A Comparative Numerical Study and Stability Analysis for a Fractional-Order SIR Model of Childhood Diseases
Abstract
:1. Introduction
- Susceptible group (S): this group is not infected but perhaps becomes infected due to the spread of the virus or stay susceptible.
- Infected group (I): This group has already been infected by the virus and may spread it to the susceptible group. An infected people may stay infected or may be removed from this group because of recovering or death.
- Removed group (R): This group has been vaccinated against the virus as well as recovered individuals with permanent immunity. The SIR model considers that the efficiency of the vaccination is one hundred percentage. The normalized dynamical system that describes the SIR model is given below [3]:
2. Explanation of the Grünwald–Letnikov Fractional Derivatives
3. Grünwald–Letnikov Discretization of Fractional-Order SIR Model
4. Stability Analysis of the Fractional-Order SIR Model
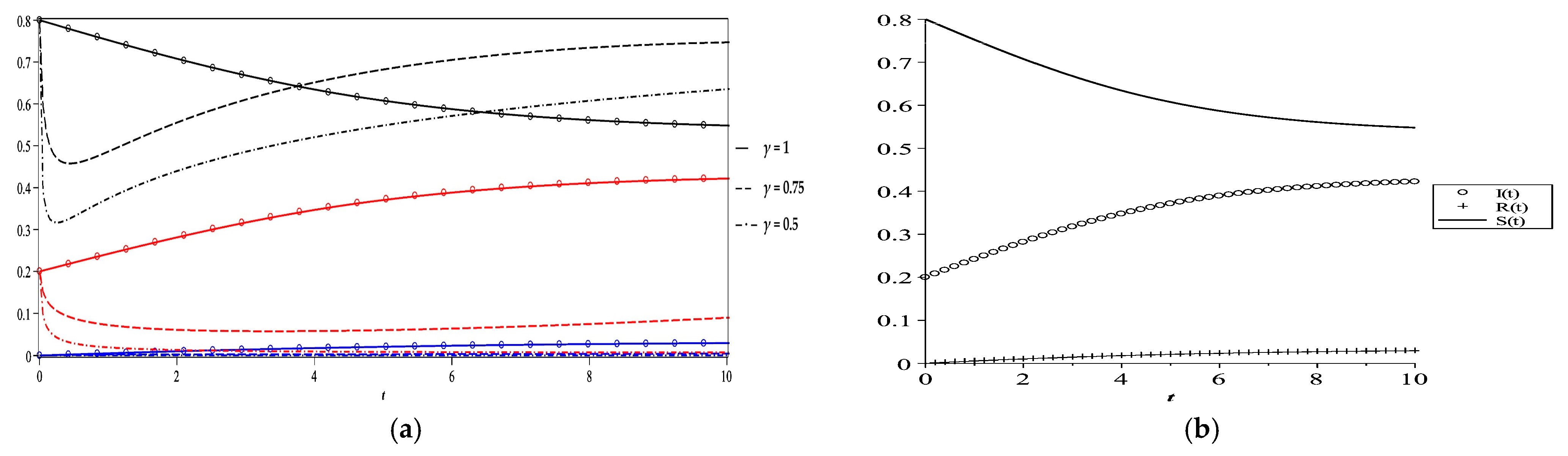
5. Numerical Results and Discussion
- Case 1
- Case 2
- Case 3
- Case 4
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, A.; Abdeljawad, T.; Ahmad, S.; Shah, K.; Karapinar, E. Study of a fractional-order epidemic model of childhood diseases. J. Funct. Spaces 2020, 2020, 5895310. [Google Scholar] [CrossRef] [PubMed]
- Haq, F.; Shahzad, M.; Muhammad, S.; Wahab, H.A.; Rahman, G.U. Numerical analysis of fractional order epidemic model of childhood diseases. Discret. Dyn. Nat. Soc. 2017, 2017, 4057089. [Google Scholar] [CrossRef]
- Makinde, O.D. Adomian decomposition approach to a SIR epidemic model with constant vaccination strategy. Appl. Math. Comput. 2007, 184, 842–848. [Google Scholar] [CrossRef]
- Rachah, A.; Torres, D.F.M. Mathematical modelling, simulation, and optimal control of the 2014 ebola outbreak in West Africa. Discret. Dyn. Nat. Soc. 2015, 2015, 842792. [Google Scholar]
- GA, N.; MI, T.-E. A Mathematical model with quarantine states for the dynamics of Ebola virus disease in human populations. Comput. Math. Methods Med. 2016, 2016, 9352725. [Google Scholar]
- Ogunlaran, O.M.; Noutchie, S.C. Mathematical model for an effective management of HIV infection. Biomed. Res. Int. 2016, 2016, 4217548. [Google Scholar] [CrossRef]
- Djordjevic, J.; Silva, C.J.; Torres, D.F.M. A stochastic SICA epidemic model for HIV transmission. Appl. Math. Lett. 2018, 84, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Arenas, A.J.; González-Parra, G.; Naranjo, J.J.; Cogollo, M.; La Espriella, N.D. Mathematical analysis and numerical solution of a model of HIV with a discrete time delay. Mathematics 2021, 9, 257. [Google Scholar] [CrossRef]
- Zafar, Z.U.A.; Rehan, K.; Mushtaq, M. HIV/AIDS epidemic fractional-order model. J. Differ. Equ. Appl. 2017, 23, 1298–1315. [Google Scholar] [CrossRef]
- Omame, A.; Abbas, M.; Onyenegecha, C.P. A fractional-order model for COVID-19 and tuberculosis co-infection using Atangana–Baleanu derivative. Chaos Solitons Fractals 2021, 153, 111486. [Google Scholar] [CrossRef] [PubMed]
- Okuonghae, D.; Omame, A. Analysis of a mathematical model for COVID-19 population dynamics in Lagos, Nigeria. Chaos Solitons Fractals 2020, 139, 110032. [Google Scholar] [CrossRef]
- Cooper, I.; Mondal, A.; Antonopoulos, C.G. A SIR model assumption for the spread of COVID-19 in different communities. Chaos Solitons Fractals 2020, 139, 110057. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Srivastava, H.M.; Hammouch, Z.; Nisar, K.S. Numerical simulation and stability analysis for the fractional-order dynamics of COVID-19. Results Phys. 2021, 20, 103722. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kumar, D.; Singh, J.; Baleanu, D. Analysis and dynamics of fractional order Covid-19 model with memory effect. Results Phys. 2021, 24, 104017. [Google Scholar] [CrossRef]
- Moreno, V.M.; Espinoza, B.; Bichara, D.; Holechek, S.A.; Castillo-Chavez, C. Role of short-term dispersal on the dynamics of Zika virus in an extreme idealized environment. Infect. Dis. Model. 2017, 2, 21–34. [Google Scholar] [CrossRef]
- Ndaïrou, F.; Area, I.; Nieto, J.J.; Silva, C.J.; Torres, D.F.M. Mathematical modeling of Zika disease in pregnant women and newborns with microcephaly in Brazil. Math. Methods Appl. Sci. 2018, 41, 8929–8941. [Google Scholar] [CrossRef] [Green Version]
- Ndaïrou, F.; Area, I.; Torres, D.F.M. Mathematical modeling of Japanese encephalitis under aquatic environmental effects. Mathematics 2020, 8, 1880. [Google Scholar] [CrossRef]
- Angstmann, C.N.; Henry, B.I.; McGann, A.V. A fractional order recovery SIR model from a stochastic process. Bull. Math. Biol. 2016, 78, 468–499. [Google Scholar] [CrossRef] [Green Version]
- Al-Sulami, H.; El-Shahed, M.; Nieto, J.J.; Shammakh, W. On fractional order dengue epidemic model. Math. Probl. Eng. 2014, 2014, 456537. [Google Scholar] [CrossRef]
- Sweilam, N.H.; AL-Mekhlafi, S.M.; Baleanu, D. Optimal control for a fractional tuberculosis infection model including the impact of diabetes and resistant strains. J. Adv. Res. 2019, 17, 125–137. [Google Scholar] [CrossRef]
- Ndaïrou, F.; Area, I.; Nieto, J.J.; Silva, C.J.; Torres, D.F.M. Fractional model of COVID-19 applied to Galicia, Spain and Portugal. Chaos Solitons Fractals 2021, 144, 110652. [Google Scholar] [CrossRef]
- Du, M.; Wang, Z.; Hu, H. Measuring memory with the order of fractional derivative. Sci. Rep. 2013, 3, 3431. [Google Scholar] [CrossRef] [PubMed]
- Lichae, B.H.; Biazar, J.; Ayati, Z. The fractional differential model of HIV-1 infection of CD4+ T-Cells with description of the effect of antiviral drug treatment, Comput. Math. Methods Med. 2019, 2019, 4059549. [Google Scholar] [CrossRef] [Green Version]
- Danane, J.; Allali, K.; Hammouch, Z. Mathematical analysis of a fractional differential model of HBV infection with antibody immune response. Chaos Solitons Fractals 2020, 136, 109787. [Google Scholar] [CrossRef]
- Li, Y.; Haq, F.; Shah, K.; Shahzad, M.; Rahman, G. Numerical analysis of fractional order Pine wilt disease model with bilinear incident rate. J. Math. Comput. Sci. 2017, 17, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Owolabi, K.M.; Atangana, A. Mathematical modelling and analysis of fractional epidemic models using derivative with exponential kernel. In Fractional Calculus in Medical and Health Science; CRC Press: Boca Raton, FL, USA, 2020; pp. 109–128. [Google Scholar]
- El-Dessoky, M.M.; Khan, M.A. Modeling and analysis of an epidemic model with fractal-fractional Atangana-Baleanu derivative. Alex. Eng. J. 2022, 61, 729–746. [Google Scholar] [CrossRef]
- Dorcak, L. Numerical models for the simulation of the fractional-order control systems. arXiv 2002, arXiv:math/0204108v1. [Google Scholar]
- Vinagre, B.M.; Chen, Y.Q.; Petráš, I. Two direct Tustin discretization methods for fractional-order differentiator/integrator. J. Frankl. Inst. 2003, 340, 349–362. [Google Scholar] [CrossRef]
- Podlubny, I. Fractional Differential Equations: An Introduction to Fractional Derivatives, Fractional Differential Equations, to Methods of Their Solution and Some of Their Applications; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Petráš, I. Fractional-Order Nonlinear Systems: Modeling, Analysis and Simulation; Springer: Berlin, Germany, 2011. [Google Scholar]
- Tavazoei, M.S.; Haeri, M. A necessary condition for double scroll attractor existence in fractional-order systems. Phys. Lett. A 2007, 367, 102–113. [Google Scholar] [CrossRef]
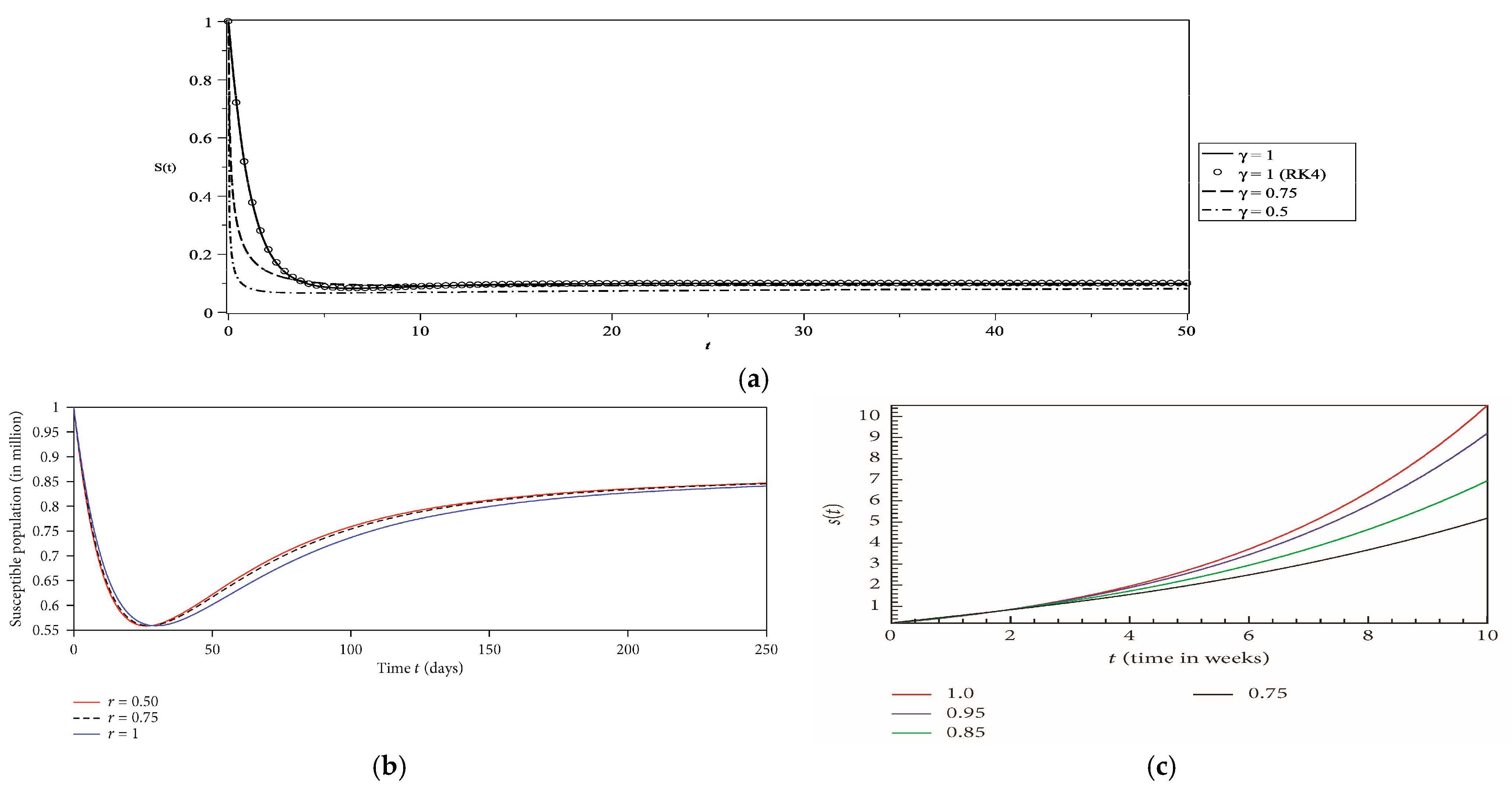
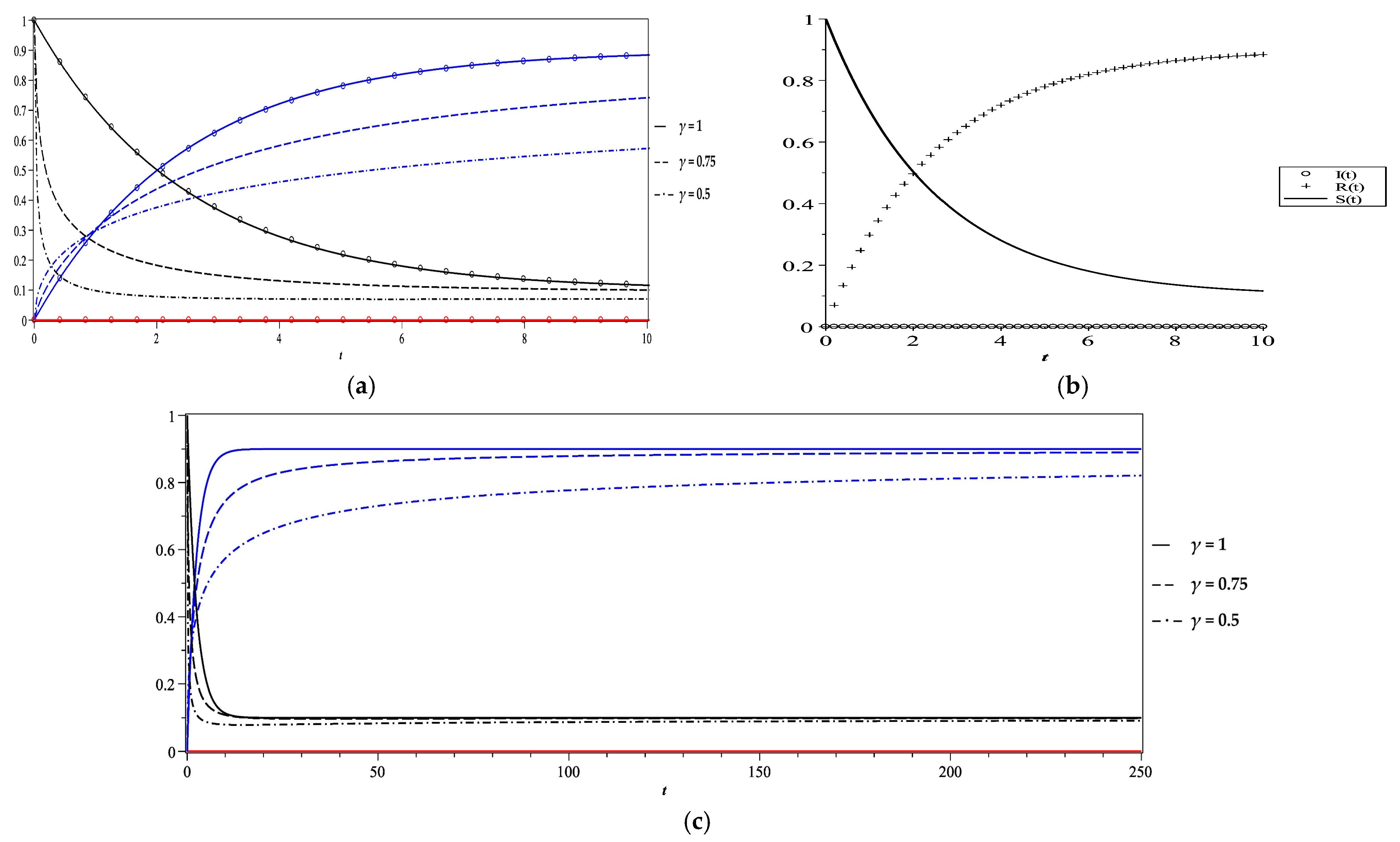
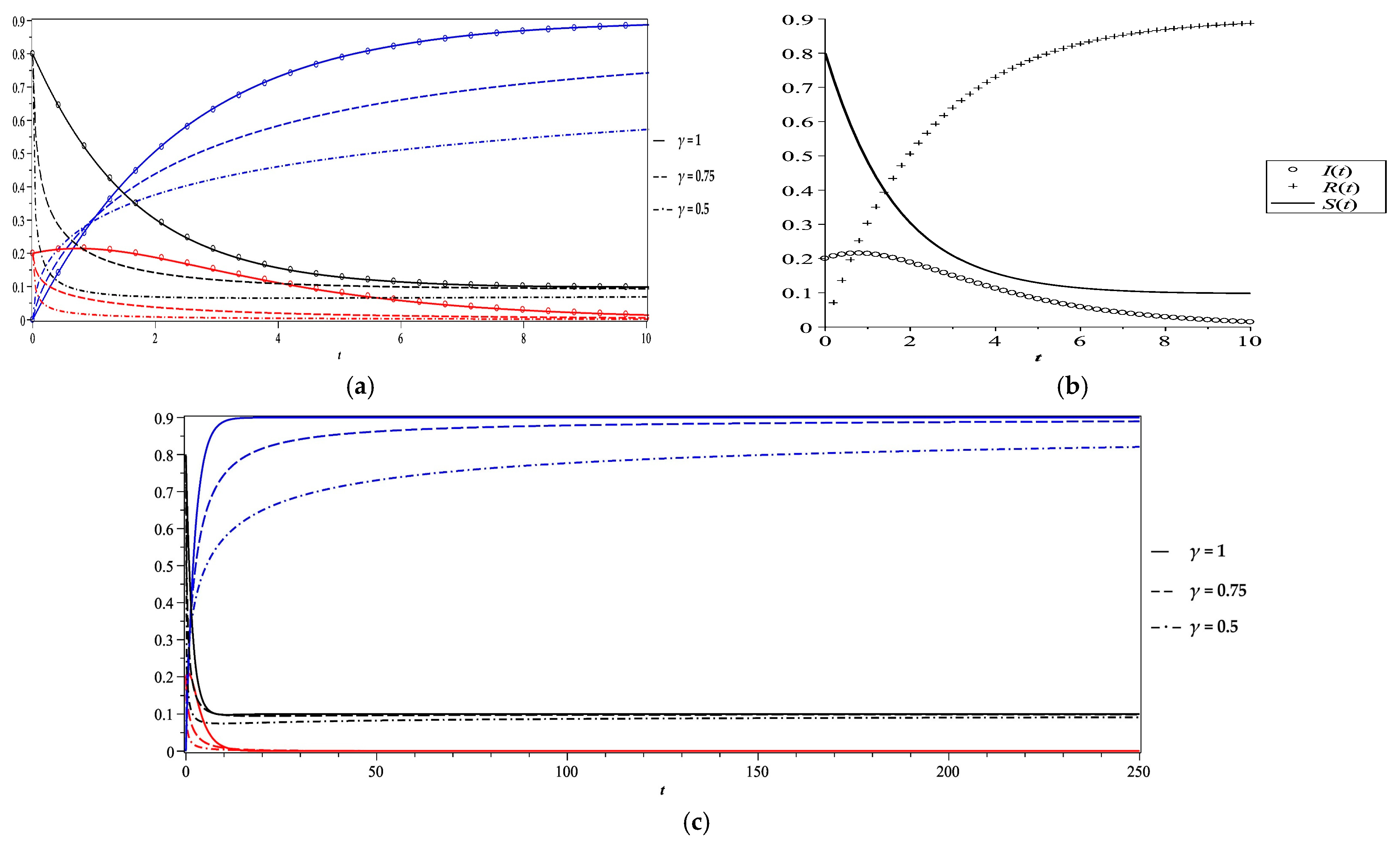

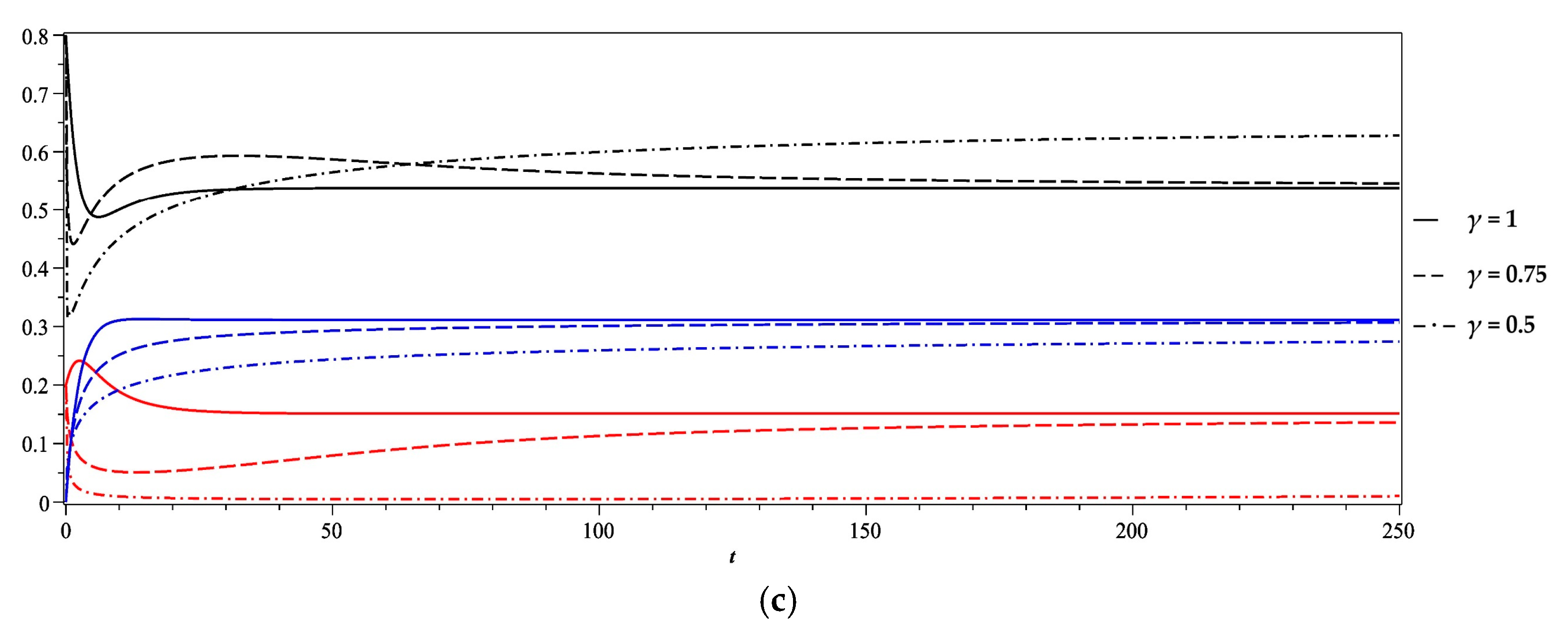

| Case | Vr | γ | Fixed Point of the Stability Analysis | Steady State Equilibrium of the Numerical Solution at T = 250 | Comment | ||||
|---|---|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | ||||
| 1 | 0.186 | 1 | 0.1 | 0.0 | 0.9 | 0.1000 | 0.0 | 0.8999 | Removal of the disease |
| 0.75 | 0.0989 | 0.0 | 0.8897 | ||||||
| 0.5 | 0.0914 | 0.0 | 0.8207 | ||||||
| 2 | 0.186 | 1 | 0.1 | 0.0 | 0.9 | 0.0999 | 0.0 | 0.9000 | |
| 0.75 | 0.0989 | 0.0 | 0.8897 | ||||||
| 0.5 | 0.0913 | 0.0 | 0.8208 | ||||||
| 3 | 1.302 | 1 | 0.5375 | 0.1512 | 0.3113 | 0.5375 | 0.1512 | 0.3113 | No removal of the disease |
| 0.75 | 0.5457 | 0.1363 | 0.3066 | ||||||
| 0.5 | 0.6277 | 0.0102 | 0.2742 | ||||||
| 4 | 1.860 | 1 | 0.5375 | 0.4302 | 0.0323 | 0.5375 | 0.4302 | 0.0323 | |
| 0.75 | 0.5440 | 0.4140 | 0.0306 | ||||||
| 0.5 | 0.6063 | 0.2870 | 0.0188 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, M.M.; Alsharari, F. A Comparative Numerical Study and Stability Analysis for a Fractional-Order SIR Model of Childhood Diseases. Mathematics 2021, 9, 2847. https://doi.org/10.3390/math9222847
Mousa MM, Alsharari F. A Comparative Numerical Study and Stability Analysis for a Fractional-Order SIR Model of Childhood Diseases. Mathematics. 2021; 9(22):2847. https://doi.org/10.3390/math9222847
Chicago/Turabian StyleMousa, Mohamed M., and Fahad Alsharari. 2021. "A Comparative Numerical Study and Stability Analysis for a Fractional-Order SIR Model of Childhood Diseases" Mathematics 9, no. 22: 2847. https://doi.org/10.3390/math9222847
APA StyleMousa, M. M., & Alsharari, F. (2021). A Comparative Numerical Study and Stability Analysis for a Fractional-Order SIR Model of Childhood Diseases. Mathematics, 9(22), 2847. https://doi.org/10.3390/math9222847







