Finite Element Analysis of Custom Shoulder Implants Provides Accurate Prediction of Initial Stability
Abstract
1. Introduction
2. Materials and Methods
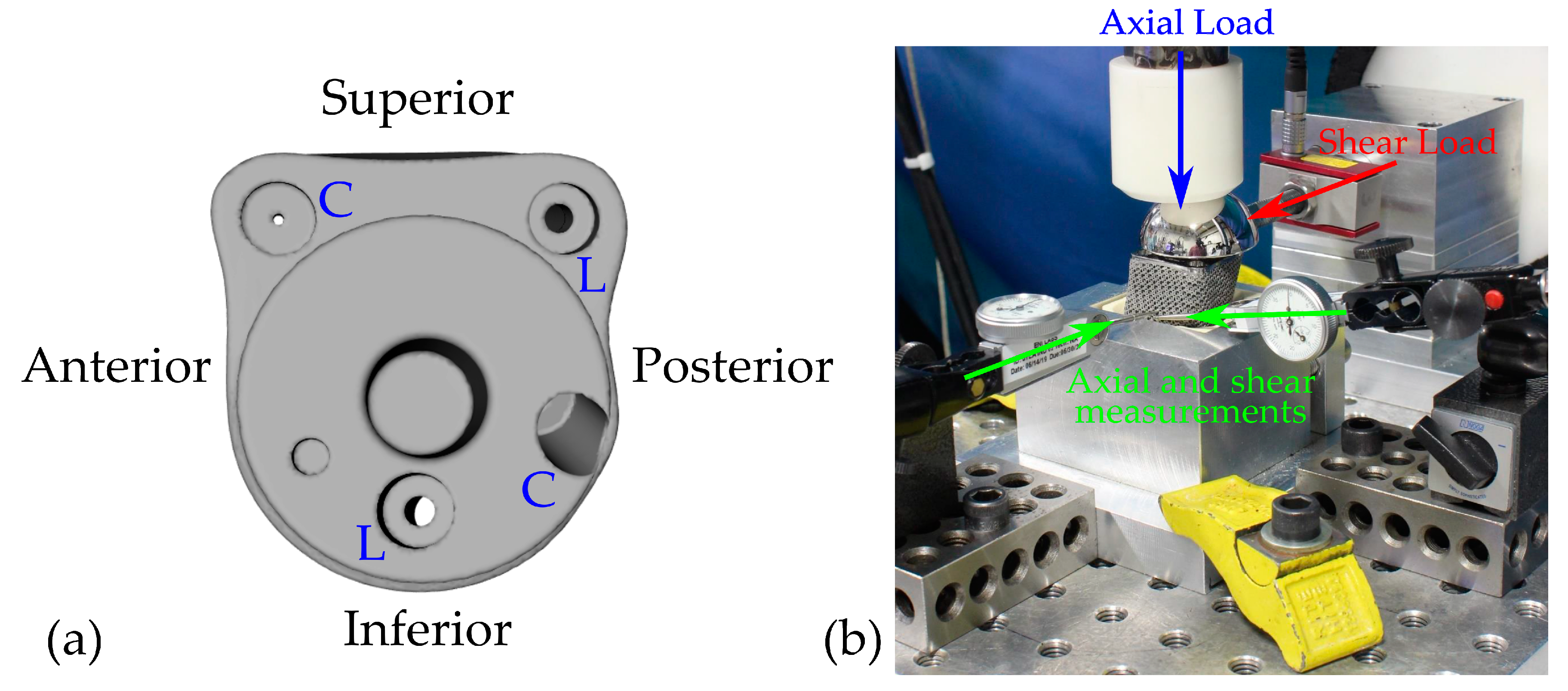
2.1. Experimental Set-Up
2.2. Generation of Finite Element Models
2.2.1. Bone and Implant Models
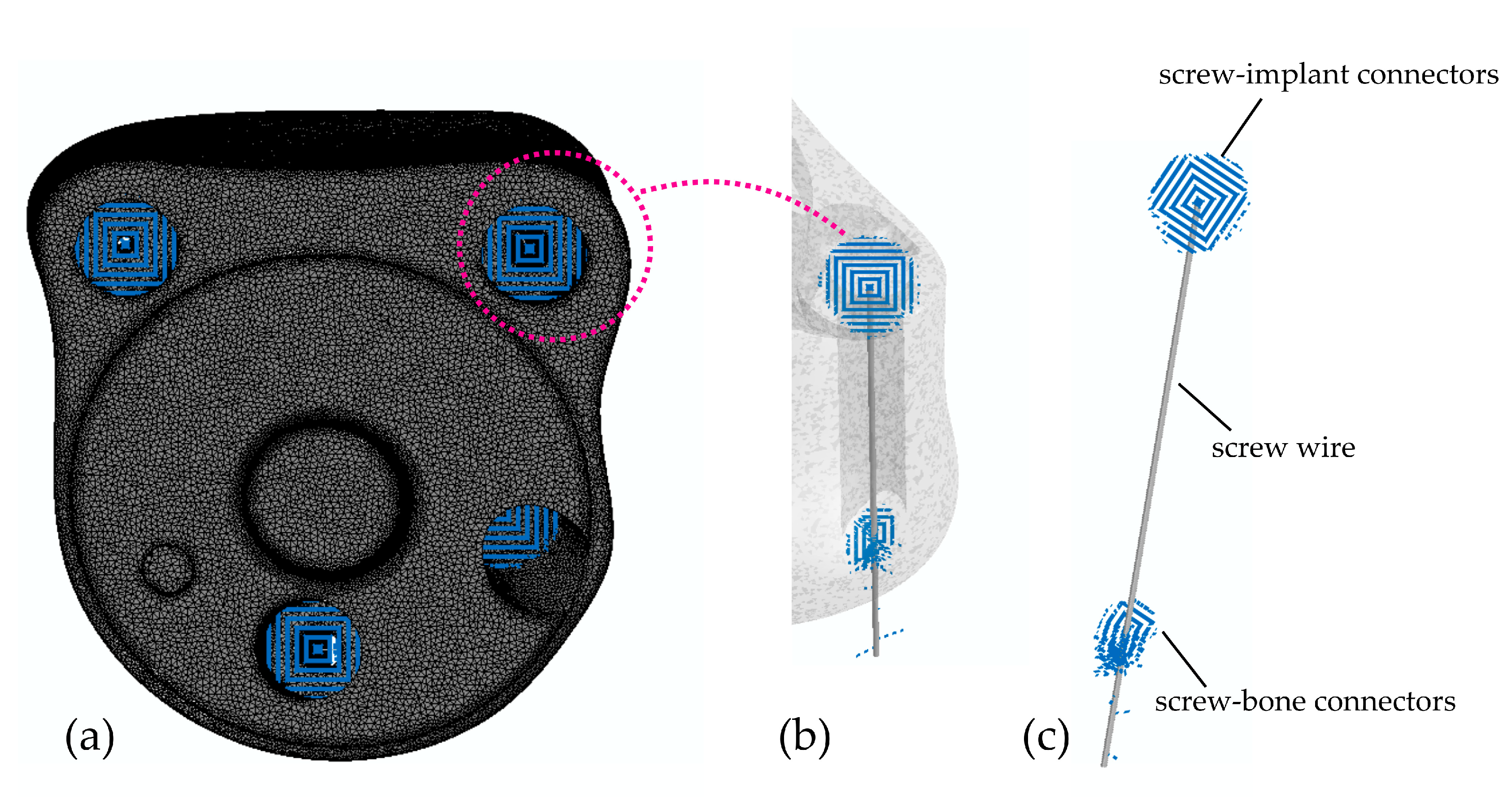
2.2.2. Screw Model
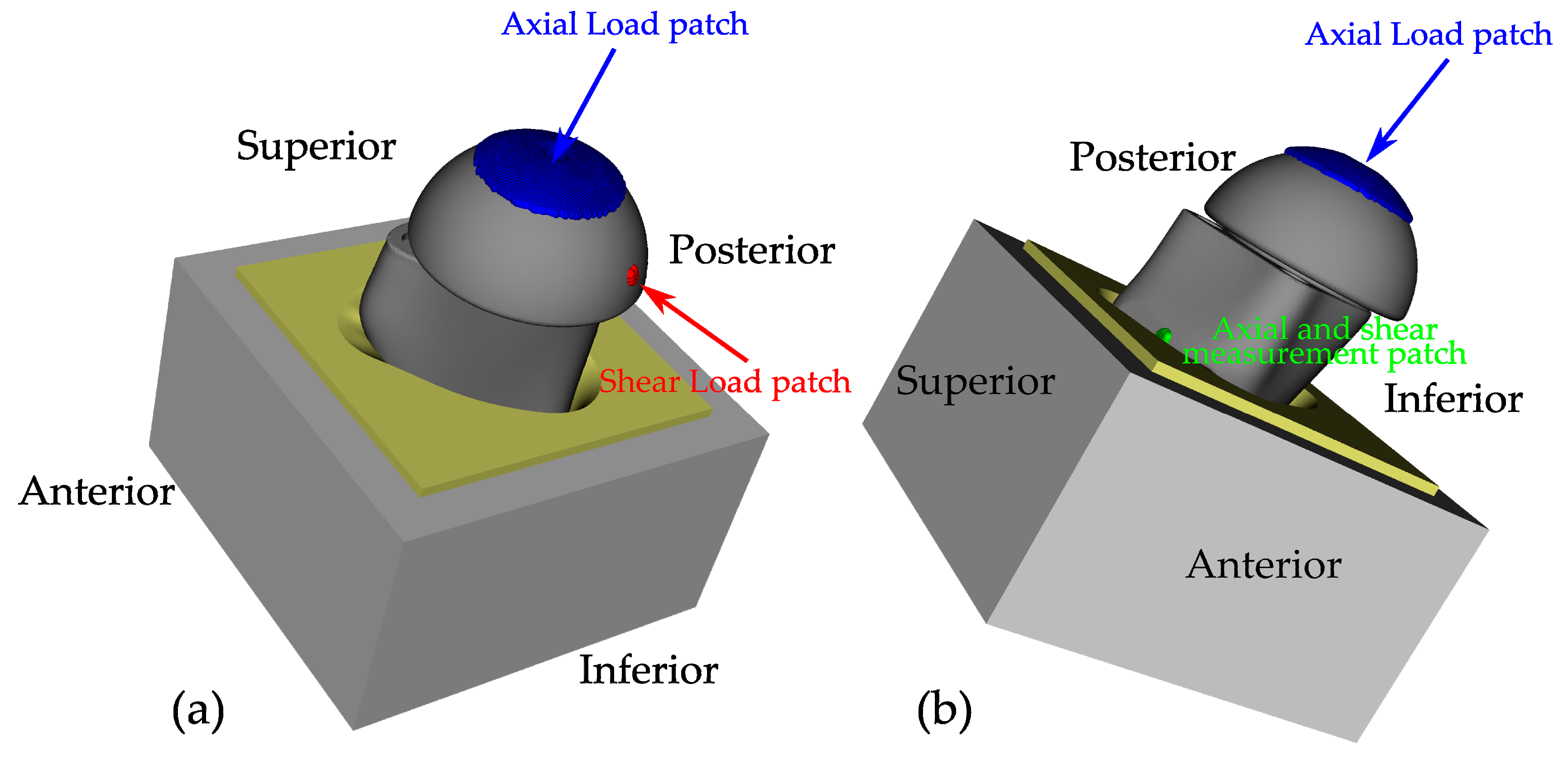
2.2.3. Boundary Conditions and Simulation Steps
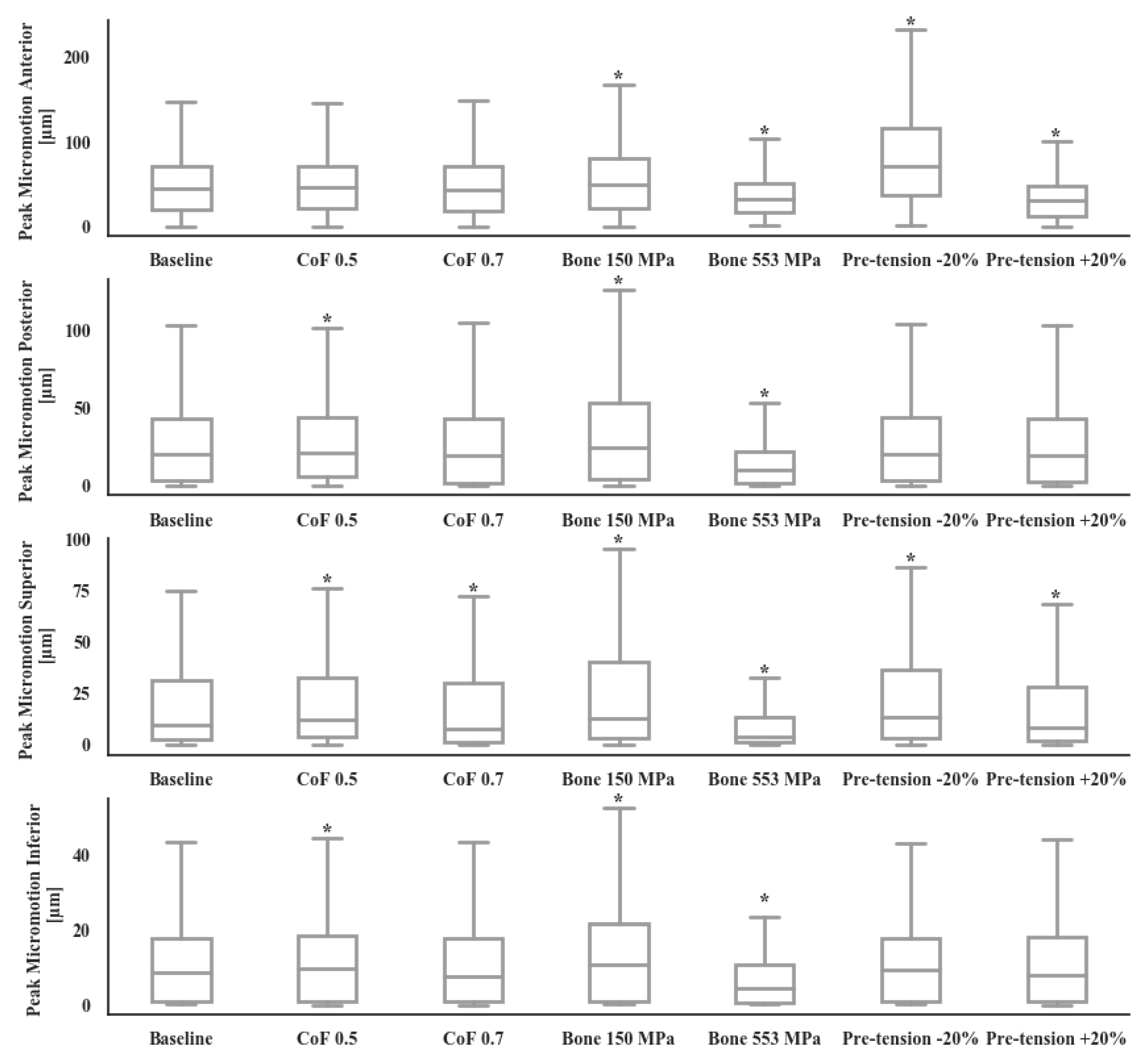
2.3. Statistical Analyses and Sensitivity Study
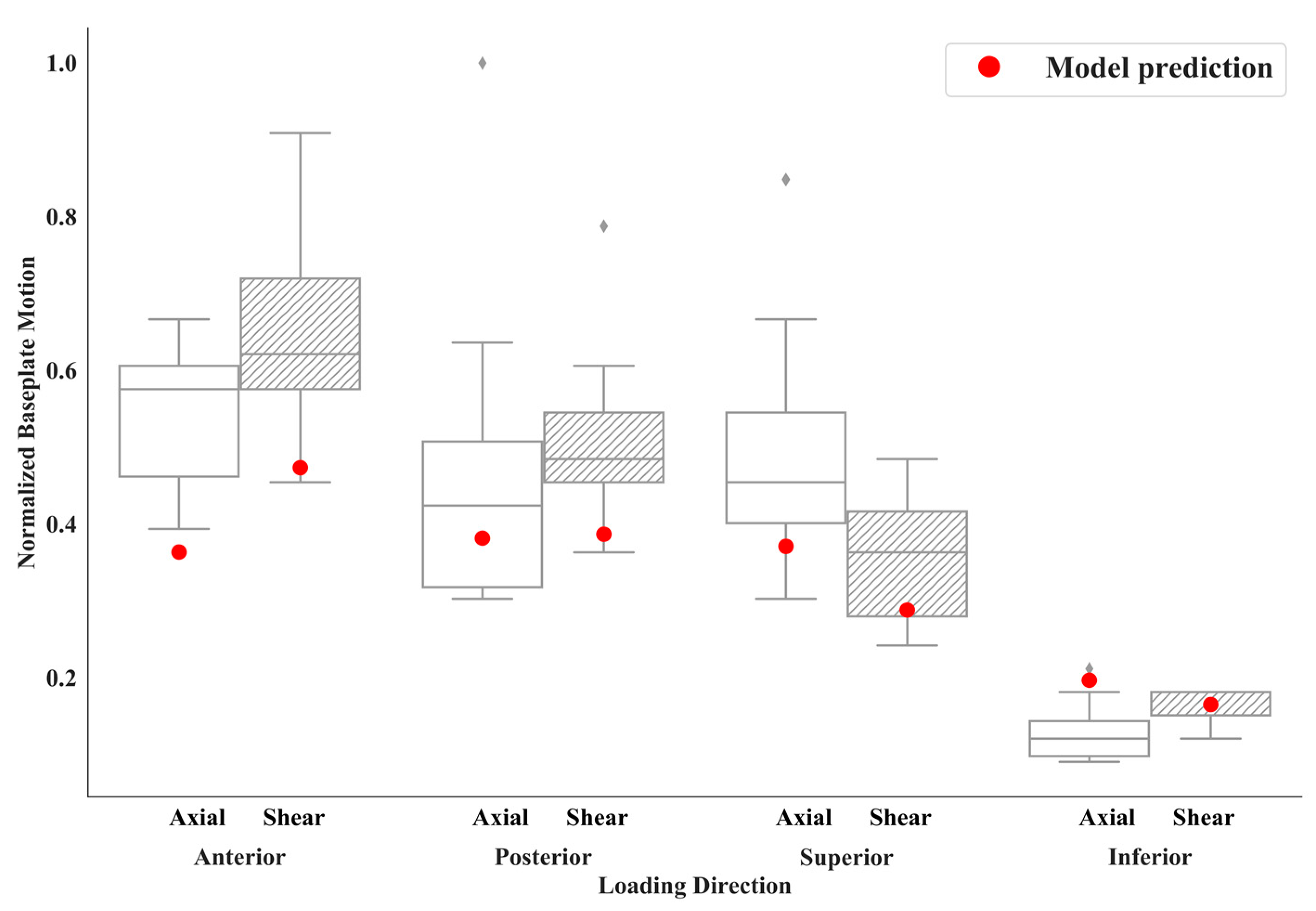
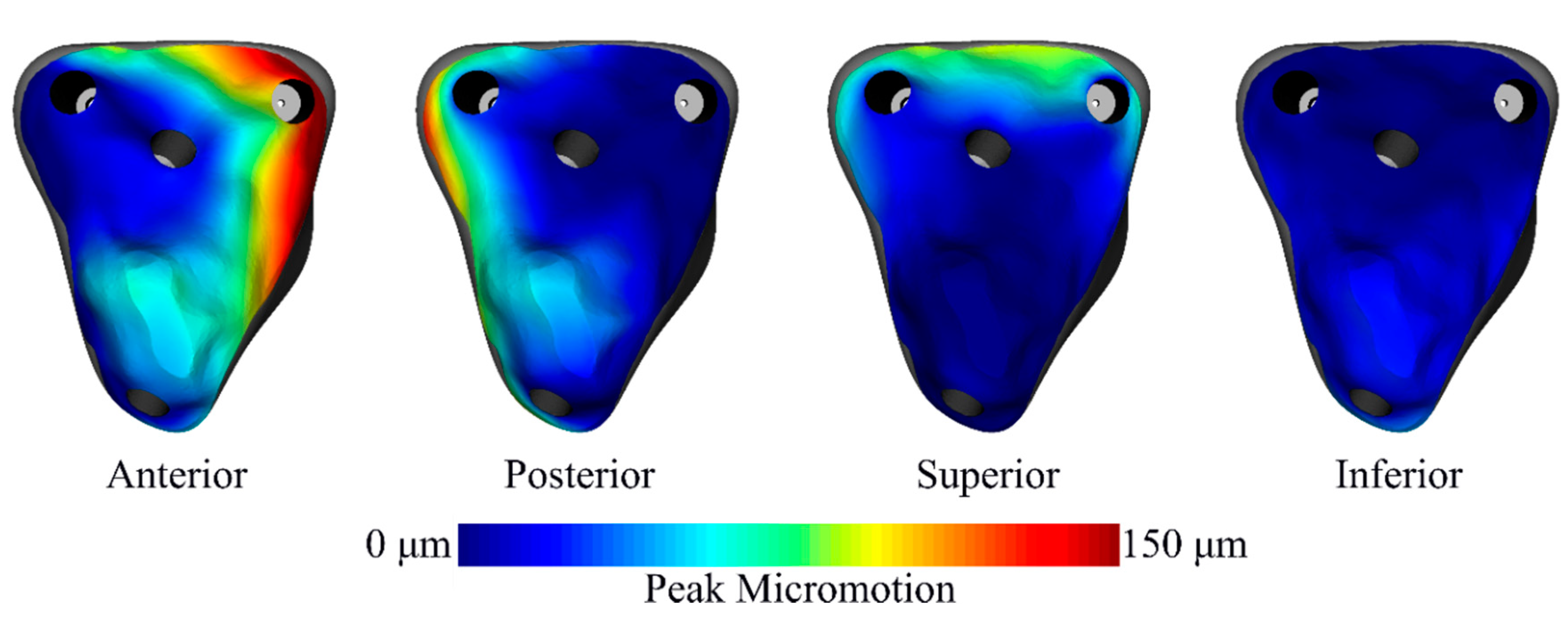
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Walker, M.; Brooks, J.; Willis, M.; Frankle, M. How Reverse Shoulder Arthroplasty Works. Clin. Orthop. Relat. Res. 2011, 469, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, C.D.; Brown, B.T.; Schmidt, C.C. Reverse Shoulder Arthroplasty. Orthop. Clin. N. Am. 2013, 44, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Iannotti, J.P. Options for glenoid bone loss: Composites of prosthetics and biologics. J. Shoulder Elb. Surg. 2007, 16, S267–S272. [Google Scholar] [CrossRef] [PubMed]
- Hoffelner, T.; Moroder, P.; Auffarth, A.; Tauber, M.; Resch, H. Outcomes after shoulder arthroplasty revision with glenoid reconstruction and bone grafting. Int. Orthop. 2014, 38, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.A.; Entezari, V.; Iannotti, J.P.; Ricchetti, E.T. Pre-operative planning for reverse shoulder replacement: The surgical benefits and their clinical translation. Ann Jt. 2019, 4, 4. [Google Scholar] [CrossRef]
- Chammaa, R.; Uri, O.; Lambert, S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J. Shoulder Elb. Surg. 2017, 26, 101–107. [Google Scholar] [CrossRef]
- Stoffelen, D.V.C.; Eraly, K.; Debeer, P. The use of 3D printing technology in reconstruction of a severe glenoid defect: A case report with 2.5 years of follow-up. J. Shoulder Elb. Surg. 2015, 24, e218–e222. [Google Scholar] [CrossRef]
- Dines, D.M.; Gulotta, L.; Craig, E.V.; Dines, J.S. Novel Solution for Massive Glenoid Defects in Shoulder Arthroplasty: A Patient-Specific Glenoid Vault Reconstruction System. Am. J. Orthop. 2017, 46, 104–108. [Google Scholar]
- Jasty, M.; Bragdon, C.; Burke, D.; O’Connor, D.; Lowenstein, J.; Harris, W.H. In Vivo Skeletal Responses to Porous-Surfaced Implants Subjected to Small Induced Motions. J. Bone Jt. Surg. 1997, 79, 707–714. [Google Scholar] [CrossRef]
- Stroud, N.; DiPaola, M.J.; Flurin, P.-H.; Roche, C.P. Reverse shoulder glenoid loosening: An evaluation of the initial fixation associated with six different reverse shoulder designs. Bull. Hosp. Jt. Dis. 2013, 71 (Suppl. S2), S12–S17. [Google Scholar]
- Formaini, N.T.; Everding, N.G.; Levy, J.C.; Santoni, B.G.; Nayak, A.N.; Wilson, C. Glenoid baseplate fixation using hybrid configurations of locked and unlocked peripheral screws. J. Orthop. Traumatol. 2017, 18, 221–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harman, M.; Frankle, M.; Vasey, M.; Banks, S. Initial glenoid component fixation in “reverse” total shoulder arthroplasty: A biomechanical evaluation. J. Shoulder Elb. Surg. 2005, 14, S162–S167. [Google Scholar] [CrossRef]
- Virani, N.A.; Harman, M.; Li, K.; Levy, J.; Pupello, D.R.; Frankle, M.A. In vitro and finite element analysis of glenoid bone/baseplate interaction in the reverse shoulder design. J. Shoulder Elb. Surg. 2008, 17, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.R.; Valstar, E.R.; Rozing, P.M.; van Keulen, F. Fracture risk and initial fixation of a cementless glenoid implant: The effect of numbers and types of screws. Proc. Inst. Mech. Eng. Part H 2013, 227, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.R.; Hansen, U.N.; Bull, A.M.J.; Emery, R.; Amis, A.A. Fixation of the reversed shoulder prosthesis. J. Shoulder Elb. Surg. 2008, 17, 974–980. [Google Scholar] [CrossRef]
- Denard, P.J.; Lederman, E.; Parsons, B.O.; Romeo, A.A. Finite element analysis of glenoid-sided lateralization in reverse shoulder arthroplasty: Lateralization in RSA. J. Orthop. Res. 2017, 35, 1548–1555. [Google Scholar] [CrossRef]
- Chae, S.-W.; Lee, H.; Kim, S.M.; Lee, J.; Han, S.-H.; Kim, S.-Y. Primary stability of inferior tilt fixation of the glenoid component in reverse total shoulder arthroplasty: A finite element study. J. Orthop. Res. 2016, 34, 1061–1068. [Google Scholar] [CrossRef]
- Suárez, D.R.; van der Linden, J.C.; Valstar, E.R.; Broomans, P.; Poort, G.; Rozing, P.M.; van Keulen, F. Influence of the positioning of a cementless glenoid prosthesis on its interface micromotions. Proc. Inst. Mech. Eng. Part H 2009, 223, 795–804. [Google Scholar] [CrossRef]
- Elwell, J.; Choi, J.; Willing, R. Quantifying the competing relationship between adduction range of motion and baseplate micromotion with lateralization of reverse total shoulder arthroplasty. J. Biomech. 2017, 52, 24–30. [Google Scholar] [CrossRef]
- F04 Committee. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Lehtinen, J.T.; Tingart, M.J.; Apreleva, M.; Warner, J.J.P. Total, trabecular, and cortical bone mineral density in different regions of the glenoid. J. Shoulder Elb. Surg. 2004, 13, 344–348. [Google Scholar] [CrossRef]
- Dharia, M.A.; Bischoff, J.E.; Schneider, D. Impact of Modeling Assumptions on Stability Predictions in Reverse Total Shoulder Arthroplasty. Front. Physiol. 2018, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Boampong, D.K.; Green, S.M.; Unsworth, A. N+ ion Implantation of Ti6Al4V Alloy and UHMWPE for total Joint Replacement Application. J. Appl. Biomater. Biomech. 2003, 1, 164–171. [Google Scholar] [CrossRef]
- Barui, S.; Chatterjee, S.; Mandal, S.; Kumar, A.; Basu, B. Microstructure and compression properties of 3D powder printed Ti-6Al-4V scaffolds with designed porosity: Experimental and computational analysis. Mater. Sci. Eng. C 2017, 70, 812–823. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Cast Cobalt-Chromium-Molybdenum Alloy for Surgical Implant Applications; ASTM International: West Conshohocken, PA, USA, 1997; pp. 4–5. [Google Scholar]
- F04 Committee. Standard Practice for Finite Element Analysis (FEA) of Non-Modular Metallic Orthopaedic Hip Femoral Stems; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Zhang, Y.; Ahn, P.B.; Fitzpatrick, D.C.; Heiner, A.D.; Poggie, R.A.; Brown, T.D. Interfacial Frictional Behavior: Cancellous Bone, Cortical Bone, And a Novel Porous Tantalum Biomaterial. J. Musculoskelet. Res. 1999, 3, 245–251. [Google Scholar] [CrossRef]
- Wieding, J.; Souffrant, R.; Fritsche, A.; Mittelmeier, W.; Bader, R. Finite Element Analysis of Osteosynthesis Screw Fixation in the Bone Stock: An Appropriate Method for Automatic Screw Modelling. PLoS ONE 2012, 7, e33776. [Google Scholar] [CrossRef] [PubMed]
- Dharia, M.; Mani, S. Impact of screw preload on primary stability in reverse shoulder arthroplasty. Orthop. Proc. 2019, 101, 48. [Google Scholar] [CrossRef]
- Pitocchi, J.; Wirix-Speetjens, R.; van Lenthe, G.H.; Pérez, M.A. Measuring tightening torque and force of non-locking screws for reverse shoulder prosthesis. Orthop. Proc. 2020, 102, 75. [Google Scholar] [CrossRef]
- Abaqus 6.14 Documentation. Available online: http://ivt-abaqusdoc.ivt.ntnu.no:2080/texis/search/?query=wetting&submit.x=0&submit.y=0&group=bk&CDB=v6.14 (accessed on 16 June 2020).
- Mukaka, M. A guide to appropriate use of Correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Favre, P.; Henderson, A.D. Prediction of stemless humeral implant micromotion during upper limb activities. Clin. Biomech. 2016, 36, 46–51. [Google Scholar] [CrossRef]
- Hoenig, M.P.; Loeffler, B.; Brown, S.; Peindl, R.; Fleischli, J.; Connor, P.; D’Alessandro, D. Reverse glenoid component fixation: Is a posterior screw necessary? J. Shoulder Elb. Surg. 2010, 19, 544–549. [Google Scholar] [CrossRef]
- Favre, P.; Perala, S.; Vogel, P.; Fucentese, S.F.; Goff, J.R.; Gerber, C.; Snedeker, J.G. In vitro assessments of reverse glenoid stability using displacement gages are misleading—Recommendations for accurate measurements of interface micromotion. Clin. Biomech. 2011, 26, 917–922. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Baseline Value | Sensitivity Values |
|---|---|---|
| Elastic Modulus Bone | 200 MPa | 150 MPa, 553 MPa |
| Coefficient of Friction (CoF) | 0.6 | 0.5, 0.7 |
| Screw pre-load | 260 N, 300 N | ±20% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitocchi, J.; Wesseling, M.; van Lenthe, G.H.; Pérez, M.A. Finite Element Analysis of Custom Shoulder Implants Provides Accurate Prediction of Initial Stability. Mathematics 2020, 8, 1113. https://doi.org/10.3390/math8071113
Pitocchi J, Wesseling M, van Lenthe GH, Pérez MA. Finite Element Analysis of Custom Shoulder Implants Provides Accurate Prediction of Initial Stability. Mathematics. 2020; 8(7):1113. https://doi.org/10.3390/math8071113
Chicago/Turabian StylePitocchi, Jonathan, Mariska Wesseling, Gerrit Harry van Lenthe, and María Angeles Pérez. 2020. "Finite Element Analysis of Custom Shoulder Implants Provides Accurate Prediction of Initial Stability" Mathematics 8, no. 7: 1113. https://doi.org/10.3390/math8071113
APA StylePitocchi, J., Wesseling, M., van Lenthe, G. H., & Pérez, M. A. (2020). Finite Element Analysis of Custom Shoulder Implants Provides Accurate Prediction of Initial Stability. Mathematics, 8(7), 1113. https://doi.org/10.3390/math8071113





