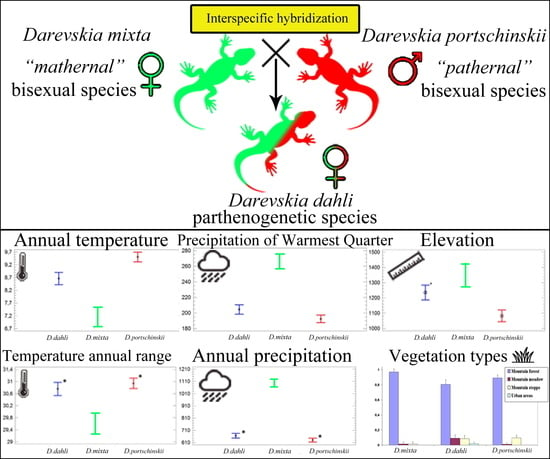
Species Distribution Models and Niche Partitioning among Unisexual Darevskia dahli and Its Parental Bisexual (D. portschinskii, D. mixta) Rock Lizards in the Caucasus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset Acquisition and Preparation of Vector and Raster Layers
2.2. Spatial Thinning of Records and Predictor Variables
2.3. Determination of the Maxent Models Parameters
2.4. Construction of Species Distribution (SDM) and Ecological Niche (ENM) Models
2.5. Comparison of Ecological Niches and Parameters of Habitat Exploitation
3. Results
3.1. Model Performance and Predictor Variables
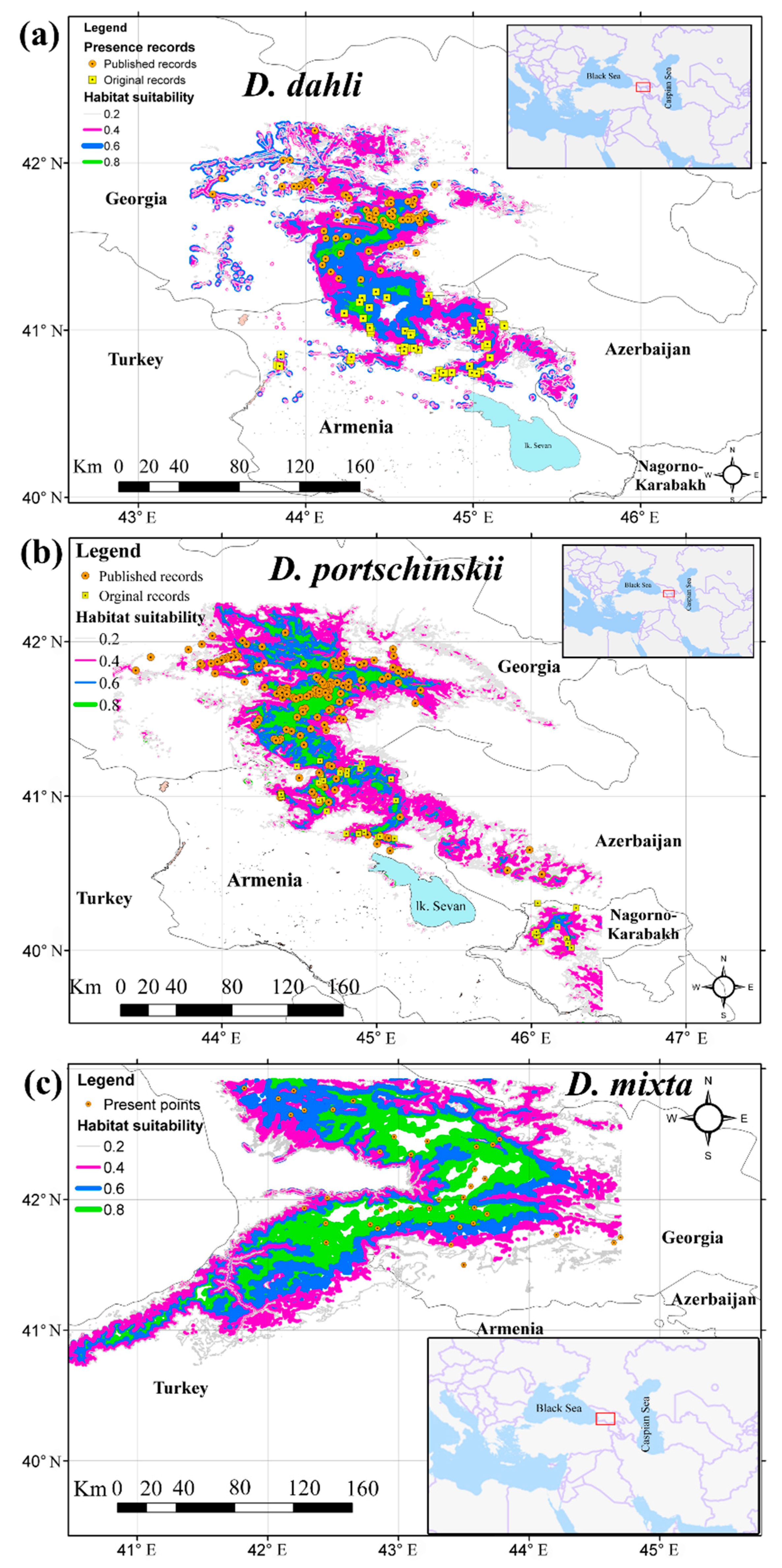
3.2. Potential Range of Studied Lizards
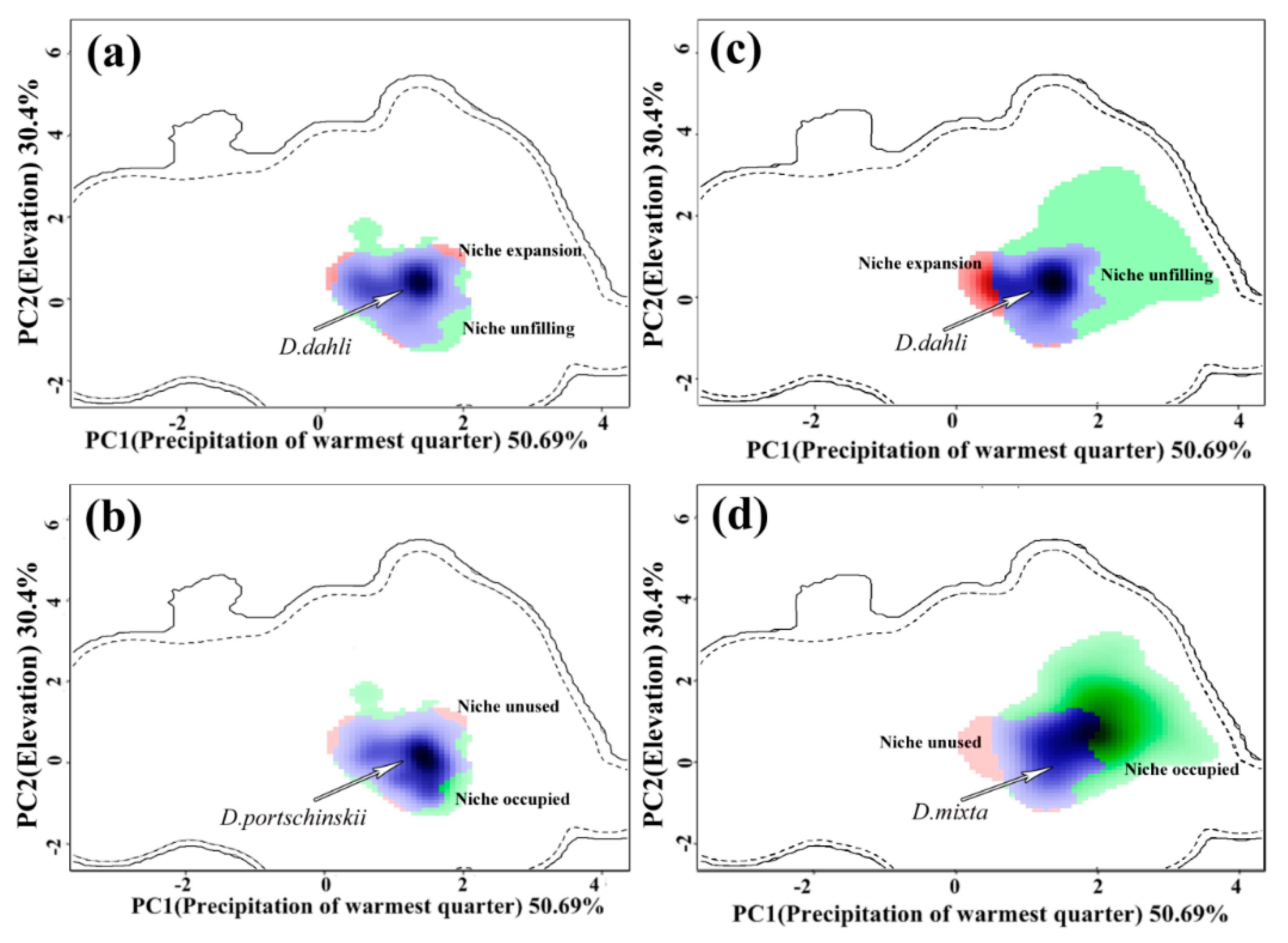
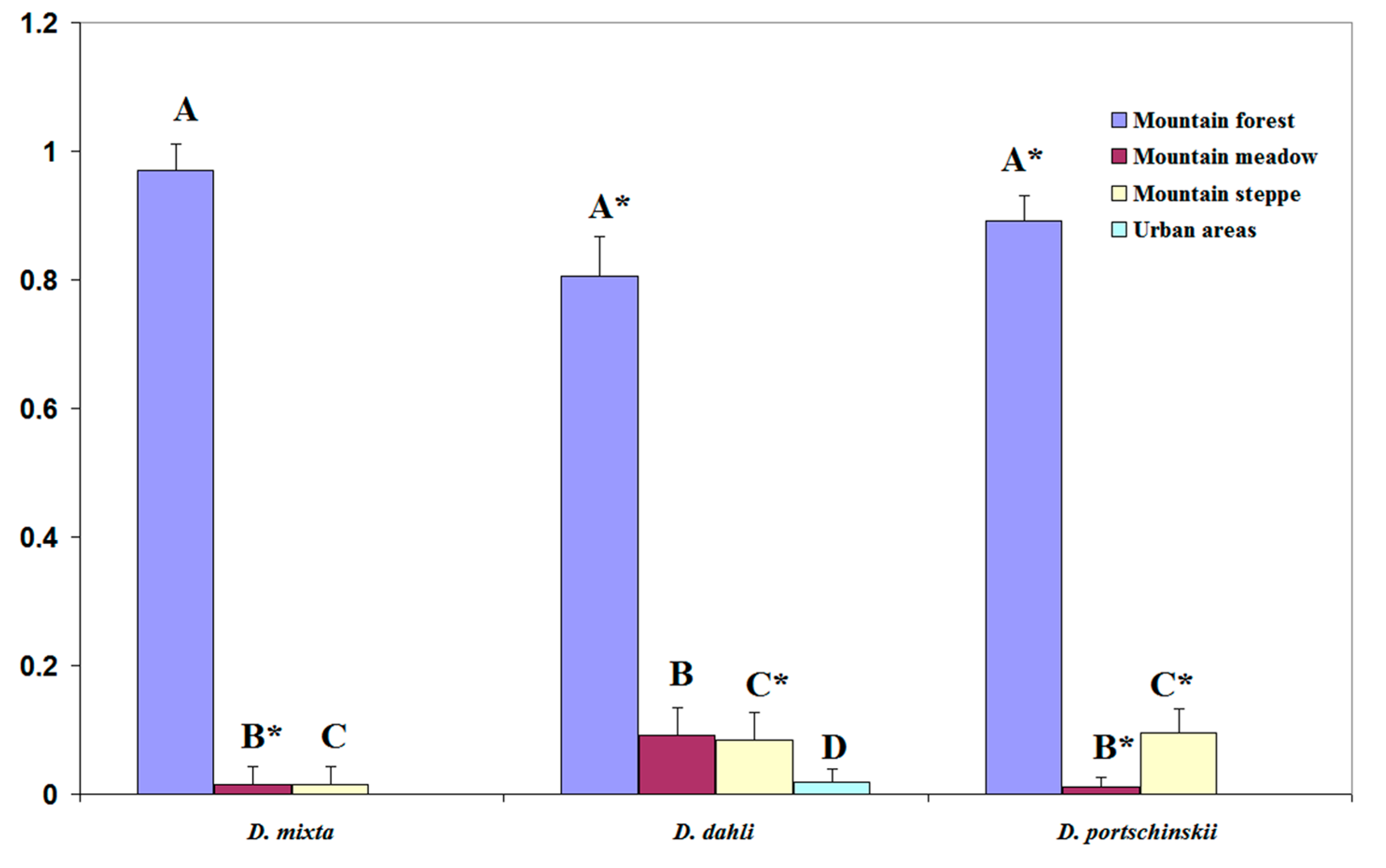
3.3. Comparative Analysis of Ecological Niches
3.4. Shifts of Ecological Niches Centroids along Environmental Gradients
3.5. Statistical Analysis of the Shifts of Niche Centroids
4. Discussion
4.1. Predicted Distribution Range with Optimal Model Parameters
4.2. Niches of the Unisexual and Bisexual Lizards: Breadth, Overlap, Similarity and Shifts
4.3. Mechanisms of Coexistence of Unisexual and Bisexual Forms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujita, M.; Moritz, C. Origin and evolution of parthenogenetic genomes in lizards: Current state and future directions. Cytogenet. Genome Res. 2009, 127, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Darevsky, I.S. Rock Lizards of The Caucasus, Systematics, Ecology and Phylogenesis of the Polymorphic Groups of Caucasian Rock Lizards of the Subgenus; Nauka: Leningrad, Russia, 1967. [Google Scholar]
- Borkin, L.J.; Darevsky, I.S. Reticular (hybridogeneous) speciation in vertebrate. Zh. Obshch. Biol. 1980, 41, 485–506. [Google Scholar]
- Kearney, M.R.; Fujita, M.K.; Ridenour, J. Lost Sex. In the Reptiles: Constraints and Correlations; Springer Science and Business Media LLC: Heidelberg, Germany, 2009; pp. 447–474. [Google Scholar]
- Sinclair, E.A.; Pramuk, J.B.; Bezy, R.L.; Crandall, K.A.; Sites, J.W. DNA evidence for non hybrid origins of parthenogenesis in natural populations of vertebrates. Evolution 2010, 64, 1346–1357. [Google Scholar] [PubMed]
- West, S. Lively; Read A pluralist approach to sex and recombination. J. Evol. Biol. 1999, 12, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Vrijenhoek, R.C. Unisexual fish: Model systems for studying ecology and evolution. Annu. Rev. Ecol. Syst. 1994, 25, 71–96. [Google Scholar] [CrossRef]
- Roughgarden, J. Evolution of niche width. Am. Nat. 1972, 106, 683–718. [Google Scholar] [CrossRef]
- Vrijenhoek, R.C.; Parker, E.D. Geographical Parthenogenesis: General Purpose Genotypes and Frozen Niche Variation. In Lost Sex; Schön, I., Martens, K., Van Dijk, P., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 99–131. [Google Scholar]
- Kearney, M.R. Hybridization, glaciation and geographical parthenogenesis. Trends Ecol. Evol. 2005, 20, 495–502. [Google Scholar] [CrossRef]
- Peck, J.R.; Yearsley, J.; Waxman, D. Explaining the geographic distributions of sexual and asexual populations. Nature 1998, 391, 889–892. [Google Scholar] [CrossRef]
- Glesener, R.R.; Tilman, D. Sexuality and the components of environmental uncertainty: Clues from geographic parthenogenesis in terrestrial animals. Am. Nat. 1978, 112, 659–673. [Google Scholar] [CrossRef]
- Kearney, M.; Wahl, R.; Autumn, K. Increased capacity for sustained locomotion at low temperature in parthenogenetic geckos of hybrid origin. Physiol. Biochem. Zool. 2005, 78, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Vandel, A. La parthénogenèse géographique contribution a l’ètude biologique et cytologique de la parthénogenèse naturelle. Bull. Biol. Fr. Belg. 1928, 62, 164–281. [Google Scholar]
- Beaton, M.J. Geographical parthenogenesis and polyploidy in daphnia pulex. Am. Nat. 1988, 132, 837–845. [Google Scholar] [CrossRef]
- Gray, M.M.; Weeks, S.C. Niche breadth in clonal and sexual fish (Poeciliopsis): A test of the frozen niche variation model. Can. J. Fish. Aquat. Sci. 2001, 58, 1313–1318. [Google Scholar] [CrossRef]
- Wright, J.W.; Lowe, C.H. Weeds, polyploids, parthenogenesis, and the geographical and ecological distribution of all-female species of cnemidophorus. Copeia 1968, 1968, 128–138. [Google Scholar] [CrossRef]
- Greenwald, K.R.; Denton, R.D.; Gibbs, H.L. Niche partitioning among sexual and unisexual Ambystoma salamanders. Ecosphere 2016, 7, 01579. [Google Scholar] [CrossRef]
- Bierzychudek, P. Patterns in plant parthenogenesis. Cell. Mol. Life Sci. 1985, 41, 1255–1264. [Google Scholar] [CrossRef]
- Hörandl, E. The complex causality of geographical parthenogenesis. New Phytol. 2006, 171, 525–538. [Google Scholar] [CrossRef]
- Darevsky, I.S. Natural parthenogenesis in polymorphic group of the Caucasian rock lizards related to Lacerta saxicola Eversman. J. Ohio Herpetol. Soc. 1966, 5, 115–152. [Google Scholar] [CrossRef]
- Murphy, R.W.; Fu, J.; MacCulloch, R.D.; Darevsky, I.S.; Kupriyanova, L.A. A fine line between sex and unisexuality: The phylogenetic constraints on parthenogenesis in lacertid lizards. Zool. J. Linn. Soc-Lond. 2000, 130, 527–549. [Google Scholar] [CrossRef]
- Vergun, A.A.; Martirosyan, I.A.; Semyenova, S.K.; Omelchenko, A.; Petrosyan, V.G.; Lazebny, O.E.; Tokarskaya, O.N.; Korchagin, V.I.; Ryskov, A. Clonal diversity and clone formation in the parthenogenetic caucasian rock lizard darevskia dahli. PLoS ONE 2014, 9, e91674. [Google Scholar] [CrossRef] [Green Version]
- Darevsky, I.S. Natural parthenogenesis in certain subspecies of rock lizards (Lacerta saxicola Eversmann). Dokl. Akad. Nauk SSSR Biol. Sci. 1958, 122, 730–732. [Google Scholar]
- Uzzell, T.; Darevsky, I.S. Biochemical evidence for the hybrid origin of the parthenogenetic species of the lacerta saxicola complex (sauria: Lacertidae), with a discussion of some ecological and evolutionary implications. Copeia 1975, 1975, 204–222. [Google Scholar] [CrossRef]
- MacCulloch, R.D.; Murphy, R.W.; Fu, J.; Darevsky, I.S.; Danielyan, F. Disjunct habitats as islands: Genetic variability in the Caucasian rock lizard Lacerta portschinskii. Genetica 1997, 101, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, I.A.; Kan, N.G.; Petrosyan, V.G.; Malysheva, D.N.; Trofimova, A.A.; Danielyan, F.D.; Darevskii, I.S.; Korochkin, L.I.; Ryskov, A.; Tokarskaya, O.N. Variation of mini- and microsatellite DNA repeats in parthenogenetic lizard darevskia armeniaca as revealed by DNA fingerprinting analysis. Russ. J. Genet. 2003, 39, 159–165. [Google Scholar] [CrossRef]
- Malysheva, D.N.; Vergun, A.A.; Tokarskaya, O.N.; Sevast’Yanova, G.A.; Darevsky, I.S.; Ryskov, A. Nucleotide sequences of the microsatellite locus Du215 (arm) allelic variants in the parthenospecies Darevskia armeniaca (Lacertidae). Russ. J. Genet. 2007, 43, 116–120. [Google Scholar] [CrossRef]
- Tarkhnishvili, D.; Gavashelishvili, A.; Avaliani, A.; Murtskhvaladze, M.; Mumladze, L. Unisexual rock lizard might be out competing its bisexual progenitors in the Caucasus. Biol. J. Linn. Soc. 2010, 101, 447–460. [Google Scholar] [CrossRef] [Green Version]
- Arakelyan, M.S.; Danielyan, F.D.; Corti, C.; Sindaco, R.; Leviton, A.E. Herpetofauna of Armenia and Nagorno-Karabakh; Society for Study of Amphibians and Reptiles: Salt Lake City, UT, USA, 2011. [Google Scholar]
- Petrosyan, V.G.; Osipov, F.A.; Bobrov, V.V.; Dergunova, N.N.; Danielyan, F.D.; Arakelyan, M.S. Analysis of geographical distribution of the parthenogenetic rock lizard Darevskia armeniaca and its parental species (D. mixta, D. valentini) based on ecological modelling. Salamandra 2019, 55, 173–190. [Google Scholar]
- Parker, E.D.; Walker, J.M.; Paulissen, M.A. Clonal Diversity in Cnemidophorus: Ecological and Morphological Consequences. In Evolution and Ecology of Unisexual vertebrates; Dawley, R.M., Bogart, J.P., Eds.; New York State Museum Bulletin: Albany, NY, USA, 1989; Volume 466, pp. 72–86. [Google Scholar]
- Species Complex of the Lacertid Lizards. Available online: https://www.lacerta.de/AS/Taxon.php?Genus=33 (accessed on 9 July 2020).
- Fu, J.; Murphy, R.W.; Darevsky, I.S. Limited genetic variation in Lacerta mixta and its parthenogenetic daughter species: Evidence from cytochrome b and ATPase 6 gene DNA sequences. Genetica 1999, 105, 227–231. [Google Scholar] [CrossRef]
- Arakelyan, M.; Danielyan, F.; Stepanyan, I. Hybrids of Darevskia valentini, D. armeniaca and D. unisexualis from a sympatric population in Armenia. Amphib. 2008, 29, 487–504. [Google Scholar] [CrossRef] [Green Version]
- Spangenberg, V.; Arakelyan, M.; Galoyan, E.; Matveevsky, S.; Petrosyan, R.; Bogdanov, Y.; Danielyan, F.; Kolomiets, O. Reticulate evolution of the rock lizards: Meiotic chromosome dynamics and spermatogenesis in diploid and triploid males of the genus darevskia. Genes 2017, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- Spangenberg, V.; Arakelyan, M.; Galoyan, E.; Pankin, M.; Petrosyan, R.; Stepanyan, I.; Grishaeva, T.; Danielyan, F.; Kolomiets, O. Extraordinary centromeres: Differences in the meiotic chromosomes of two rock lizards species Darevskia portschinskii and Darevskia raddei. Peer J. 2019, 7, e6360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarkhnishvili, D.; Murtskhvaladze, M.; Anderson, C.L. Coincidence of genotypes at two loci in two parthenogenetic rock lizards: How backcrosses might trigger adaptive speciation. Biol. J. Linn. Soc. 2017, 121, 365–378. [Google Scholar] [CrossRef]
- Kaliontzopoulou, A.; Brito, J.C.; Carretero, M.; Larbes, S.; Harris, D.J. Modelling the partially unknown distribution of wall lizards (Podarcis) in North Africa: Ecological affinities, potential areas of occurrence, and methodological constraints. Can. J. Zool. 2008, 86, 992–1001. [Google Scholar] [CrossRef]
- Pleguezuelos, J.M.; Mora, E.; De Pous, P.; Escoriza, D.; Metallinou, M.; Donaire, D.; Comas, M.; Carranza, S. Elusive but widespread? The potential distribution and genetic variation of Hyalosaurus koellikeri (Günther, 1873) in the Maghreb. Amphib-reptil 2011, 32, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadeh, F.; Carretero, M.A.; Rödder, D.; Harris, D.J.; Freitas, S.N.; Perera, A.; Böhme, W. Inferring the effects of past climate fluctuations on the distribution pattern of Iranolacerta (Reptilia, Lacertidae): Evidence from mitochondrial DNA and species distribution models. Zool. Anz. A J. Comp. Zool. 2013, 252, 141–148. [Google Scholar] [CrossRef]
- Doronin, I.V. Distribution data of rock lizards from the Darevskia (praticola) complex (Sauria: Lacertidae). Curr. Stud. Herpetol. 2015, 15, 3–38. [Google Scholar]
- Freitas, S.N.; Rocha, S.; Campos, J.C.; Ahmadzadeh, F.; Corti, C.; Sillero, N.; Ilgaz, Ç.; Kumlutas, Y.; Arakelyan, M.; Harris, D.J.; et al. Parthenogenesis through the ice ages: A biogeographic analysis of Caucasian rock lizards (genus Darevskia). Mol. Phylogenetics Evol. 2016, 102, 117–127. [Google Scholar] [CrossRef]
- Ćorović, J.; Popovic, M.; Cogălniceanu, D.; Carretero, M.; Crnobrnja-Isailović, J. Distribution of the meadow lizard in Europe and its realized ecological niche model. J. Nat. Hist. 2018, 52, 1909–1925. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J. Species distribution modeling and ecological niche modeling: Getting the concepts right. Nat. Conserv 2012, 10, 102–107. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, P.A.; Graham, C.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Wisz, M.; Hijmans, R.; Elith, J.; Peterson, A.T.; Graham, C.; Guisan, A. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility. Available online: https://doi.org/10.15468/dl.4wnnka (accessed on 9 July 2020).
- Global Biodiversity Information Facility. Available online: https://doi.org/10.15468/dl.ml4da9 (accessed on 9 July 2020).
- Global Biodiversity Information Facility. Available online: https://doi.org/10.15468/dl.i83b94 (accessed on 9 July 2020).
- Global Climate and Weather Data. Available online: https://www.worldclim.org/data/worldclim21.html (accessed on 9 July 2020).
- Hijmans, R.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Shuttle Radar Topographic Mission. Available online: https://www2.jpl.nasa.gov/srtm (accessed on 9 July 2020).
- Open Street Map. Available online: http://www.openstreetmap.org/ (accessed on 11 December 2011).
- Acopian Center for the Environment GIS Data. Available online: http://ace.aua.am/gis-and-remote-sensing/vector-data (accessed on 9 July 2020).
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P.; Silva, D.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute. Arc GIS Desktop 10.6.1. Available online: https://support.esri.com/en/products/desktop/arcgis-desktop/arcmap/10-6-1 (accessed on 9 July 2020).
- Barbosa, A.M. fuzzySim: Applying fuzzy logic to binary similarity indices in ecology. Methods Ecol. Evol. 2015, 6, 853–858. [Google Scholar] [CrossRef]
- Hair, J.F.; Anderson, R.E., Jr.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis, 3rd ed.; Macmillan: New York, NY, USA, 1995. [Google Scholar]
- Radosavljevic, A.; Anderson, R.P. Making better Maxentmodels of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2013, 41, 629–643. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo Package for R. 2017. Available online: https://cran.r-project.org/package=dismo (accessed on 9 July 2020).
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.E.; Schmiegelow, F.K. Evaluating resource selection functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef] [Green Version]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [Green Version]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2011, 21, 481–497. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Manly, B.F. Multivariate Statistical Methods, A Primer, 2nd ed.; Chapman and Hall: London, UK, 1994. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: New Jersey, NJ, USA, 2010. [Google Scholar]
- Feltz, C.J.; Miller, G.E. An asymptotic test for the equality of coefficients of variation from k population. Stat. Med. 1996, 15, 647–658. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anonymous. The R Project for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 13 February 2012).
- RStudio is an Integrated Development Environment (IDE) for R Language. Available online: https://www.rstudio.com (accessed on 9 July 2020).
- Tuniyev, B.S.; Lotiev, K.Y.; Tuniyev, S.B.; Gabaev, V.N.; Kidov, A.A. Amphibians and reptiles of South Ossetia. Nat. Conserv. Res. 2017, 2, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Tuniyev, B.S.; Tuniyev, S.B.; Avci, A.; Ilgaz, Ç. Herpetological studies in eastern and north-eastern Turkey. Curr. Herpetol. 2014, 14, 44–53. [Google Scholar]
- Breiner, F.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Darevsky, I.S.; Shcherbak, N.N. Acclimatization of parthenogenetic lizards in Ukraine. Priroda 1967, 3, 93–94. [Google Scholar]
- Darevsky, I.S.; Kan, N.G.; Ryabinina, N.L.; Martirosyan, I.A.; Tokarskaya, O.N.; Grechko, V.V.; Shcherbak, N.N.; Danielyan, F.D.; Ryskov, A.P. Biochemical, molecular, and genetic characteristics of parthenogenetic lizards Lacerta armeniaca (Mehely), introduced from Armenia to Ukraine. Dokl Akad Nauk. 1998, 363, 846–848. [Google Scholar]
- Dotsenko, I.B.; Peskov, V.N.; Miropolskaya, M.V. Comparative analysis of genus Darevskia rock lizards external morphology from the territory of Ukraine, and the species belonging of them. Proc. Zool. Mus. 2008–2009, 40, 130–142. [Google Scholar]
- Darevsky, I.S.; Kulikova, V.N. Natürliche Parthenogenese in der polymorphen Gruppe der Kaukasischen Felseidechse (Lacerta saxicola Eversmann). Zool. Jahrb. Syst. 1961, 89, 119–176. [Google Scholar]
- Parker, E.D.; Selander, R.K.; Hudson, R.O.; Lester, L.J. Genetic diversity in colonizing parthenogenetic cockroaches. Evolution 1977, 31, 836–842. [Google Scholar] [CrossRef]
- Lynch, M.; Bürger, R.; Butcher, D.; Gabriel, W. The Mutational meltdown in asexual populations. J. Hered. 1993, 84, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Nekrasova, O.D.; Kostiushyn, V.A. Current Distribution of the Introduced Rock Lizards of the Darevskia (Saxicola) Complex (Sauria, Lacertidae, Darevskia) in Zhytomyr Region (Ukraine). Vestnik Zool. 2016, 50, 225–230. [Google Scholar] [CrossRef]
- Vrijenhoek, R.C. Factors affecting clonal diversity and coexistence. Am. Zool. 1979, 19, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Vrijenhoek, R.C. Ecological Differentiation Among Clones: The Frozen Niche Variation Model. In Population Biology and Evolution; Springer: Berlin/Heidelberg, Germany, 1984; pp. 217–231. [Google Scholar]
- Weeks, S.C. The effects of recurrent clonal formation on clonal invasion patterns and sexual persistence: A monte carlo simulation of the frozen niche-variation model. Am. Nat. 1993, 141, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Girnyk, A.E.; Vergun, A.A.; Semyenova, S.K.; Guliaev, A.; Arakelyan, M.; Danielyan, F.D.; Martirosyan, I.A.; Murphy, R.W.; Ryskov, A. Multiple interspecific hybridization and microsatellite mutations provide clonal diversity in the parthenogenetic rock lizard Darevskia armeniaca. BMC Genom. 2018, 19, 979. [Google Scholar] [CrossRef] [PubMed]
- Shine, R. Seasonal shifts in nest temperature can modify the phenotypes of hatchling lizards, regardless of overall mean incubation temperature. Funct. Ecol. 2004, 18, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Nei, M. The new mutation theory of phenotypic evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 12235–12242. [Google Scholar] [CrossRef] [Green Version]
- Dessauer, H.C.; Cole, C.J. Diversity between and within Nominal Forms of Unisexual Teiid Lizards. In Evolution and Ecology of Unisexual Vertebrates; Dawley, R.M., Bogart, J.P., Eds.; New York State Museum Bulletin: Albany, NY, USA, 1989; Volume 466, pp. 49–71. [Google Scholar]
- Moritz, C.; Uzzell, T.; Spolsky, C.; Hotz, H.; Darevsky, I.; Kupriyanova, L.; Danielyan, F. The material ancestry and approximate age of parthenogenetic species of Caucasian rock lizards (Lacerta: Lacertidae). Genetica 1992, 87, 53–62. [Google Scholar] [CrossRef]

| Variable | Code | R2 | VIF |
|---|---|---|---|
| D. dahli, RP = 0.63 | |||
| Solar irradiation (kJ m−2 day−1) | C_SRad | 0.72 | 3.53 |
| Precipitation in warmest quarter (mm) | C_PWarmQ | 0.63 | 2.69 |
| Elevation (m) | T_EL | 0.42 | 1.72 |
| Isothermality, % | C_ISOT | 0.26 | 1.35 |
| Distance to road (m) | L_DHW | 0.09 | 1.1 |
| D. portschinskii, RP = 0.72 | |||
| Solar irradiation (kJ m−2 day−1) | C_SRad | 0.86 | 7.28 |
| Elevation (m) | T_EL | 0.77 | 4.43 |
| Mean temperature in driest quarter (°C) | C_MeanTDrQ | 0.77 | 4.27 |
| Precipitation in warmest quarter (mm) | C_PWarmQ | 0.76 | 4.18 |
| Annual temperature range (°C) | C_TAnR | 0.72 | 3.56 |
| Precipitation seasonality (coefficient of variation) (%) | C_PCoefVar | 0.56 | 2.28 |
| Isothermality, % | C_ISOT | 0.56 | 2.27 |
| Vegetation type | L_VEG | 0.56 | 0.44 |
| Distance to road (m) | L_DHW | 0.12 | 1.14 |
| D. mixta, RP = 0.69 | |||
| Solar irradiation (kJ m−2 day−1) | C_Srad | 0.76 | 4.13 |
| Precipitation in warmest quarter | C_PWarmQ | 0.65 | 2.87 |
| Precipitation seasonality (coefficient of variation) (%) | C_PCoefVar | 0.54 | 2.16 |
| Elevation (m) | T_EL | 0.47 | 1.9 |
| Vegetation type | L_VEG | 0.41 | 1.7 |
| Distance to road (m) | L_DHW | 0.07 | 1.08 |
| Predictor Variables | D. dahli | D. portschinskii | D. mixta | |||
|---|---|---|---|---|---|---|
| PC | PI | PC | PI | PC | PI | |
| C_ISOT | 24.4 | 10.4 | 12.6 | 21.9 | 0.3 | 1 |
| C_TAnR | 0.1 | 0.1 | 1.5 | 5.6 | 0.8 | 0.7 |
| C_MeanTDrQ | 0.2 | 0 | 9.1 | 8.6 | 0.1 | 0 |
| C_PCoefVar | 1.7 | 0.8 | 3.9 | 5.3 | 36.8 | 44.5 |
| C_PWarmQ | 30 | 50.2 | 32.7 | 22.3 | 30.1 | 20.4 |
| C_SRad | 12.4 | 22.7 | 17.8 | 26.4 | 8.7 | 17.4 |
| T_EL | 13.6 | 13.7 | 6.6 | 1.8 | 4.9 | 9.1 |
| L_DHW | 9 | 0.1 | 5.4 | 0.1 | 0.2 | 0.1 |
| L_VEG | 0.2 | 0.3 | 5.1 | 1 | 8.3 | 0.8 |
| Boyce index (±SE) | 0.95(±0.004) | 0.93 (±0.004) | 0.94 (±0.003) | |||
| Parental Species | Schoener’s D Index | p-Value | E | S | U |
|---|---|---|---|---|---|
| D. mixta | 0.22 | 0.09 | 0.12 | 0.88 | 0.57 |
| D. portschinskii | 0.72 | 0.009 | 0.02 | 0.98 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrosyan, V.; Osipov, F.; Bobrov, V.; Dergunova, N.; Omelchenko, A.; Varshavskiy, A.; Danielyan, F.; Arakelyan, M. Species Distribution Models and Niche Partitioning among Unisexual Darevskia dahli and Its Parental Bisexual (D. portschinskii, D. mixta) Rock Lizards in the Caucasus. Mathematics 2020, 8, 1329. https://doi.org/10.3390/math8081329
Petrosyan V, Osipov F, Bobrov V, Dergunova N, Omelchenko A, Varshavskiy A, Danielyan F, Arakelyan M. Species Distribution Models and Niche Partitioning among Unisexual Darevskia dahli and Its Parental Bisexual (D. portschinskii, D. mixta) Rock Lizards in the Caucasus. Mathematics. 2020; 8(8):1329. https://doi.org/10.3390/math8081329
Chicago/Turabian StylePetrosyan, Varos, Fedor Osipov, Vladimir Bobrov, Natalia Dergunova, Andrey Omelchenko, Alexander Varshavskiy, Felix Danielyan, and Marine Arakelyan. 2020. "Species Distribution Models and Niche Partitioning among Unisexual Darevskia dahli and Its Parental Bisexual (D. portschinskii, D. mixta) Rock Lizards in the Caucasus" Mathematics 8, no. 8: 1329. https://doi.org/10.3390/math8081329
APA StylePetrosyan, V., Osipov, F., Bobrov, V., Dergunova, N., Omelchenko, A., Varshavskiy, A., Danielyan, F., & Arakelyan, M. (2020). Species Distribution Models and Niche Partitioning among Unisexual Darevskia dahli and Its Parental Bisexual (D. portschinskii, D. mixta) Rock Lizards in the Caucasus. Mathematics, 8(8), 1329. https://doi.org/10.3390/math8081329