Bayesian Network Analysis for Prediction of Unplanned Hospital Readmissions of Cancer Patients with Breakthrough Cancer Pain and Complex Care Needs
Abstract
:1. Introduction
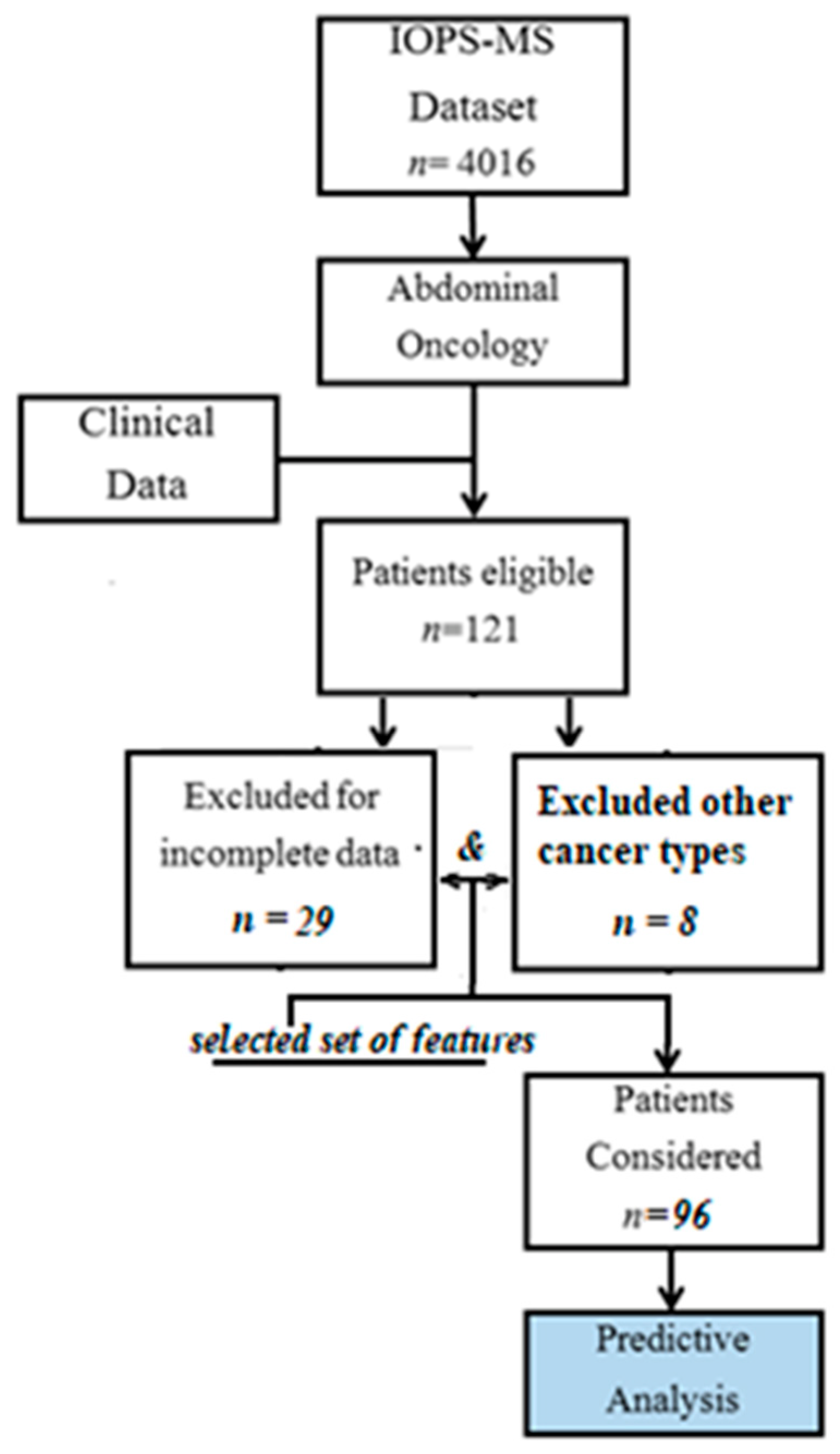
2. Materials and Methods
2.1. Data Sources
2.2. Data Preprocessing and Model Building
- Data preprocessing and discretization using cut points of clinical interest (e.g., serum albumin values and the number of hospital accesses).
- Selection of a subset of variables.
- Evaluation of significant associations (Pearson’s Chi-square test).
- Development of white- and blacklists relating to arches (variables associations), according to logic and clinical criteria.
- Design of a “knowledge-based” model containing the associations (causality, therefore directed) between variables as a causal directed acyclic graph (DAG).
- Significance analysis of the arches (relationships).
- Estimation of the Bayesian network model according to goodness indicators for the Bayesian Information Criteria (BIC) (also termed as Schwarz Criterion). It is linked to the likelihood of the model regarding the estimated parameters and contains associations between variables. Theoretical models are validated according to the BIC minimization or other indicators (e.g., Akaike Information Criteria, AIC, Bayesian Dirichlet Equivalent) [27].
- Choice of the BIC model due to observation penalization balance (its reliability decreases as the number of observations increases) and implementation of the Bayesian network for exact inference [28]. See the following formula where k indicates the number of parameters estimated by the model; n is the number of observations; θ is the set of parameters; and L(θ) represents the maximized value of the likelihood function of the model:
- 9.
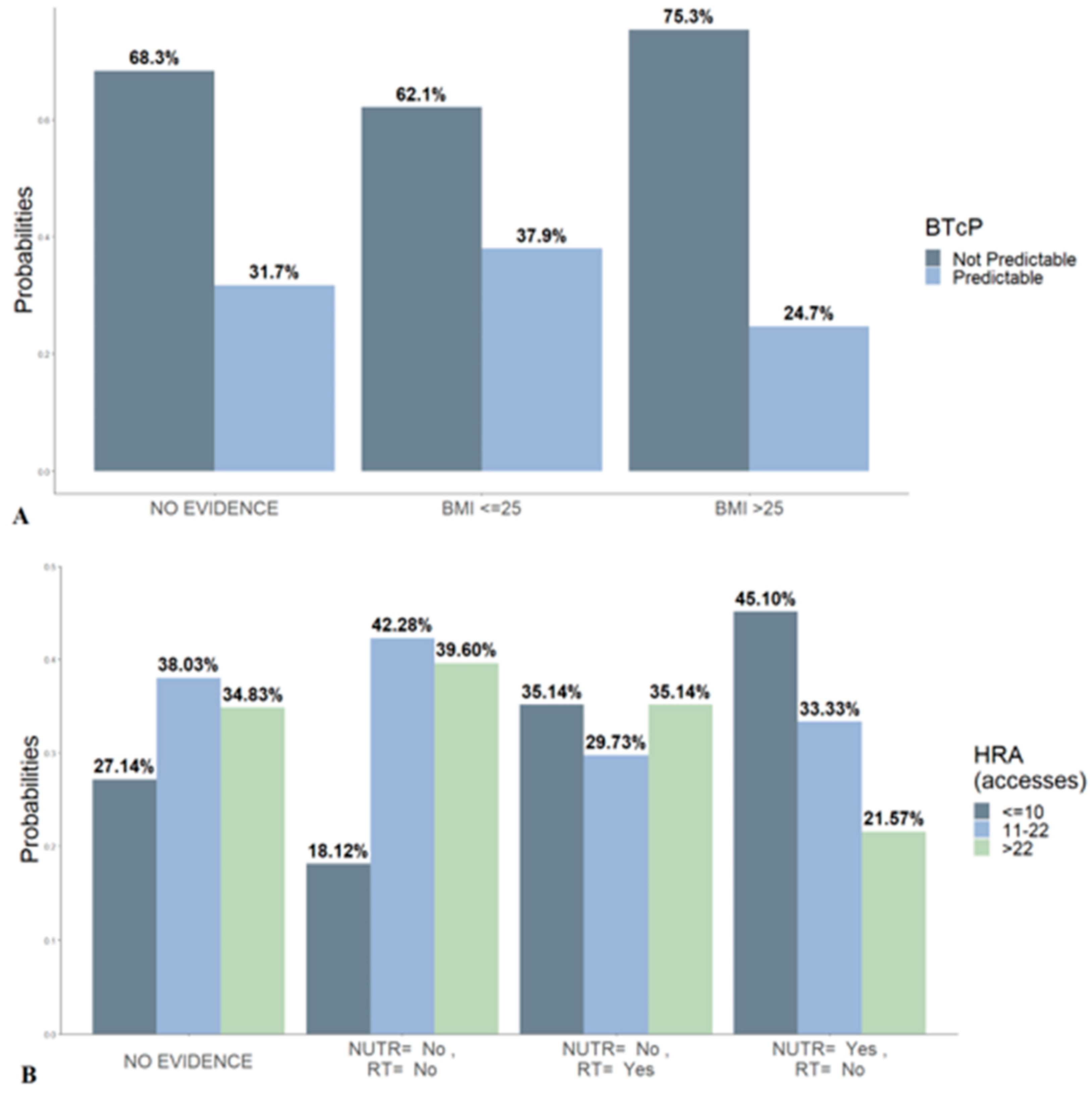
- Causal inference on the sample for main clinical interest queries.
3. Results
- ▪
- Cancer type
- ▪
- Body mass index (BMI)
- ▪
- Bone metastasis (MTX)
- ▪
- Albumin (ALB)
- ▪
- Nutritional support (NUTR)
- ▪
- Breakthrough cancer pain (BTcP)
- ▪
- Radiotherapy (RADIO)
- ▪
- Hospital readmission (HRA)
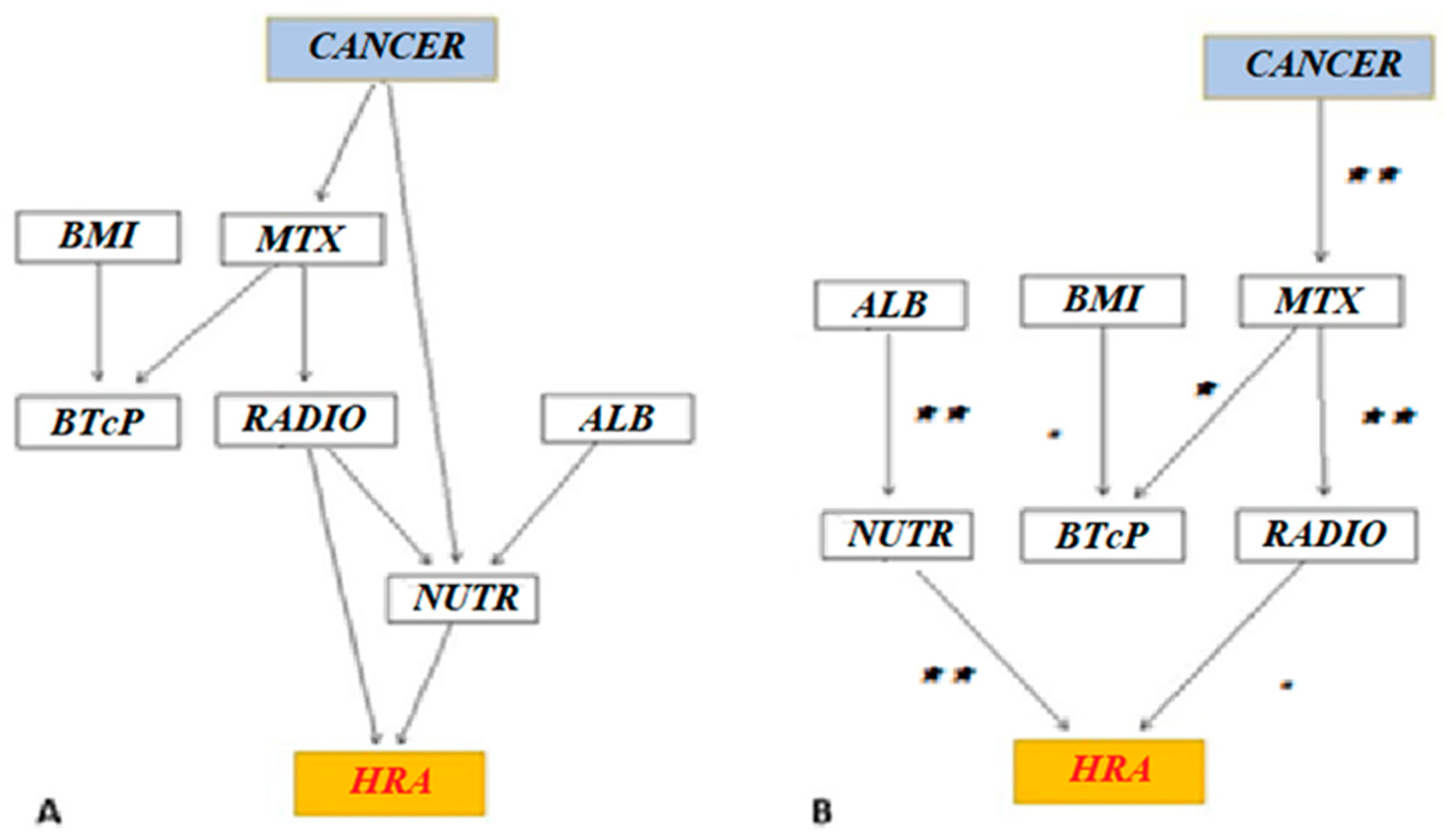
- Whitelist. Certain relationships must be necessarily valid. Even if the relationship is not certain, it must be reported in the graph model, because, validated by the theory:
- ○
- About BTcP, higher BMI can be linked to greater pain severity [29].
- ○
- In some types of cancer (prostate cancer, breast cancer, and others), bone metastases are more common; in others, the metabolic effort is more evident (e.g., pancreatic cancer). Thus, the correlations of cancer type and bone metastases with HRAs were included in the whitelist.
- ○
- Bone metastases induce BTcP, as well as palliative radiotherapy and nutritional needs [30].
- ○
- There is a clinical correlation between albumin values and nutritional support.
- Blacklist. Impossible relationships.
- ○
- A tumor (“cancer”) cannot be caused by the other considered variables.
- ○
- BTcP cannot be caused by albumin and nutritional support.
- ○
- Albumin and nutritional support cannot cause bone metastasis and nutritional needs.
- BMI and BTcP.
- Cancer type and bone metastasis, nutritional support, and HRAs.
- Bone metastasis and cancer type, nutritional support, BTcP, and radiotherapy.
- Albumin with nutritional support. Motivation: clinical relationships.
- Nutritional support and cancer type, bone metastasis, albumin, radiotherapy, and HRAs.
- BTcP with BMI and bone metastasis.
- Radiotherapy with bone metastasis and nutritional support.
- HRAs with cancer type and nutritional support.
4. Discussion
4.1. Limitations
4.2. Clinical and Research Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zibelli, A.; Holland, K.; Wei, E. Causes of Cancer Re-Admissions: A Patient-Centered Approach. JCO Oncol. Pract. 2020, 16, e734–e740. [Google Scholar] [CrossRef]
- Rocque, G.B.; Barnett, A.E.; Illig, L.C.; Eickhoff, J.C.; Bailey, H.H.; Campbell, T.C.; Stewart, J.A.; Cleary, J.F. Inpatient Hospitalization of Oncology Patients: Are We Missing an Opportunity for End-of-Life Care? J. Oncol. Pract. 2012, 9, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Numico, G.; Cristofano, A.; Mozzicafreddo, A.; Cursio, O.E.; Franco, P.; Courthod, G.; Trogu, A.; Malossi, A.; Cucchi, M.; Sirotovà, Z.; et al. Hospital Admission of Cancer Patients: Avoidable Practice or Necessary Care? PLoS ONE 2015, 10, e0120827. [Google Scholar] [CrossRef]
- Manzano, J.G.; Luo, R.; Elting, L.S.; George, M.; Suarez-Almazor, M.E. Patterns and predictors of unplanned hospitalization in a population-based cohort of elderly patients with GI cancer. J. Clin. Oncol. 2014, 32, 3527–3533. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Maekawa, Y.; Sugimoto, K.; Mizushima, T.; Eguchi, H.; Ogihara, T.; Shintani, A.; Rakugi, H.; Mori, M.; Doki, Y. Development of a geriatric prognostic scoring system for predicting survival after surgery for elderly patients with gastrointestinal cancer. Ann. Surg. Oncol. 2019, 26, 3644–3651. [Google Scholar] [CrossRef] [PubMed]
- Fessele, K.L.; Hayat, M.J.; Mayer, D.; Atkins, R.L. Factors associated with unplanned hospitalizations among patients with nonmetastatic colorectal cancers intended for treatment in the ambulatory setting. Nurs. Res. 2016, 65, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Hermosillo-Rodriguez, J.; Anaya, D.A.; Sada, Y.; Walder, A.; Amspoker, A.B.; Berger, D.H.; Naik, A.D. The effect of age and comorbidity on patient centered health outcomes in patients receiving adjuvant chemotherapy for colon cancer. J. Geriatr. Oncol. 2013, 4, 99–106. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.-L.; Allen, R.; Crook, C.J.; Levi, A.; Ballena, R.; Klepin, H.D.; Elias, R.; Mohile, S.G.; Tew, W.P.; et al. Risk Factors for Hospitalizations Among Older Adults with Gastrointestinal Cancers. Oncologist 2022, 27, e37–e44. [Google Scholar] [CrossRef]
- Cuomo, A.; Cascella, M.; Forte, C.A.; Bimonte, S.; Esposito, G.; De Santis, S.; Cavanna, L.; Fusco, F.; Dauri, M.; Natoli, S.; et al. Careful Breakthrough Cancer Pain Treatment through Rapid-Onset Transmucosal Fentanyl Improves the Quality of Life in Cancer Patients: Results from the BEST Multicenter Study. J. Clin. Med. 2020, 9, 1003. [Google Scholar] [CrossRef] [Green Version]
- Davies, A.N. Breakthrough cancer pain. Curr. Pain Headache Rep. 2014, 18, 420. [Google Scholar] [CrossRef]
- Cascella, M.; Vittori, A.; Petrucci, E.; Marinangeli, F.; Giarratano, A.; Cacciagrano, C.; Tizi, E.S.; Miceli, L.; Natoli, S.; Cuomo, A. Strengths and Weaknesses of Cancer Pain Management in Italy: Findings from a Nationwide SIAARTI Survey. Healthcare 2022, 10, 441. [Google Scholar] [CrossRef]
- Mercadante, S.; Adile, C.; Ferrera, P.; Casuccio, A. Characteristics of advanced cancer patients who were readmitted to an acute palliative/supportive care unit. Support. Care Cancer 2017, 25, 1947–1952. [Google Scholar] [CrossRef]
- Whitney, R.L.; Bell, J.F.; Tancredi, D.; Romano, P.S.; Bold, R.J.; Wun, T.; Joseph, J.G. Unplanned Hospitalization Among Individuals with Cancer in the Year After Diagnosis. J. Oncol. Pract. 2018, 15, e20–e29. [Google Scholar] [CrossRef]
- Galinier, M.; Itier, R.; Matta, A.; Massot, M.; Fournier, P.; Galtier, G.; Ayot, S.; Nader, V.; Rene, M.; Lecourt, L.; et al. Benefits of Interventional Telemonitoring on Survival and Unplanned Hospitalization in Patients with Chronic Heart Failure. Front. Cardiovasc. Med. 2022, 9, 943778. [Google Scholar] [CrossRef]
- Cascella, M.; Marinangeli, F.; Vittori, A.; Scala, C.; Piccinini, M.; Braga, A.; Miceli, L.; Vellucci, R. Open Issues and Practical Suggestions for Telemedicine in Chronic Pain. Int. J. Environ. Res. Public Health 2021, 18, 12416. [Google Scholar] [CrossRef]
- Chu, J.; Sun, N.A.; Hu, W.; Chen, X.; Yi, N.; Shen, Y. The Application of Bayesian Methods in Cancer Prognosis and Prediction. Cancer Genom. Proteom. 2022, 19, 1–11. [Google Scholar] [CrossRef]
- Parker, R.A.; Sande, T.A.; Laird, B.; Hoskin, P.; Fallon, M.; Colvin, L. Bayesian methods in palliative care research: Cancer-induced bone pain. BMJ Support Palliat. Care 2022, 12, e5–e9. [Google Scholar] [CrossRef]
- Ryan, E.G.; Couturier, D.L.; Heritier, S. Bayesian adaptive clinical trial designs for respiratory medicine. Respirology 2022, 27, 834–843. [Google Scholar] [CrossRef]
- Zafra, A.O.; Martins, B.; Ponseti-Verdaguer, F.J.; Ruiz-Barquín, R.; García-Mas, A. It Is Not Just Stress: A Bayesian Approach to the Shape of the Negative Psychological Features Associated with Sport Injuries. Healthcare 2022, 10, 236. [Google Scholar] [CrossRef]
- Cao, K.-H.; Damien, P.; Woo, C.-K.; Zarnikau, J. A Bayes Decision Rule to Assist Policymakers during a Pandemic. Healthcare 2021, 9, 1023. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.A.; Sakoparnig, T.; Gerber, M.; Hug, B.L. Bayesian networks to identify potential high-risk multimorbidity and intervention clusters in inpatients: An explorative data mining study. Swiss Med. Wkly. 2020, 150, w20299. [Google Scholar] [CrossRef] [PubMed]
- Witrick, B.; Kalbaugh, C.A.; Shi, L.; Mayo, R.; Hendricks, B. Geographic Disparities in Readmissions for Peripheral Artery Disease in South Carolina. Int. J. Environ. Res. Public Health 2021, 19, 285. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; Campbell, K. Bayesian statistical learning for big data biology. Biophys. Rev. 2019, 11, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Lazzari, M.; Reale, C.; Cuomo, A.; Fusco, F.; Marchetti, P.; Mediati, R.D.; Chiurazzi, B.; Ciuffedra, L.; Caraceni, A.; et al. Italian Oncological Pain Survey (IOPS): A multicentre Italian study of breakthrough pain performed in different. Clin. J. Pain 2015, 31, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Racca, E. Dataset_Racca. Available online: https://zenodo.org/record/6769798#.YyxWEqTP2Uk (accessed on 28 June 2022).
- Zhang, Y.; Trippa, L.; Parmigiani, G. Frequentist operating characteristics of Bayesian optimal designs via simulation. Stat. Med. 2019, 38, 4026–4039. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ma, S. A selective review of robust variable selection with applications in bioinformatics. Brief. Bioinform. 2015, 16, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, T.H.; Jonas, B.M.; Sevcik, M.A.; Kubota, K.; Halvorson, K.G.; Ghilardi, J.R.; Kuskowski, M.A.; Stelow, E.B.; Mukherjee, P.; Gendler, S.J.; et al. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain 2005, 119, 233–246. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, S.; Wang, X.; Yu, S. Factors associated with bone metastasis in breast cancer: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4435–4452. [Google Scholar] [CrossRef]
- Scutari, M.; Denis, J.-B. Bayesian Networks: With Examples in R, 1st ed.; Chapman and Hall: London, UK; CRC: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Dziak, J.J.; Coffman, D.L.; Lanza, S.T.; Li, R.; Jermiin, L.S. Sensitivity and specificity of information criteria. Brief. Bioinform. 2020, 21, 553–565. [Google Scholar] [CrossRef]
- Ouyang, X.; Dang, Y.; Zhang, F.; Huang, Q. Low Serum Albumin Correlates with Poor Survival in Gastric Cancer Patients. Clin. Lab. 2018, 64, 239–245. [Google Scholar] [CrossRef]
- Tey, J.; Soon, Y.Y.; Koh, W.Y.; Leong, C.N.; Choo, B.A.; Ho, F.; Vellayappan, B.; Lim, K.; Tham, I.W. Palliative radiotherapy for gastric cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 25797–25805. [Google Scholar] [CrossRef]
- Cascella, M.; Monaco, F.; Nocerino, D.; Chinè, E.; Carpenedo, R.; Picerno, P.; Migliaccio, L.; Armignacco, A.; Franceschini, G.; Coluccia, S.; et al. Bibliometric Network Analysis on Rapid-Onset Opioids for Breakthrough Cancer Pain Treatment. J. Pain Symptom Manag. 2022, 63, 1041–1050. [Google Scholar] [CrossRef]
- Ferrer Albiach, C.; Villegas Estévez, F.; López Alarcón, M.D.; de Madariaga, M.; Carregal, A.; Arranz, J.; Trinidad Martín-Arroyo, J.M.; Jiménez López, A.J.; Sanz Yagüe, A. Real-life management of patients with breakthrough cancer pain caused by bone metastases in Spain. J. Pain Res. 2019, 12, 2125–2135. [Google Scholar] [CrossRef]
- Fan, R.; Li, X.; Yang, S.; Bu, X.; Chen, Y.; Wang, Y.; Qiu, C. Retrospective Observational Study on the Characteristics of Pain and Associated Factors of Breakthrough Pain in Advanced Cancer Patients. Pain Res. Manag. 2022, 2022, 8943292. [Google Scholar] [CrossRef]
- Cascella, M.; Crispo, A.; Esposito, G.; Forte, C.A.; Coluccia, S.; Porciello, G.; Amore, A.; Bimonte, S.; Mercadante, S.; Caraceni, A.; et al. Multidimensional Statistical Technique for Interpreting the Spontaneous Breakthrough Cancer Pain Phenomenon. A Secondary Analysis from the IOPS-MS Study. Cancers 2021, 13, 4018. [Google Scholar] [CrossRef]
- Cascella, M.; Coluccia, S.; Grizzuti, M.; Romano, M.C.; Esposito, G.; Crispo, A.; Cuomo, A. Satisfaction with Telemedicine for Cancer Pain Management: A Model of Care and Cross-Sectional Patient Satisfaction Study. Curr. Oncol. 2022, 29, 5566–5578. [Google Scholar] [CrossRef]
| Data Collected | Variable(s) |
|---|---|
| Demographic information | Age Gender |
| Anthropometric data | Weight Height BMI |
| Clinical data | Type of primary tumor Surgical resection Metastases Bone metastases Cancer stage Line of chemotherapy (first or subsequent lines) ECOG-PS Radiotherapy treatment |
| Biochemical parameters | Serum albumin ^ ESR Leukocytes/neutrophils ratio |
| Nutritional support prescription | Enteral/parenteral nutrition |
| Pain information | Type of background pain BTcP features * |
| Analgesic therapy | Type and dosage of opioids for background pain and BTcP |
| HRAs | Number |
| Features | n (%) |
|---|---|
| Age | |
| Mean (SD) | 70 (11) |
| Median (IQR) | 72 (61, 79) |
| Gender | |
| Female | 48 (50%) |
| Male | 48 (50%) |
| BMI | |
| Mean (SD) | 24.8 (4.1) |
| Median (IQR) | 25.0 (22.0, 27.7) |
| <25 kg/m2 | 52 (54%) |
| ≥25 kg/m2 | 44 (56%) |
| Cancer type | |
| Esophageal or gastric | 17 (18%) |
| Colon-rectum | 51 (53%) |
| Pancreas, | 28 (29%) |
| Gallbladder, biliary tract | |
| Bone Metastasis | |
| Yes | 15 (16%) |
| No | 81 (84%) |
| Serum Albumin | |
| ≤3.5 g/dL | 49 (51%) |
| >3.5 g/dL | 47 (49%) |
| Nutritional Support | |
| Yes | 18 (19%) |
| No | 78 (81%) |
| BTcP | |
| Not predictable | 71 (74%) |
| Predictable | 25 (26%) |
| Type of BTcP | |
| Nociceptive | 51 (53%) |
| Neuropathic or both | 45 (47%) |
| Radiotherapy | |
| Yes | 11 (11%) |
| No | 85 (89%) |
| HRA | |
| ≤10 | 24 (25%) |
| 11–22 | 38 (40%) |
| <22 | 34 (35%) |
| BMI | CANCER | MTX | ALB | NUTR | BTcP | RADIO | HRA | |
|---|---|---|---|---|---|---|---|---|
| BMI | - | - | - | - | - | - | - | - |
| CANCER | 0 | - | - | - | - | - | - | - |
| MTX | 0 | 1 | - | - | - | - | - | - |
| ALB | 0 | 0 | 0 | - | - | - | - | - |
| NUTR | 0 | 1 | 1 | 1 | - | - | - | - |
| BTcP | 1 | 0 | 1 | 0 | 0 | - | - | - |
| RADIO | 0 | 0 | 1 | 0 | 1 | 0 | - | - |
| HRA | 0 | 1 | 0 | 0 | 1 | 0 | 0 | - |
| Knowledge-Based | BIC-Based | |
|---|---|---|
| Directed arches | 11 | 8 |
| Average Markov-Blanket size | 4.50 | 2.25 |
| Average neighborhood size | 2.75 | 1.75 |
| Average branching factor | 1.38 | 0.88 |
| Penalization coefficient | - | 2.28 |
| Step of the learning procedure | - | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascella, M.; Racca, E.; Nappi, A.; Coluccia, S.; Maione, S.; Luongo, L.; Guida, F.; Avallone, A.; Cuomo, A. Bayesian Network Analysis for Prediction of Unplanned Hospital Readmissions of Cancer Patients with Breakthrough Cancer Pain and Complex Care Needs. Healthcare 2022, 10, 1853. https://doi.org/10.3390/healthcare10101853
Cascella M, Racca E, Nappi A, Coluccia S, Maione S, Luongo L, Guida F, Avallone A, Cuomo A. Bayesian Network Analysis for Prediction of Unplanned Hospital Readmissions of Cancer Patients with Breakthrough Cancer Pain and Complex Care Needs. Healthcare. 2022; 10(10):1853. https://doi.org/10.3390/healthcare10101853
Chicago/Turabian StyleCascella, Marco, Emanuela Racca, Anna Nappi, Sergio Coluccia, Sabatino Maione, Livio Luongo, Francesca Guida, Antonio Avallone, and Arturo Cuomo. 2022. "Bayesian Network Analysis for Prediction of Unplanned Hospital Readmissions of Cancer Patients with Breakthrough Cancer Pain and Complex Care Needs" Healthcare 10, no. 10: 1853. https://doi.org/10.3390/healthcare10101853
APA StyleCascella, M., Racca, E., Nappi, A., Coluccia, S., Maione, S., Luongo, L., Guida, F., Avallone, A., & Cuomo, A. (2022). Bayesian Network Analysis for Prediction of Unplanned Hospital Readmissions of Cancer Patients with Breakthrough Cancer Pain and Complex Care Needs. Healthcare, 10(10), 1853. https://doi.org/10.3390/healthcare10101853








