A New ODE-Based Model for Tumor Cells and Immune System Competition
Abstract
1. Introduction
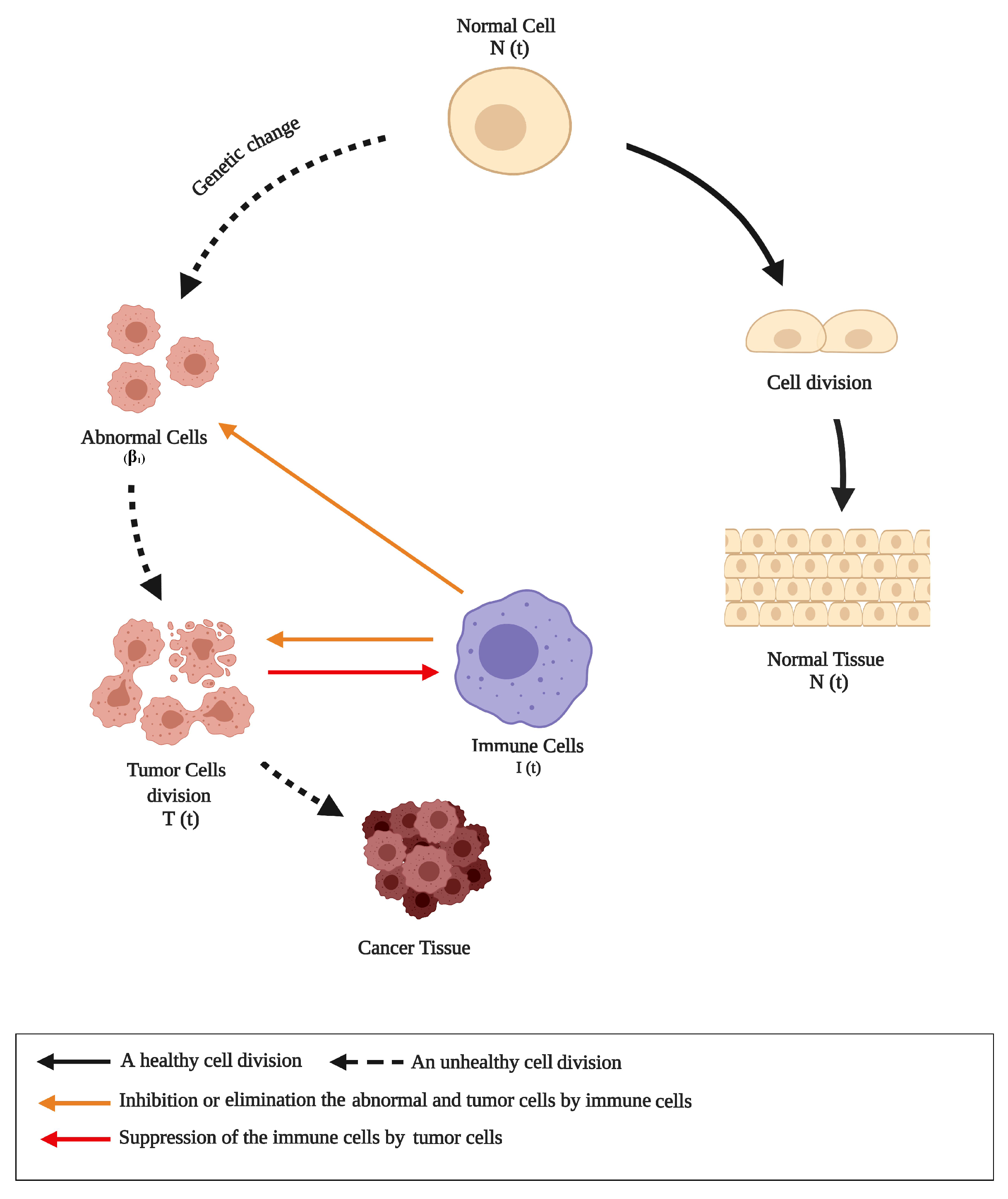
2. Normal-Tumor-Immune-Unhealthy Diet Model (Ntiunhdm)
3. The Model Analysis
3.1. Conditions and Solution Positivity
3.2. Equilibrium Points
- (1)
- The response stage, : This response stage indicates that the immune system is capable of responding when abnormal cells start appearing within a tissue. The progression of abnormal cells and their tumors counterparts is governed by the stability case of this particular stage. Thus, the equilibrium point of the response stage is given as follows:
- (2)
- The coexisting stage, : The NTIUNHDM is built based on the IUNHDM [36] which is characterised by the loss of the interaction stage. In this case, the immune cells respond to abnormal cells as if they were normal cells, which gives abnormal cells a chance to occur in the tissue as non-active cells for a long time (10–15 years). The equilibrium point of this stage is given as follows:
- (3)
- The resisting stage, : This stage is generated when abnormal cells begin to turn into tumor cells. The equilibrium point at this stage is given as follows:wherewhere
- The tumor cells compete for survival at the resisting stage by rapidly dividing and growing. Therefore,
- There is a suppression to the activity of immune cells as a consequence of rapid growth and division of tumors cells [23]. Therefore,
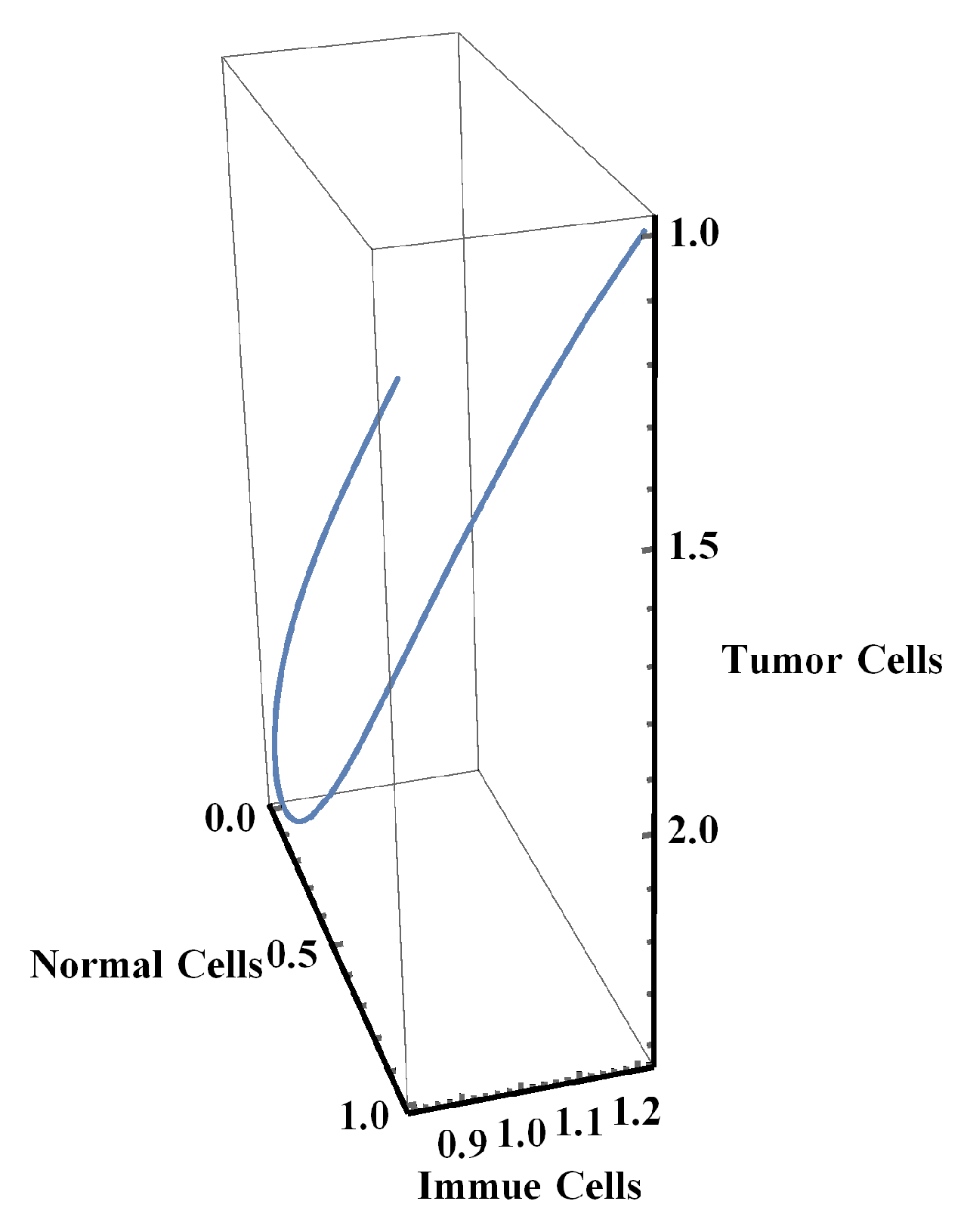
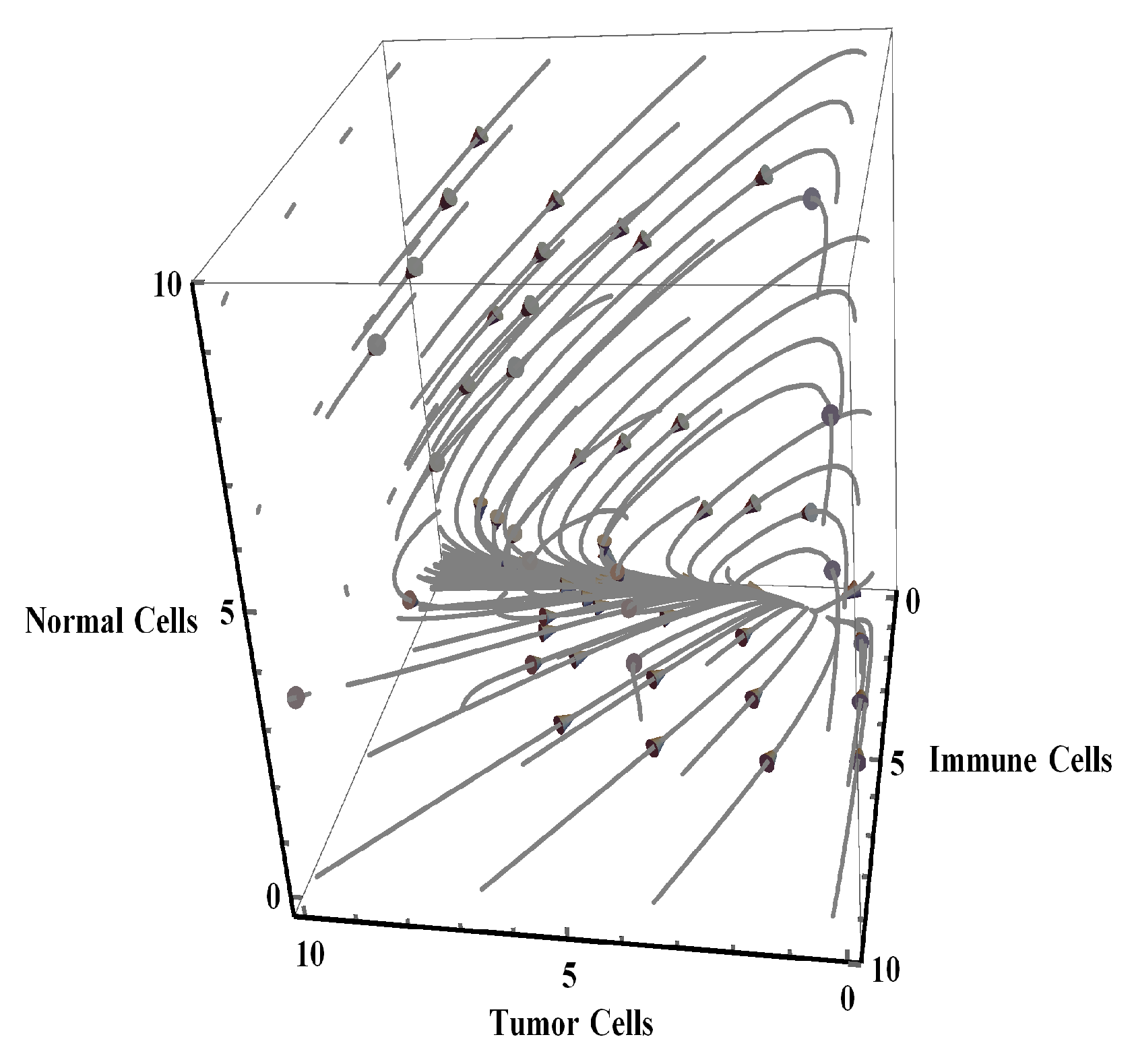
3.3. Stability of Equilibrium Points
- The immune system in the NTIUNHDM failed to eliminate or inhibit the abnormal cells.
- The semi-stable state of the coexisting stage indicated a probability of abnormal cells progressing into their tumors counterparts at any stage.
- The appearance of tumors cells stimulated the immune cells to react, but the growth and division of tumor cells was rapid when compared to the number of immune cells generated to inhibit or remove tumor cells. This generation of cells might convert to immune cell cancer [23].
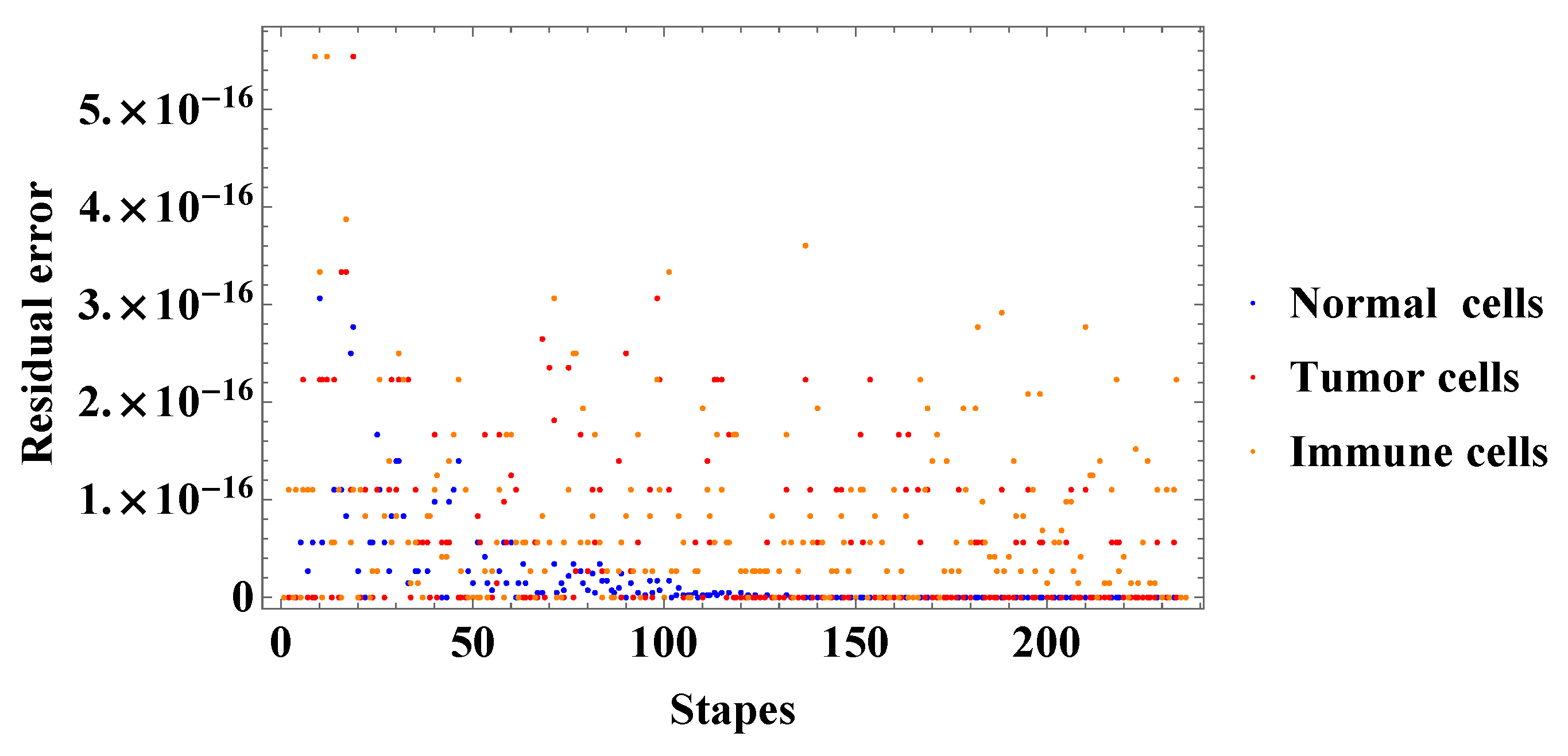
4. Numerical Simulation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Witten, M. Modeling cellular agign and tumorigenic transformation. Math. Comput. Simul. 1982, 24, 572–584. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Makalkin, I.A.; Taylor, M.A.; Perelson, A.S. Nonlinear dynamics of immunogenic tumors: Parameter estimation and global bifurcation analysis. Bull. Math. Biol. 1994, 56, 295–321. [Google Scholar] [CrossRef]
- Kirschner, D.; Panetta, J.C. Modeling immunotherapy of the tumor–immune interaction. J. Math. Biol. 1998, 37, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Zaenker, K.; An Der Heiden, U. A basic mathematical model of the immune response. Chaos Interdiscip. J. Nonlinear Sci. 1995, 5, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, M.; Ghosh, M. Modeling treatment of cancer using virotherapy with generalized logistic growth of tumor cells. Stoch. Anal. Appl. 2018, 36, 1068–1086. [Google Scholar] [CrossRef]
- Feizabadi, M.S.; Volk, C.; Hirschbeck, S. A two-compartment model interacting with dynamic drugs. Appl. Math. Lett. 2009, 22, 1205–1209. [Google Scholar] [CrossRef]
- Feizabadi, M.S.; Witten, T.M. Chemotherapy in conjoint aging-tumor systems: Some simple models for addressing coupled aging-cancer dynamics. Theor. Biol. Med. Model. 2010, 7, 21. [Google Scholar] [CrossRef]
- Sharma, S.; Samanta, G. Analysis of the dynamics of a tumor–immune system with chemotherapy and immunotherapy and quadratic optimal control. Differ. Equ. Dyn. Syst. 2016, 24, 149–171. [Google Scholar] [CrossRef]
- Lavi, O.; Gottesman, M.M.; Levy, D. The dynamics of drug resistance: A mathematical perspective. Drug Resist. Updat. 2012, 15, 90–97. [Google Scholar] [CrossRef]
- Feizabadi, M.S.; Witten, T.M. Modeling drug resistance in a conjoint normal-tumor setting. Theor. Biol. Med. Model. 2015, 12, 3. [Google Scholar] [CrossRef]
- Sameen, S.; Barbuti, R.; Milazzo, P.; Cerone, A.; Del Re, M.; Danesi, R. Mathematical modeling of drug resistance due to KRAS mutation in colorectal cancer. J. Theor. Biol. 2016, 389, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Feizabadi, M.S. Modeling multi-mutation and drug resistance: Analysis of some case studies. Theor. Biol. Med. Model. 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Gałach, M. Dynamics of the Tumor—Immune System Competition—The Effect of Time Delay. Int. J. Appl. Math. Comput. Sci. 2003, 13, 395–406. [Google Scholar]
- Ji, Z.; Yan, K.; Li, W.; Hu, H.; Zhu, X. Mathematical and computational modeling in complex biological systems. BioMed Res. Int. 2017, 2017, 5958321. [Google Scholar] [CrossRef] [PubMed]
- Kolev, M. Mathematical Analysis of an Autoimmune Diseases Model: Kinetic Approach. Mathematics 2019, 7, 1024. [Google Scholar] [CrossRef]
- Bianca, C.; Mogno, C. Modelling pedestrian dynamics into a metro station by thermostatted kinetic theory methods. Math. Comput. Model. Dyn. Syst. 2018, 24, 207–235. [Google Scholar] [CrossRef]
- Vizzari, G.; Crociani, L.; Bandini, S. An agent-based model for plausible wayfinding in pedestrian simulation. Eng. Appl. Artif. Intell. 2020, 87, 103241. [Google Scholar] [CrossRef]
- De Montigny, J.; Iosif, A.; Breitwieser, L.; Manca, M.; Bauer, R.; Vavourakis, V. An in silico hybrid continuum-/agent-based procedure to modelling cancer development: Interrogating the interplay amongst glioma invasion, vascularity and necrosis. Methods 2020, in press. [Google Scholar] [CrossRef]
- Harris, L.A.; Beik, S.; Ozawa, P.M.; Jimenez, L.; Weaver, A.M. Modeling heterogeneous tumor growth dynamics and cell–cell interactions at single-cell and cell-population resolution. Curr. Opin. Syst. Biol. 2019, 17, 24–34. [Google Scholar] [CrossRef]
- Menale, M.; Carbonaro, B. The mathematical analysis towards the dependence on the initial data for a discrete thermostatted kinetic framework for biological systems composed of interacting entities. AIMS Biophys. 2020, 7, 204. [Google Scholar] [CrossRef]
- Bianca, C.; Brézin, L. Modeling the antigen recognition by B-cell and T-cell receptors through thermostatted kinetic theory methods. Int. J. Biomath. 2017, 10, 1750072. [Google Scholar] [CrossRef]
- Masurel, L.; Bianca, C.; Lemarchand, A. On the learning control effects in the cancer-immune system competition. Phys. A Stat. Mech. Appl. 2018, 506, 462–475. [Google Scholar] [CrossRef]
- Mufudza, C.; Sorofa, W.; Chiyaka, E.T. Assessing the effects of estrogen on the dynamics of breast cancer. Comput. Math. Methods Med. 2012, 2012, 473572. [Google Scholar] [CrossRef] [PubMed]
- Glick, A.E.; Mastroberardino, A. An optimal control approach for the treatment of solid tumors with angiogenesis inhibitors. Mathematics 2017, 5, 49. [Google Scholar] [CrossRef]
- Alqudah, M.A. Cancer treatment by stem cells and chemotherapy as a mathematical model with numerical simulations. Alex. Eng. J. 2020. [Google Scholar] [CrossRef]
- Simbawa, E. Mechanistic model for cancer growth and response to chemotherapy. Comput. Math. Methods Med. 2017, 2017, 3676295. [Google Scholar] [CrossRef]
- Zheng, M. Quantitative Analysis for the Spread Range of Malignant Tumor Based on Lie Symmetry. Complexity 2020, 2020, 8468024. [Google Scholar] [CrossRef]
- Khajanchi, S.; Perc, M.; Ghosh, D. The influence of time delay in a chaotic cancer model. Chaos Interdiscip. J. Nonlinear Sci. 2018, 28, 103101. [Google Scholar] [CrossRef]
- Elaiw, A.; Al Agha, A. Analysis of a delayed and diffusive oncolytic M1 virotherapy model with immune response. Nonlinear Anal. Real World Appl. 2020, 55, 103116. [Google Scholar] [CrossRef]
- Alqarni, A.J.; Rambely, A.S.; Hashim, I. Dynamic Modelling of Interactions between Microglia and Endogenous Neural Stem Cells in the Brain during a Stroke. Mathematics 2020, 8, 132. [Google Scholar] [CrossRef]
- Cooper, G. The Cell: A Molecular Approach; ASM Press: Washington, DC, USA, 2000. [Google Scholar]
- Yusof, A.S.; Isa, Z.M.; Shah, S.A. Dietary patterns and risk of colorectal cancer: A systematic review of cohort studies (2000-2011). Asian Pac. J. Cancer Prev. 2012, 13, 4713–4717. [Google Scholar] [CrossRef] [PubMed]
- Marwitz, S.E.; Woodie, L.N.; Blythe, S.N. Western-style diet induces insulin insensitivity and hyperactivity in adolescent male rats. Physiol. Behav. 2015, 151, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sample, C.H.; Martin, A.A.; Jones, S.; Hargrave, S.L.; Davidson, T.L. Western-style diet impairs stimulus control by food deprivation state cues: Implications for obesogenic environments. Appetite 2015, 93, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.; Rambely, A.S.; Alsuhaimi, O. Effect of dietary factor on response of the immune system numerically. J. Phys. Conf. Ser. 2019, 1212, 012025. [Google Scholar] [CrossRef]
- Alharbi, S.A.; Rambely, A.S. A Dynamic Simulation of the Immune System Response to Inhibit And Eliminate Abnormal Cells. Symmetry 2019, 11, 572. [Google Scholar] [CrossRef]
- Alharbi, S.A.; Rambely, A.S. Dynamic Simulation for Analyzing the Effects of the Intervention of Vitamins on Delaying the Growth of Tumor Cells. IEEE Access 2019, 7, 128816–128827. [Google Scholar] [CrossRef]
- Jiménez, R.P.; Hernandez, E.O. Tumour–host dynamics under radiotherapy. Chaos Solitons Fractals 2011, 44, 685–692. [Google Scholar] [CrossRef]
- Hartman, P. Ordinary Differential Equations; John Wiley & Sons: New York, NY, USA; London, UK; Sydney, Australia, 1964. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, S.A.; Rambely, A.S. A New ODE-Based Model for Tumor Cells and Immune System Competition. Mathematics 2020, 8, 1285. https://doi.org/10.3390/math8081285
Alharbi SA, Rambely AS. A New ODE-Based Model for Tumor Cells and Immune System Competition. Mathematics. 2020; 8(8):1285. https://doi.org/10.3390/math8081285
Chicago/Turabian StyleAlharbi, Sana Abdulkream, and Azmin Sham Rambely. 2020. "A New ODE-Based Model for Tumor Cells and Immune System Competition" Mathematics 8, no. 8: 1285. https://doi.org/10.3390/math8081285
APA StyleAlharbi, S. A., & Rambely, A. S. (2020). A New ODE-Based Model for Tumor Cells and Immune System Competition. Mathematics, 8(8), 1285. https://doi.org/10.3390/math8081285




