Methodology to Calibrate the Dissection Properties of Aorta Layers from Two Sets of Experimental Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
2.2. Elastic Properties of Aortic Tissue
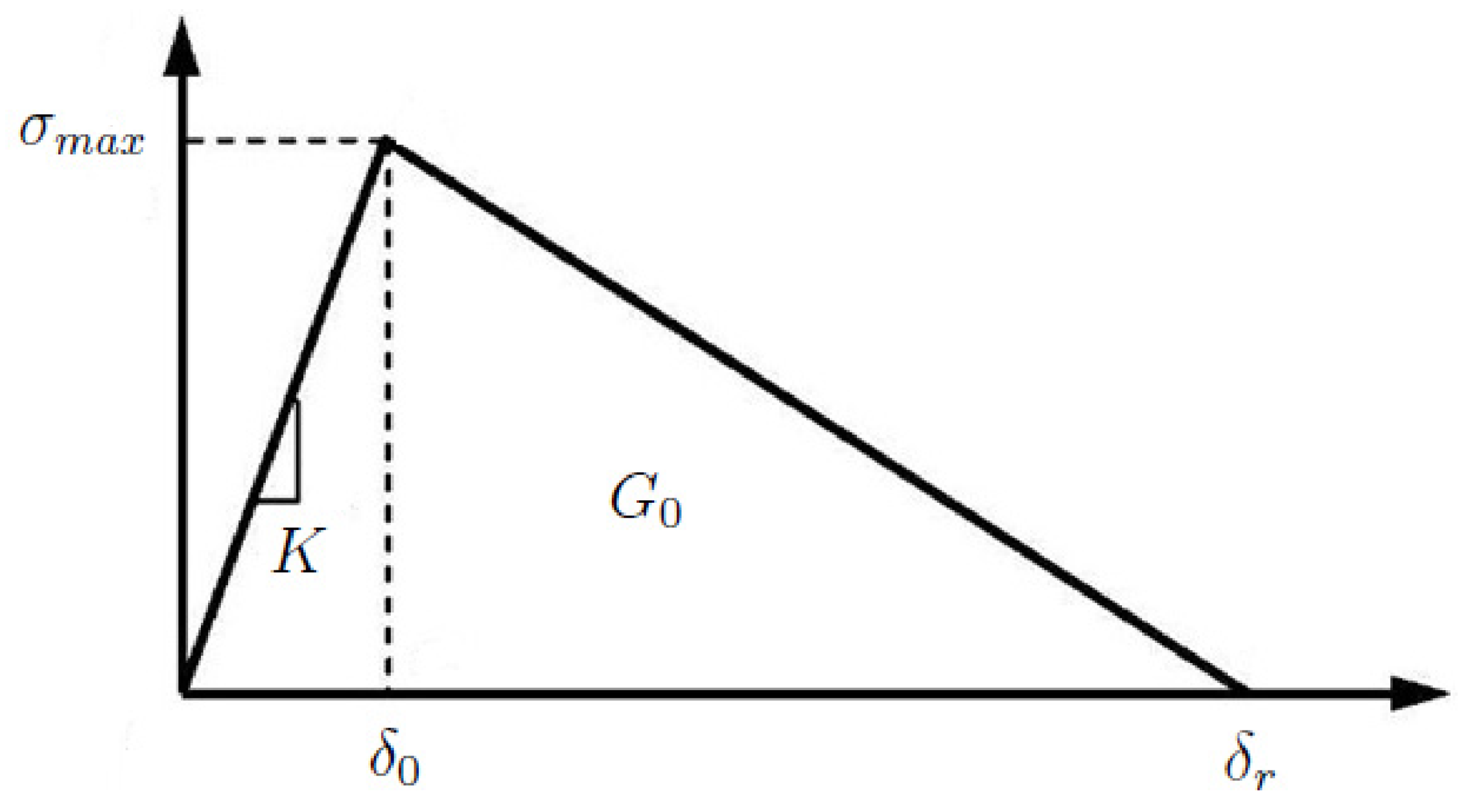
2.3. Fracture Properties of Aortic Tissue
2.4. Methodology to Calibrate the Failure Properties from Experimental Measurements
2.5. Numerical Implementation
3. Results
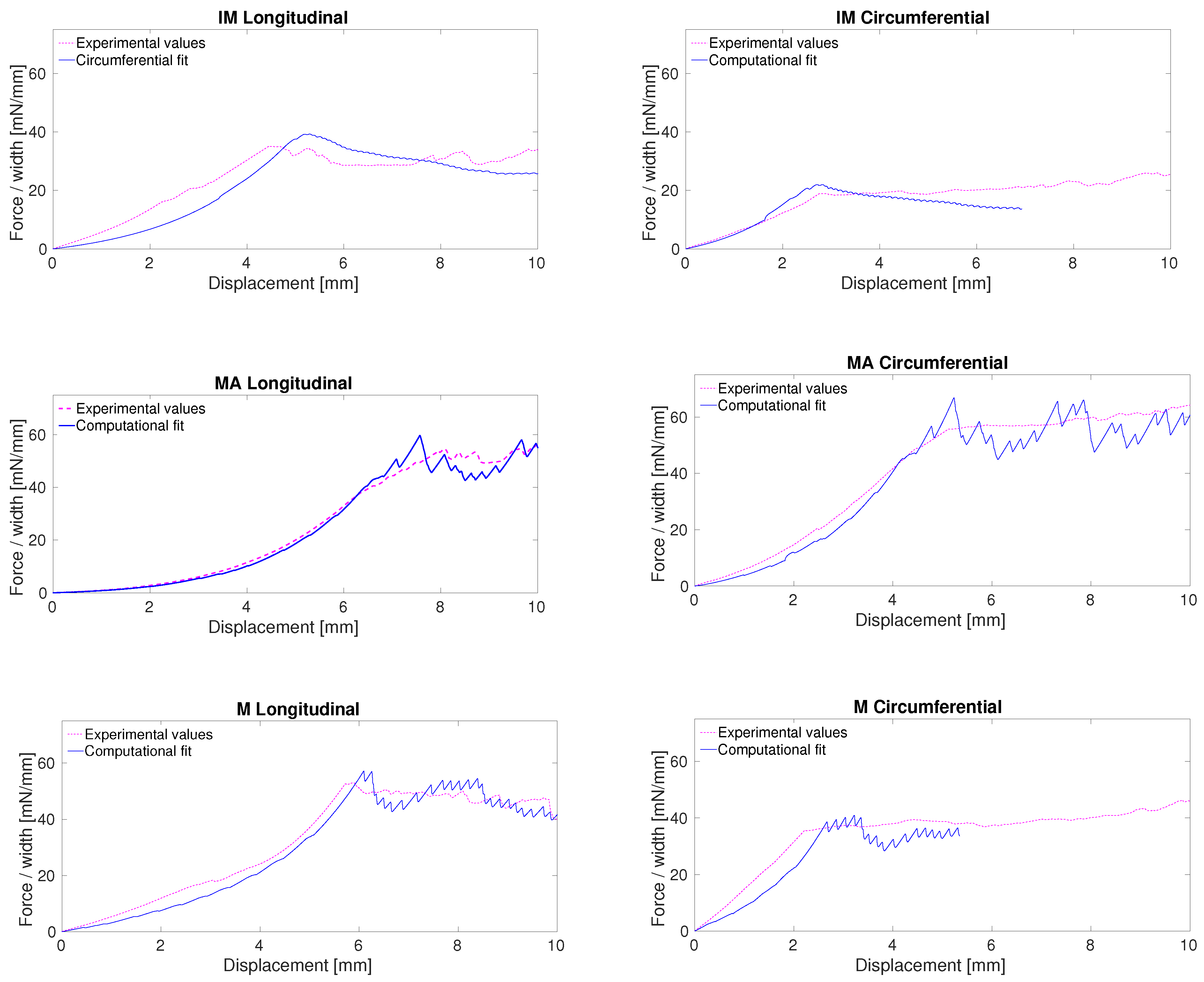
3.1. Elastic Properties of Aortic Tissue
3.2. Fracture Properties of Aortic Tissue
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Estimates 2019: Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability; Technical Report; World Health Organization (WHO): Geneva, Switzerland, 2020. [Google Scholar]
- Mikich, M. Dissection of the aorta: A new approach. Heart 2003, 89, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Sherifova, S.; Holzapfel, G.A. Biochemomechanics of the thoracic aorta in health and disease. Prog. Biomed. Eng. 2020, 99, 1–17. [Google Scholar]
- Elefteriades, J.A. Thoracic aortic aneurysm: Reading enemy’s playbook. J. Biol. Med. 2008, 81, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Alfonso, F.; Boileau, C.; Dirsch, O.; Eber, B.; Haverich, A.; Rakowski, H.; Struyven, J.; Radegran, K.; Sechtem, U.; et al. Diagnosis and management of aortic dissection: Recommendations of the Task Force in Aortic Dissection. Eur. Heart J. 2001, 22, 1642–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; Eusanio, M.D.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. Insights From the International Registry of Acute Aortic Dissection. Circulation 2018, 137, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
- Sommer, G.; Gasser, T.C.; Regitnig, P.; Auer, M.; Holzapfel, G.A. Dissection Properties of the Human Aortic Media: An Experimental Study. J. Biomech. Eng. 2008, 130, 021007. [Google Scholar] [CrossRef]
- Sommer, G.; Sherifova, S.; Oberwalder, P.; Dapunt, O.; Ursomanno, P.; De Anda, A.; Griffith, B.; Holzapfel, G.A. Mechanical strength of aneurysmatic and dissected human thoracic aortas at different shear loading modes. J. Biomech. 2016, 49, 2374–2382. [Google Scholar] [CrossRef] [Green Version]
- Pasta, S.; Phillippi, J.A.; Gleason, T.G.; Vorp, D.A. Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. Thoracic Cardiovasc. Surg. 2012, 143, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Angouras, D.C.; Kritharis, E.; Sokolis, D. Regional distribution of delamination strength in ascending thoracic aortic aneurysms. J. Mech. Behav. Biomed. Mater. 2019, 98, 58–70. [Google Scholar] [CrossRef]
- Pasta, S.; Phillippi, J.A.; Tsamis, A.; D’Amore, A.; Raffa, G.M.; Pilato, M.; Scardulla, C.; Watkins, S.C.; Wagner, W.R.; Gleason, T.G.; et al. Constitutive modeling of ascending thoracic aortic aneurysms using microstructural parameter. Med. Eng. Phys. 2016, 38, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Sassani, S.G.; Tsangaris, S.; Sokolis, D.P. Layer- and region-specific material characterization of ascending thoracic aortic aneurysms by microstructure-based models. J. Biomech. 2015, 48, 3757–3765. [Google Scholar] [CrossRef] [PubMed]
- Manopoulos, C.; Karathanasis, I.; Kouerinis, I.; Angouras, D.; Lazaris, A.; Tsangaris, S.; Sokolis, D. Identification of regional/layer differences in failure properties and thickness as important biomechanical factors responsible for the initiation of aortic dissections. J. Biomech. 2018, 80, 102–110. [Google Scholar] [CrossRef]
- Amabili, M.; Arena, G.O.; Balasubramanian, P.; Breslavsky, I.D.; Cartier, R.; Ferrari, G.; Holzapfel, G.A.; Kassab, A.; Mongrain, R. Biomechanical characterization of a chronic type a dissected human aorta. J. Biomech. 2020, 110, 109978. [Google Scholar] [CrossRef]
- Leng, X.; Zhou, B.; Deng, X.; Davis, L.; Lessner, S.M.; Sutton, M.A.; Shazly, T. Experimental and numerical studies of two arterial wall delamination modes. J. Mech. Behav. Biomed. Mater. 2018, 77, 321–330. [Google Scholar] [CrossRef] [PubMed]
- FitzGibbon, B.; McGarry, P. Development of a test method to investigate mode II fracture and dissection of arteries. Acta Biomat. 2021, 121, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T.C.; Holzapfel, G.A. Modeling the propagation of arterial dissection. Eur. J. Mech. Solids 2006, 25, 617–633. [Google Scholar] [CrossRef]
- Ferrara, A.; Pandolfi, A. A numerical study of arterial media dissection processes. Int. J. Fract. 2010, 166, 21–33. [Google Scholar] [CrossRef]
- Noble, C.; van der Sluis, O.; Voncken, R.M.; Burke, O.; Franklin, S.E.; Lewis, R.; Taylor, Z.A. Simulation of arterial dissection by a penetrating external body using cohesive zone modelling. J. Mech. Behav. Biomed. 2017, 71, 95–105. [Google Scholar] [CrossRef]
- Peña, J.A.; Martínez, M.A.; Peña, E. Layer-specific residual deformations and uniaxial and biaxial mechanical properties of thoracic porcine aorta. J. Mech. Behav. Biomed. 2015, 50, 55–69. [Google Scholar] [CrossRef]
- Gasser, T.C.; Ogden, R.W.; Holzapfel, G.A. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 2006, 3, 15–35. [Google Scholar] [CrossRef]
- Spencer, A.J.M. Theory of Invariants. In Continuum Physics; Academic Press: New York, NY, USA, 1971; pp. 239–253. [Google Scholar]
- Forsell, C.; Gasser, T.C. Numerical simulation of the failure of ventricular tissue due to deeper penetration, the impact of constitutive properties. J. Biomech. 2011, 44, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, J. A continuous damage mechanics model for ductile fracture. J. Eng. Mater. Tchnol. 1985, 107, 83–89. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Carew, T.E.; Vaishnav, R.N.; Patel, D.J. Compressibility of the arterial wall. Circ. Res. 1968, 23, 61–86. [Google Scholar] [CrossRef] [Green Version]
- Hernández, Q.; Peña, E. Failure properties of vena cava tissue due to deep penetration during filter insertion. Biomech. Model. Mechanobiol. 2016, 15, 845–856. [Google Scholar] [CrossRef] [PubMed]
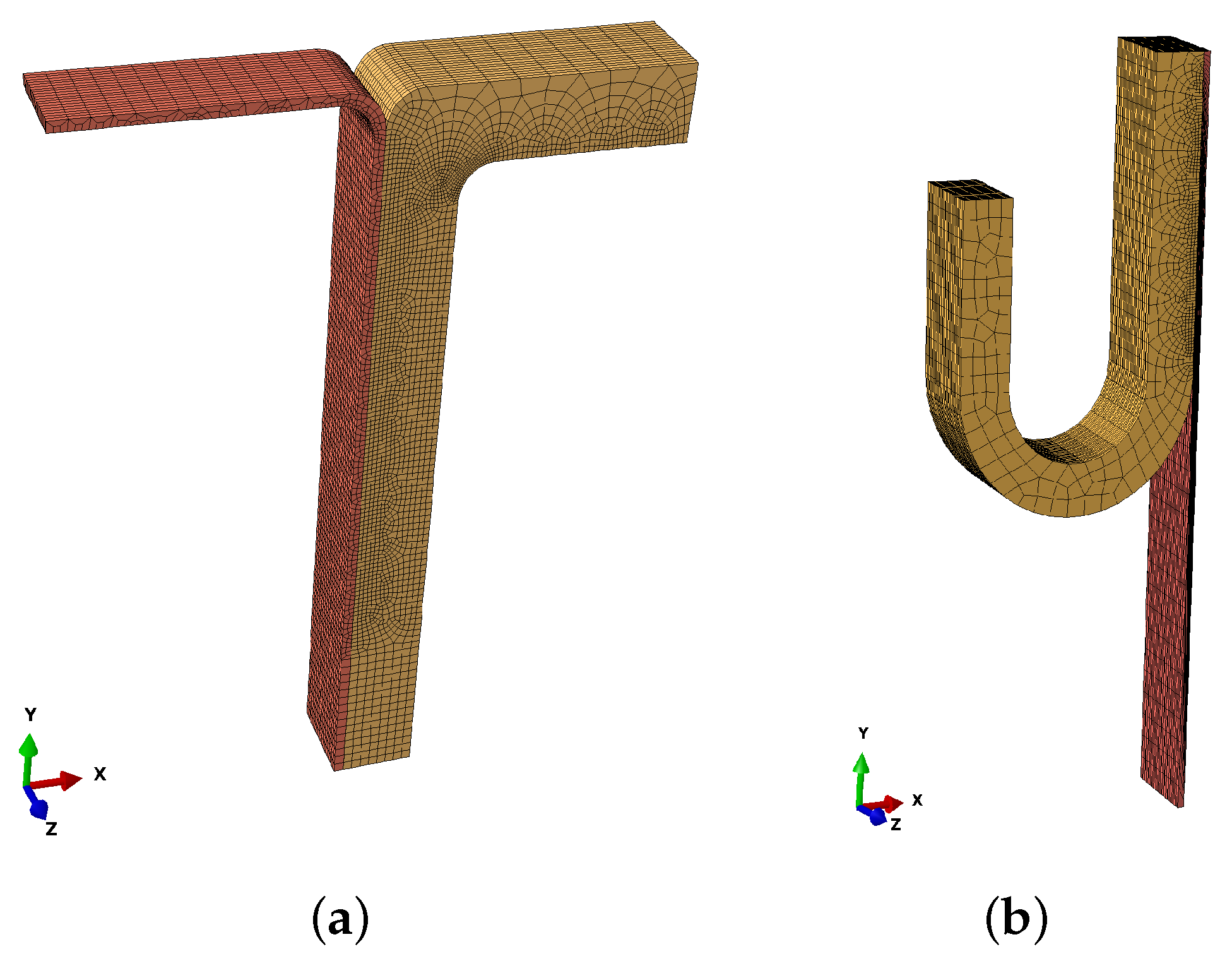


| Specimen | Width [mm] | Thickness [mm] | Number of Elements | |
|---|---|---|---|---|
| Peel test | IM | 4.0 | 1.70 | 50,340 |
| MA | 4.0 | 2.00 | 54,700 | |
| M | 4.0 | 2.10 | 57,040 | |
| Mixed test | IM | 5.0 | 1.57 | 25,265 |
| MA | 4.8 | 2.30 | 20,370 | |
| M | 5.0 | 2.00 | 27,962 | |
| [kPa] | [kPa] | |||||
|---|---|---|---|---|---|---|
| 18.0606 | 504.9060 | 44.8462 | 0.24299 | 35 | 0.9893080 | 0.0652 |
| Interface | ||||||
|---|---|---|---|---|---|---|
| [mm] | [mm] | [kPa] | [mN/mm] | [mN/mm] | ||
| IM | Longitudinal | 0.023 | 0.070 | 230 | 10,000 | 8 |
| Circumferential | 0.014 | 0.100 | 200 | 14,000 | 10 | |
| MA | Longitudinal | 0.019 | 0.086 | 185 | 10,000 | 8 |
| Circumferential | 0.020 | 0.063 | 160 | 8000 | 5 | |
| M | Longitudinal | 0.013 | 0.092 | 130 | 10,000 | 6 |
| Circumferential | 0.010 | 0.100 | 80 | 8000 | 4 | |
| Interface | ||||||
|---|---|---|---|---|---|---|
| [mm] | [mm] | [kPa] | [mN/mm] | [mN/mm] | ||
| IM | Longitudinal | 0.040 | 0.100 | 800 | 20,000 | 40 |
| Circumferential | 0.034 | 0.073 | 550 | 16,000 | 20 | |
| MA | Longitudinal | 0.078 | 0.104 | 1250 | 16,000 | 65 |
| Circumferential | 0.088 | 0.107 | 1400 | 16,000 | 75 | |
| M | Longitudinal | 0.081 | 0.100 | 1300 | 16,000 | 65 |
| Circumferential | 0.075 | 0.100 | 1200 | 16,000 | 60 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-Ruiz, I.; Cilla, M.; Martínez, M.A.; Peña, E. Methodology to Calibrate the Dissection Properties of Aorta Layers from Two Sets of Experimental Measurements. Mathematics 2021, 9, 1593. https://doi.org/10.3390/math9141593
Ríos-Ruiz I, Cilla M, Martínez MA, Peña E. Methodology to Calibrate the Dissection Properties of Aorta Layers from Two Sets of Experimental Measurements. Mathematics. 2021; 9(14):1593. https://doi.org/10.3390/math9141593
Chicago/Turabian StyleRíos-Ruiz, Itziar, Myriam Cilla, Miguel A. Martínez, and Estefanía Peña. 2021. "Methodology to Calibrate the Dissection Properties of Aorta Layers from Two Sets of Experimental Measurements" Mathematics 9, no. 14: 1593. https://doi.org/10.3390/math9141593
APA StyleRíos-Ruiz, I., Cilla, M., Martínez, M. A., & Peña, E. (2021). Methodology to Calibrate the Dissection Properties of Aorta Layers from Two Sets of Experimental Measurements. Mathematics, 9(14), 1593. https://doi.org/10.3390/math9141593






