of the European Association of Preventive Cardiology and University of Siena, in collaboration with the European Association of Cardiovascular Imaging, the European Heart Rhythm Association and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Prev. Cardiol. 2021, 28, 1071–1077. [Google Scholar]
- Angelini, P.; Muthupillai, R.; Lopez, A.; Cheong, B.; Uribe, C.; Hernandez, E.; Tomanek, R. Young athletes: Preventing sudden death by adopting a modern screening approach? A critical review and the opening of a debate. Int. J. Cardiol. Heart Vasc. 2021, 34, 100790. [Google Scholar] [CrossRef]



| Variable | Male (n = 388) | Female (n = 312) | Total (n = 700) |
|---|---|---|---|
| Demographic Data | |||
| Age, years (range) | 9.07 ± 4.97 (0–17.87) | 8.21 ± 4.87 (0–17.98) | 9.53 ± 5.95 (0–17.98) |
| Weight, kg (range) | 36.59 ± 21.14 (2.57–110) | 32.7 ± 19.03 (3–82) | 36.62 ± 20.33 (2.57–110) |
| Height, cm (range) | 132.33 ± 34.96 (11–196) | 128.47 ± 33.51 (48–178) | 130.7 ± 51.6 (46–196) |
| BSA Haycock, m2 (range) | 1.14 ± 0.46 (0.18–2.43) | 1.05 ± 0.45 (0.21–2) | 1.1 ± 0.46 (0.18–2.43) |
| Anomalous aortic origin of coronary arteries | |||
| LAD and CFx: separate ostia, nr | 9 | 1 | 10 |
| CFx from right coronary sinus, nr | 3 | 0 | 3 |
| Single ostium from the right coronary sinus, nr | 2 | 1 | 3 |
| Coronary artery fistula, nr | 1 | 0 | 1 |
| Coronary artery position within the aortic root | |||
| RCA above the STJ, nr | 15 | 14 | 29 |
| RCA at the level of the STJ, nr | 29 | 37 | 66 |
| RCA below the STJ, nr | 317 | 288 | 605 |
| LMCA above the STJ, nr | 8 | 9 | 17 |
| LMCA at the level of the STJ, nr | 6 | 6 | 12 |
| LMCA below the STJ, nr | 344 | 327 | 671 |
| (A) RCA position within the aortic root in absolute value, percentage terms, and indexed by BSA. | |||||||
| Mean | SD | Min | Max | Median | IQR 25th | IQR 75th | |
| RCA Below the STJ | |||||||
| RCA distance from AoV (mm)—oblique | 9.53 | 3.50 | 1.83 | 26.10 | 9.70 | 6.80 | 11.90 |
| RCA distance from AoV (mm/m2) | 9.24 | 3.59 | 2.27 | 29.53 | 8.73 | 6.92 | 10.78 |
| RCA distance from AoV (%) | 60.45 | 14.08 | 15.64 | 90.31 | 62.98 | 51.88 | 70.85 |
| RCA distance from STJ (mm) | 4.32 | 2.62 | 0.44 | 14.99 | 3.75 | 2.29 | 6.00 |
| RCA distance from STJ (mm/m2) | 4.56 | 3.53 | 0.56 | 27.72 | 3.70 | 2.07 | 5.85 |
| RCA distance from STJ (%) | 28.22 | 15.83 | 5.05 | 82.85 | 25.86 | 15.56 | 38.17 |
| RCA diameter (mm) | 2.46 | 0.82 | 0.60 | 7.50 | 2.34 | 1.90 | 2.97 |
| RCA emergence degree (°) | 35.73 | 8.99 | 9.7 | 116.6 | 34.75 | 30 | 40.78 |
| RCA at the STJ | |||||||
| RCA distance from AoV (mm)—oblique | 12.21 | 2.78 | 5.47 | 18.02 | 12.40 | 10.90 | 13.70 |
| RCA distance from AoV (mm/m2) | 11.34 | 4.12 | 6.59 | 30.58 | 10.64 | 8.76 | 12.78 |
| RCA distance from AoV (%) | 79.66 | 5.50 | 68.18 | 98.47 | 80.09 | 76.67 | 83.01 |
| RCA distance from STJ (mm) | 0.40 | 0.25 | 0.00 | 0.94 | 0.40 | 0.20 | 0.60 |
| RCA distance from STJ (mm/m2) | 0.37 | 0.25 | 0.00 | 1.02 | 0.34 | 0.17 | 0.55 |
| RCA distance from STJ (%) | 2.61 | 1.48 | 0.00 | 4.82 | 2.74 | 1.48 | 3.92 |
| RCA diameter (mm) | 2.83 | 0.97 | 1.00 | 5.40 | 2.67 | 2.21 | 3.50 |
| RCA emergence degree (°) | 30.97 | 9.8 | 12.5 | 54.5 | 30.15 | 23.83 | 38.85 |
| RCA above the STJ | |||||||
| RCA distance from AoV (mm)—oblique | 13.29 | 3.55 | 4.70 | 19.50 | 12.70 | 10.70 | 15.85 |
| RCA distance from AoV (mm/m2) | 12.28 | 3.42 | 6.63 | 20.63 | 11.40 | 9.85 | 13.27 |
| RCA distance from AoV (%) | 89.80 | 9.54 | 73.91 | 106.58 | 88.79 | 81.28 | 99.46 |
| RCA distance from STJ (mm) | −1.40 | 1.36 | −4.46 | −0.01 | −1.04 | −2.29 | −0.23 |
| RCA distance from STJ (mm/m2) | −1.32 | 1.21 | −4.27 | −0.01 | −0.90 | −2.34 | −0.17 |
| RCA distance from STJ (%) | −10.02 | 9.20 | −26.39 | −0.07 | −7.37 | −19.88 | −1.41 |
| RCA diameter (mm) | 3.01 | 0.95 | 1.36 | 4.90 | 3.00 | 2.43 | 3.79 |
| RCA emergence degree (°) | 7.27 | 14.06 | −37.2 | 18 | 13.2 | 4.4 | 16.7 |
| (B) LMCA position within the aortic root in absolute value, percentage terms, and indexed by BSA. | |||||||
| LMCA below the STJ | |||||||
| LMCA distance from AoV (mm)—oblique | 4.86 | 2.35 | 1.00 | 16.80 | 4.42 | 3.24 | 5.70 |
| LMCA distance from AoV (mm/m2) | 4.78 | 2.38 | 1.08 | 16.55 | 4.14 | 3.18 | 5.64 |
| LMCA distance from AoV (%) | 31.57 | 12.73 | 8.04 | 93.71 | 28.50 | 23.26 | 36.34 |
| LMCA distance from STJ (mm) | 8.62 | 3.62 | 0.73 | 22.49 | 8.49 | 6.00 | 10.90 |
| LMCA distance from STJ (mm/m2) | 8.68 | 4.29 | 0.51 | 31.10 | 8.09 | 5.88 | 10.59 |
| LMCA distance from STJ (%) | 55.27 | 16.26 | 3.86 | 88.99 | 56.54 | 47.74 | 66.15 |
| LMCA diameter (mm) | 2.68 | 0.88 | 0.76 | 6.00 | 2.60 | 2.00 | 3.21 |
| LMCA emergence degree (°) | 111.97 | 12.59 | 11.60 | 163.00 | 113.00 | 105.00 | 120.00 |
| LMCA at the STJ | |||||||
| LMCA distance from AoV (mm)—oblique | 14.65 | 2.22 | 11.60 | 17.90 | 14.45 | 12.80 | 16.58 |
| LMCA distance from AoV (mm/m2) | 10.03 | 1.23 | 8.48 | 12.33 | 9.85 | 8.99 | 10.83 |
| LMCA distance from AoV (%) | 79.22 | 5.51 | 67.03 | 84.57 | 81.69 | 75.42 | 82.96 |
| LMCA distance from STJ (mm) | 0.30 | 0.19 | 0.00 | 0.50 | 0.30 | 0.16 | 0.50 |
| LMCA distance from STJ (mm/m2) | 0.19 | 0.14 | 0.00 | 0.42 | 0.17 | 0.07 | 0.28 |
| LMCA distance from STJ (%) | 1.65 | 1.07 | 0.00 | 3.18 | 1.70 | 0.74 | 2.57 |
| LMCA diameter (mm) | 3.52 | 0.97 | 2.64 | 5.50 | 3.15 | 2.74 | 4.43 |
| LMCA emergence degree (°) | 131.15 | 12.20 | 113.60 | 152.60 | 130.55 | 121.23 | 140.28 |
| LMCA above the STJ | |||||||
| LMCA distance from AoV (mm)—oblique | 13.24 | 3.61 | 5.20 | 19.60 | 13.60 | 12.44 | 15.39 |
| LMCA distance from AoV (mm/m2) | 11.68 | 3.02 | 3.10 | 16.14 | 12.02 | 10.59 | 13.08 |
| LMCA distance from AoV (%) | 90.35 | 17.50 | 28.71 | 104.00 | 96.48 | 86.34 | 98.65 |
| LMCA distance from STJ (mm) | −3.77 | 7.69 | −33.40 | −0.10 | −2.10 | −2.86 | −1.20 |
| LMCA distance from STJ (mm/m2) | −2.65 | 4.30 | −17.87 | −0.13 | −1.74 | −2.34 | −0.90 |
| LMCA distance from STJ (%) | −23.81 | 38.58 | −165.35 | −0.73 | −14.29 | −17.87 | −9.03 |
| LMCA diameter (mm) | 5.44 | 10.96 | 1.50 | 47.80 | 2.60 | 2.35 | 3.05 |
| LMCA emergence degree (°) | 129.34 | 18.45 | 94.40 | 164.00 | 131.40 | 114.00 | 141.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantinotti, M.; Marchese, P.; Franchi, E.; Pizzuto, A.; Corana, G.; Viacava, C.; Barnes, B.T.; Kutty, S.; Assanta, N.; McMahon, C.J.; et al. Screening of Coronary Artery Origin by Echocardiography: Definition of Normal (and Abnormal) Take-Off by Standard Echocardiographic Views in a Healthy Pediatric Population. Healthcare 2022, 10, 1890. https://doi.org/10.3390/healthcare10101890
Cantinotti M, Marchese P, Franchi E, Pizzuto A, Corana G, Viacava C, Barnes BT, Kutty S, Assanta N, McMahon CJ, et al. Screening of Coronary Artery Origin by Echocardiography: Definition of Normal (and Abnormal) Take-Off by Standard Echocardiographic Views in a Healthy Pediatric Population. Healthcare. 2022; 10(10):1890. https://doi.org/10.3390/healthcare10101890
Chicago/Turabian StyleCantinotti, Massimiliano, Pietro Marchese, Eliana Franchi, Alessandra Pizzuto, Giulia Corana, Cecilia Viacava, Benjamin T. Barnes, Shelby Kutty, Nadia Assanta, Colin J. McMahon, and et al. 2022. "Screening of Coronary Artery Origin by Echocardiography: Definition of Normal (and Abnormal) Take-Off by Standard Echocardiographic Views in a Healthy Pediatric Population" Healthcare 10, no. 10: 1890. https://doi.org/10.3390/healthcare10101890
APA StyleCantinotti, M., Marchese, P., Franchi, E., Pizzuto, A., Corana, G., Viacava, C., Barnes, B. T., Kutty, S., Assanta, N., McMahon, C. J., Koestenberger, M., & Giordano, R. (2022). Screening of Coronary Artery Origin by Echocardiography: Definition of Normal (and Abnormal) Take-Off by Standard Echocardiographic Views in a Healthy Pediatric Population. Healthcare, 10(10), 1890. https://doi.org/10.3390/healthcare10101890









