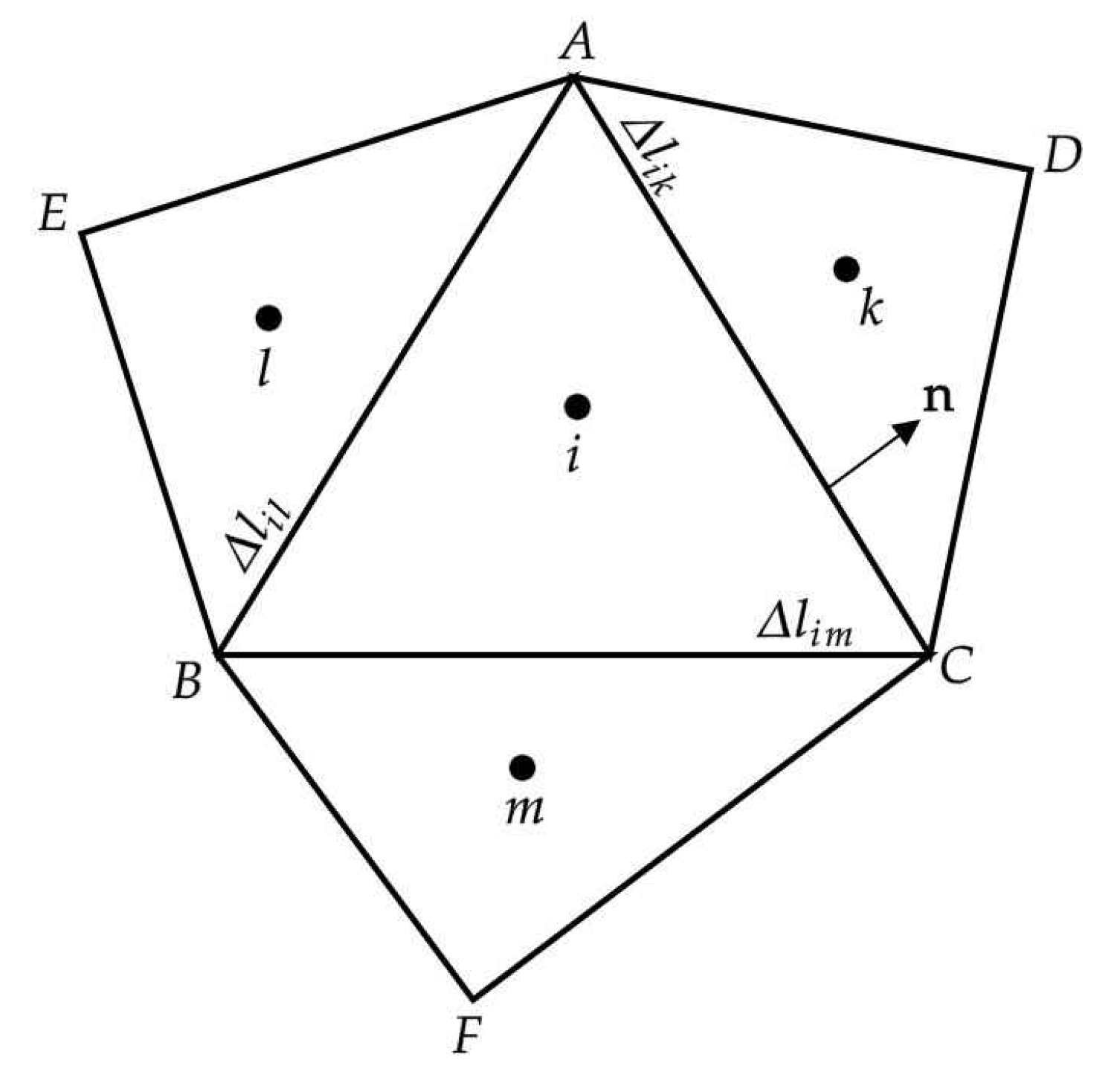
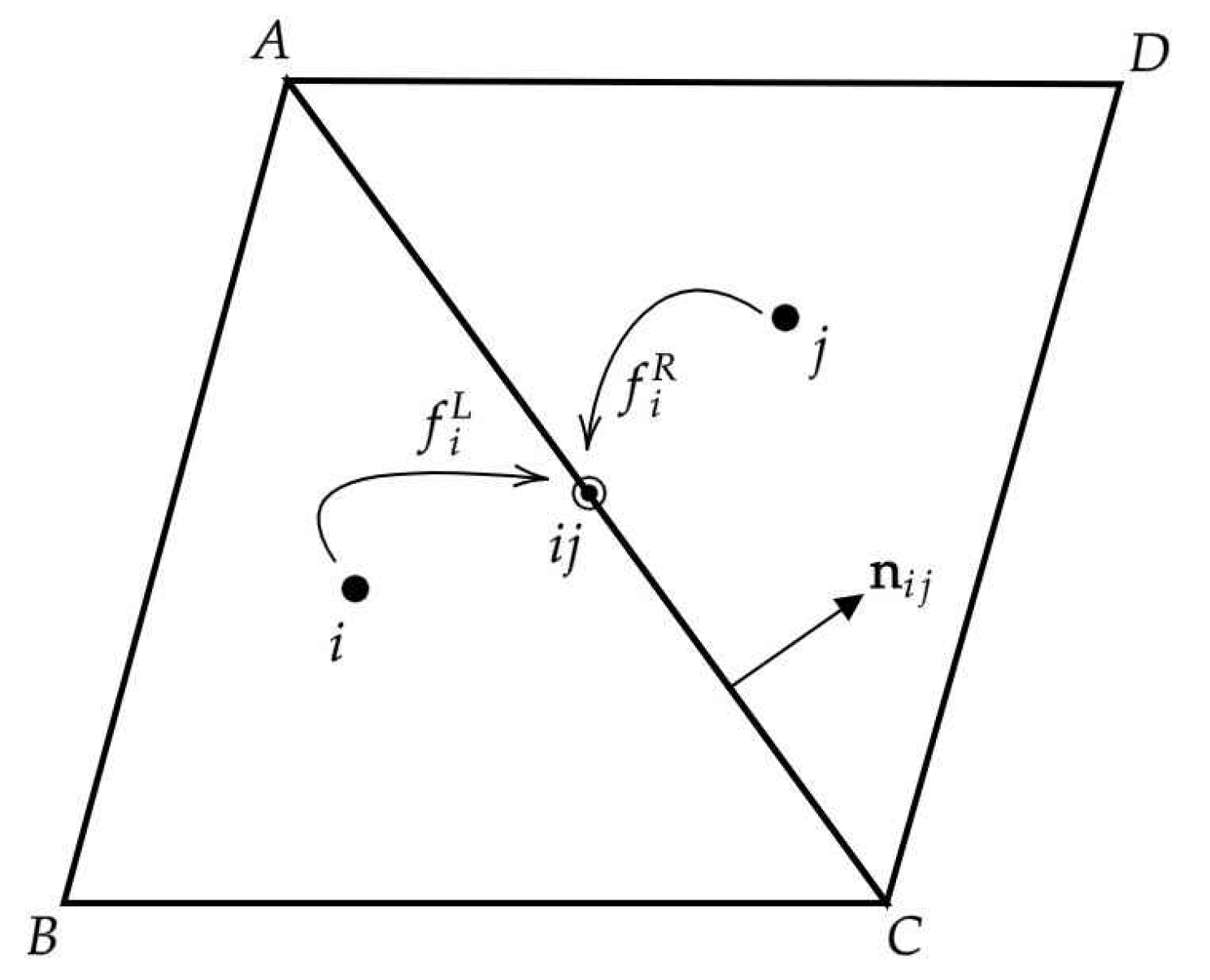
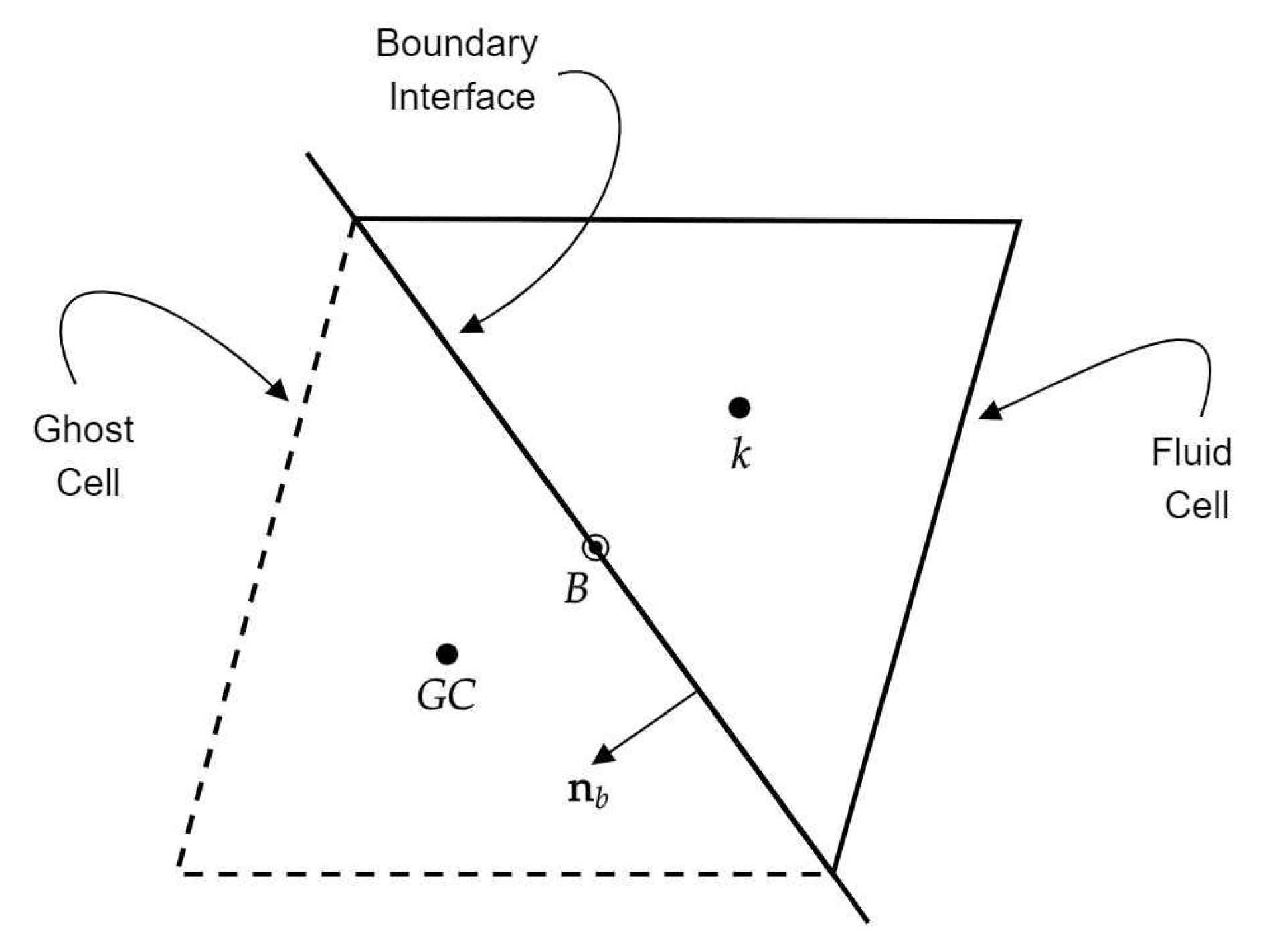
This section presents the results of validation test cases to assess the accuracy and applicability of the FV-iLBM solver developed in-house. Simulations include Taylor–Green vortex flow, shear wave decay, developing flow through a 2D channel, Womersley flow, lid-driven cavity flow, and flow over the NACA0012 airfoil. Additionally, validation results for non-Newtonian flows through both straight and stenosed channels are discussed. Finally, the FV-iLBM solver is applied to simulate non-Newtonian blood flow through an artery with multiple stenoses.
3.1. Taylor–Green Vortex Flow
The Taylor–Green vortex (TGV) is a canonical benchmark in fluid dynamics that captures essential mechanisms of vortex dynamics, turbulent transition, and energy cascade. Its three-dimensional vortex stretching and reconnection processes are representative of phenomena observed in real-world turbulent flows, such as those in ocean currents, atmospheric systems, and industrial mixing [
51]. In engineering, the TGV problem provides a rigorous validation test for numerical schemes targeting large-eddy simulation (LES) and direct numerical simulation (DNS) of turbulent flows, with applications in aerospace (e.g., flow over wings, turbomachinery), combustion chambers, and biomedical devices. Thus, the results from the TGV case are instrumental in evaluating the solver’s ability to accurately resolve complex, unsteady flow features relevant to practical scenarios.
The evolution of the Taylor–Green vortex flow in a 2D square domain has a time-dependent analytical solution that can be used to perform error estimation using
and
error norms [
52] to evaluate the order-of-accuracy of the reconstruction methods used. Here, the Taylor–Green vortex flow in a square domain
has been studied for different control volume sizes. Periodic boundary conditions are applied on both x- and y-direction boundaries. The analytical solution [
53,
54] for this case is given by
where
U is set to
and
represents the wave number, which is set to
. The initial conditions are obtained by substituting
in the above equations. As time progresses, at any instant
, viscosity will cause the initial magnitude of velocity to decay. The decay of velocity in x- and y-direction can be obtained using Equation (
47) and Equation (
48), respectively.
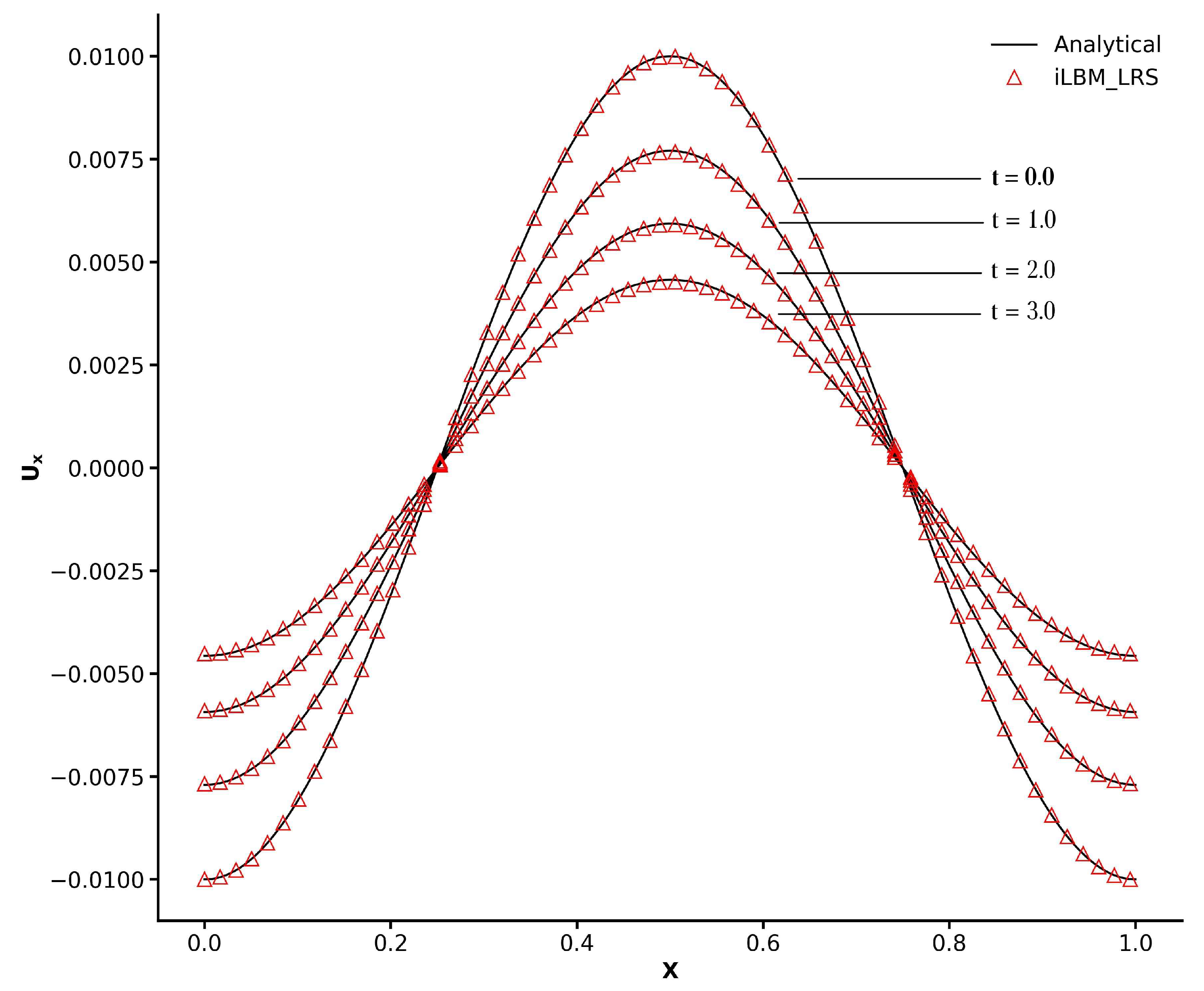
Figure 5 shows the x-direction velocity profiles along the location
against the non-dimensional time
t obtained using the FV-iLBM model in conjunction with the LR scheme. The non-dimensional time
t is computed as
, where
is the number of time steps. The time step
is taken as
, whereas the relaxation parameter has been kept fixed at 0.01. The analytical solution at four different time instances—
and
—has been plotted. The iLBM model in conjunction with the LR scheme has produced excellent agreement with the analytical solution at different time instances. The excellent agreement between our numerical results and the analytical solution demonstrates that the FV-iLBM model with the LR scheme can accurately capture complex flow physics.
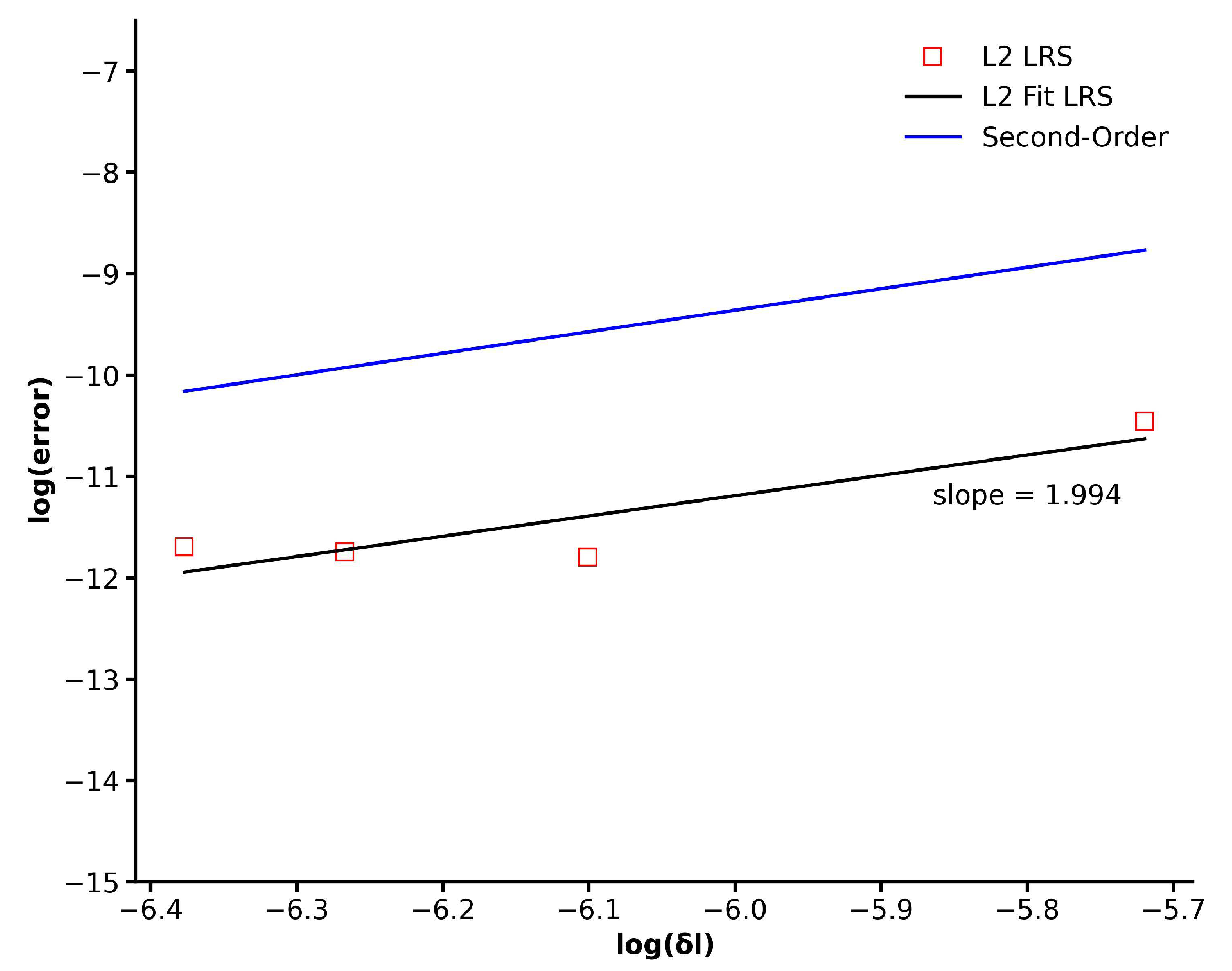
In order to perform error estimation to evaluate the order-of-accuracy, simulations have been performed using four different grids with increasing mesh refinement. Four grids with a total cell count of 37,986, 67,836, 139,792, and 253,942 cells have been used for this purpose. As the mesh is refined, the characteristic length
, representing the smallest length of an edge in mesh, as well as the error obtained using
and
norms should decrease, giving us an estimate of the order-of-accuracy. In
Figure 6, the error assessment, determined through the
norm, is presented for the four grids mentioned above. It is evident that as the mesh undergoes refinement, the
error diminishes in a consistent second-order manner. This observation validates the second-order precision of the LR scheme integrated into the existing finite volume implementation of the iLBM model.
Table 1 quantifies the convergence behavior of the numerical scheme for the Taylor–Green vortex flow by reporting
and
error norms across the progressively refined grids mentioned above. A systematic reduction in all error norms with decreasing characteristic length
indicates a consistent convergence of the solution. This trend validates the spatial accuracy of the method and its ability to resolve the flow field with increasing fidelity under grid refinement.
3.4. Womersley Flow
The capabilities of the FV-iLBM model to simulate the transient flow has been tested using the Womersley flow [
56] in a 2D channel. NEE no-slip conditions are applied on the top and bottom walls of the channel. A time-dependent pressure difference
is applied on the inlet and the flow is driven by the fluctuation of pressure gradient
given by
where
and the analytical solution is given by [
56]
where
,
is the frequency of the pressure pulse, and
is the Womersley number representing the relationship of transient inertial forces to viscous forces, given by
The Womersley number (
) considered here is
and the pressure gradient
applied is
in LBM units. The Reynolds number simulated is 25.
and
represent the domain length in x- and y-direction, respectively, where
. An unstructured mesh with a total cell count of 85602 elements has been used to perform simulations. The maximum centerline velocity
is calculated using the Hagen–Poiseuille equation. Once the Womersley number is chosen, the frequency of the pressure pulse is determined, which in turn determines the time period (
T) as
.
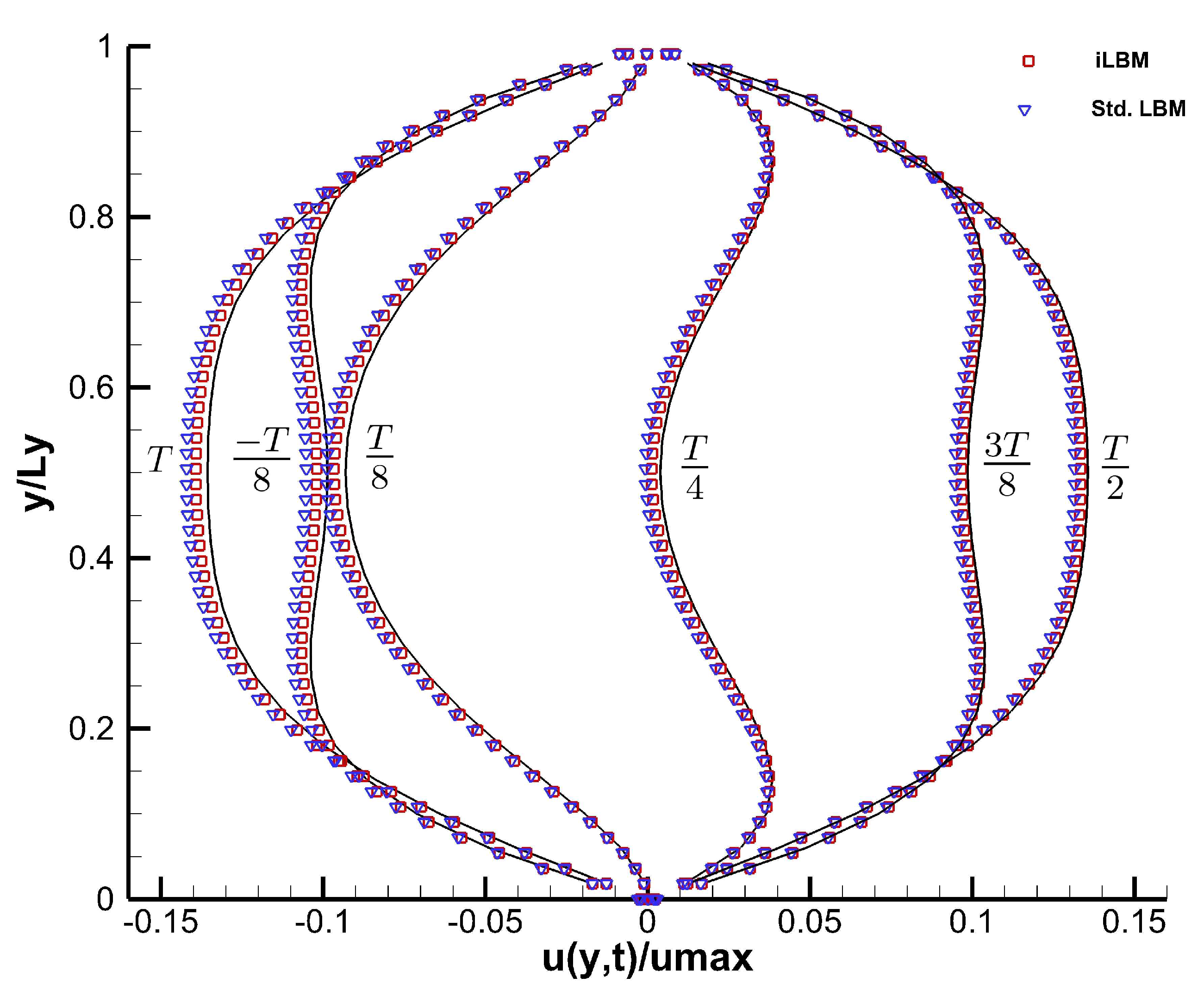
Figure 10 shows the non-dimensional velocity profiles at different time periods (in factors of
) obtained using the iLBM model, in conjunction with the LR scheme, compared against the analytical solution of Equation (
56). The velocity profiles have been extracted along the y-direction at
. The velocity profiles obtained using the standard LBM model have also been plotted to show a comparison with the iLBM model. As can be seen from
Figure 10, velocity profiles obtained using the iLBM model are more accurate than those obtained using the standard LBM model.
Table 2 shows the comparison of the maximum centerline velocity (
) obtained using the iLBM and standard LBM models with the analytical centerline velocity, where the proficiency of the iLBM model over the standard LBM model is evident. The analytical value for centerline velocity at time period
is very close to zero, where the numerical models will struggle to capture the velocity profiles. Thus, the centerline velocity values obtained using the iLBM and standard LBM models deviate from the expected results [
17].
To further evaluate the performance of the iLBM model, a quantitative comparison was conducted against the standard LBM model (both models implemented in the finite volume framework) for the Womersley flow case. As summarized in
Table 3, for a serial implementation, the iLBM model incurs a modest increase in memory usage per cell (∼104 bytes vs. ∼96 bytes) due to the inclusion of pressure terms. However, it achieves a significant improvement in both computational efficiency and accuracy, with a ∼15.2% reduction in runtime per iteration and a ∼38.9% lower L2 error. These results demonstrate that the FV-iLBM model offers a favorable trade-off between memory footprint and overall simulation performance, highlighting its potential for accurate and efficient incompressible flow simulations.
The proficiency of the iLBM model to limit the compressibility error while simulating pulsating flows is a significant finding of this study. Pulsating flows play a pivotal role in a wide array of fluid dynamics scenarios with applications ranging from cardiovascular health assessment to thermal energy engineering, electronic engineering, machinery manufacturing, chemical engineering, etc. Reciprocating compressors, pulse combustion chamber, microdiffusers, micropumps, and microchannels all feature pulsating flows. In electronic engineering, the rate of heat transfer is enhanced using pulsating flows. The iLBM model can be effectively used to simulate flows across these applications and explore the flow physics involved.
3.5. Lid-Driven Flow in a 2D Square Cavity
The steady state solution of a lid-driven flow in a 2D square cavity
at different Reynolds numbers has been studied to assess the capabilities of the FV-iLBM solver. The Reynolds number, in this case, is based on the width of the cavity as
, where
L is the cavity width and
U is the velocity of the top plate. Four different Reynolds numbers have been simulated: 100, 400, 1000, and 3200. The wall velocity boundary condition given by Equation (
39) has been applied to all the four walls of the cavity with a velocity of the top wall
U set to 0.1. For the remaining three walls, the no-slip boundary condition has been imposed for velocity and the normal gradient of pressure has been taken as zero. The time step has been taken as
. The NSE results of Ghia et al. [
57] have been considered as the benchmark solution here. The results obtained using the FV-iLBM model in conjunction with the LR scheme and AB2 time integration method are presented here.
To simulate lid-driven flow for
, an unstructured grid with 200 triangular cells on each wall and a total cell count of 105,692 cells has been used.
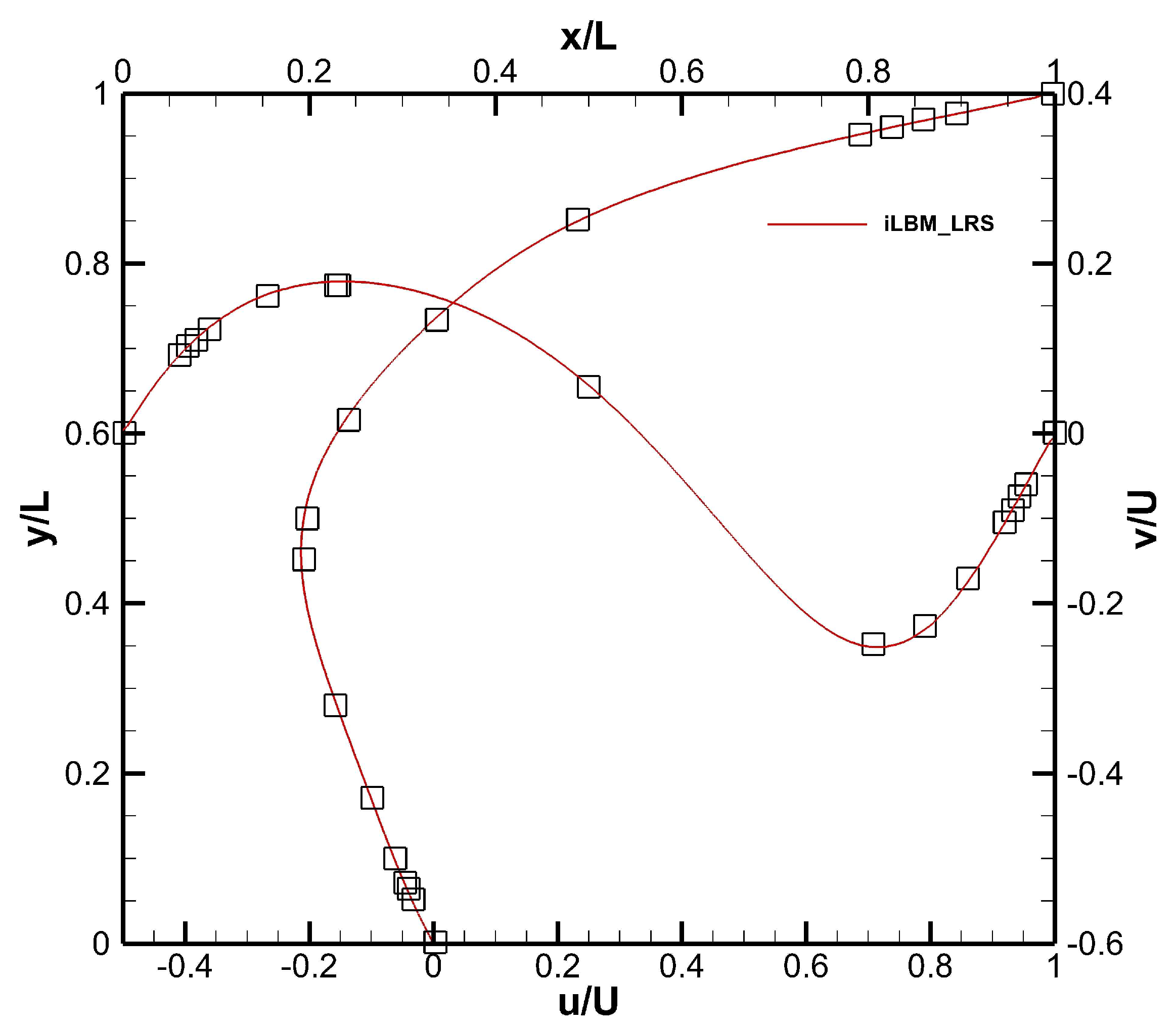
Figure 11 shows the normalized axial and radial velocity profiles for
. As can be observed from the figure, the FV-iLBM predictions agree well with the benchmark solution [
57].
Table 4 lists the x- and y-coordinates of the centers of primary vortex, bottom right vortex, and bottom left vortex obtained for
. The results obtained using the FV-iLBM approach are compared with the benchmark solution and those obtained by Patil et al. [
15]. As observed, the FV-iLBM approach accurately captures the location of all the vortices.
As the Reynolds number increases, the numerical predictions for the lid-driven flow depend on how well the boundary layer is resolved. Therefore, for simulating lid-driven flow with
and 3200, an unstructured grid with 250 triangular cells on each wall and a total cell count of 164,756 cells has been used.
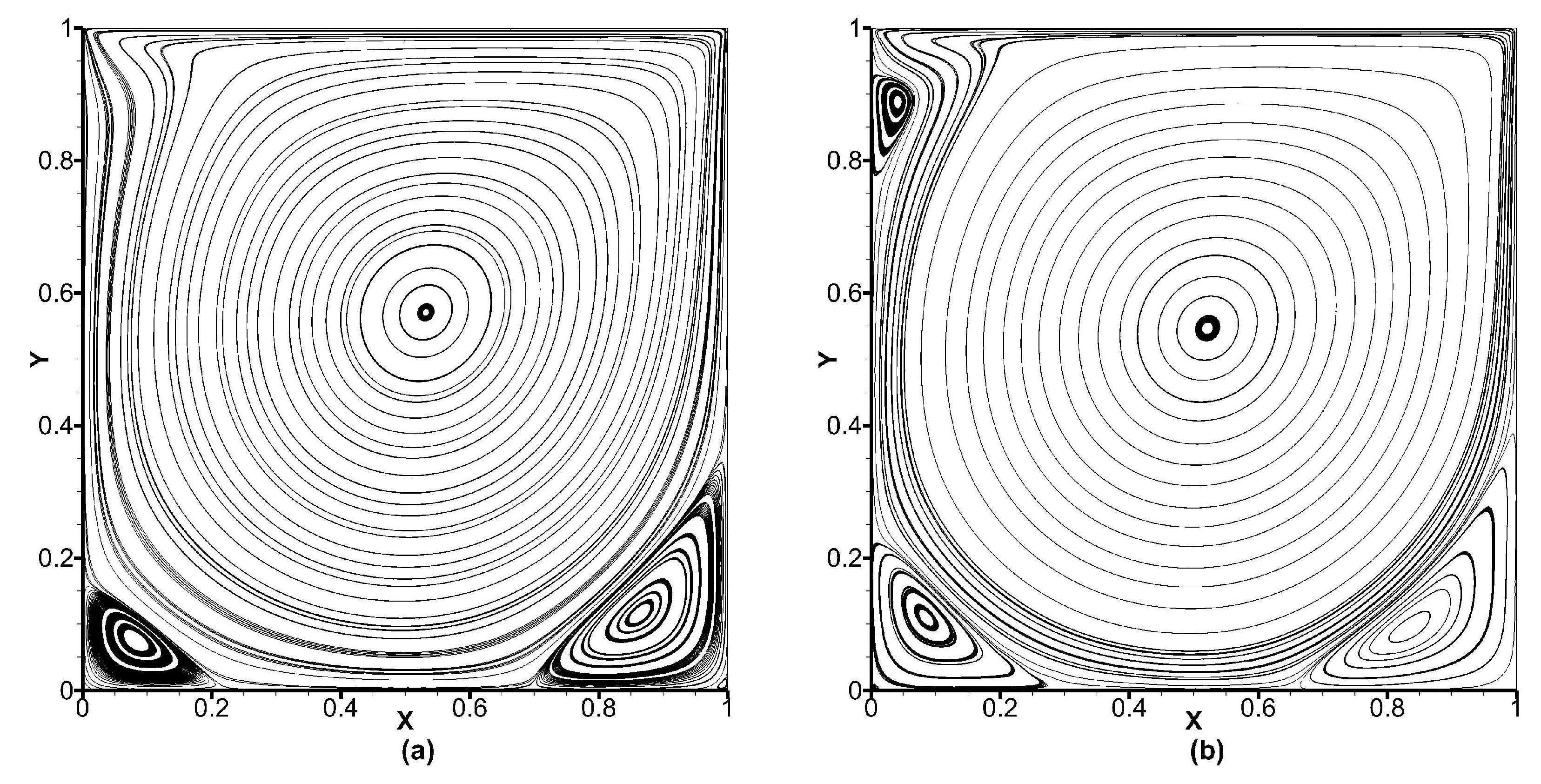
Figure 12 shows the streamlines obtained for the lid-driven flow with
and 3200, and as observed, important flow features such as corner vortices including the top left corner vortex for
has been captured by the FV-iLBM model, implying that the boundary layer has been adequately resolved.
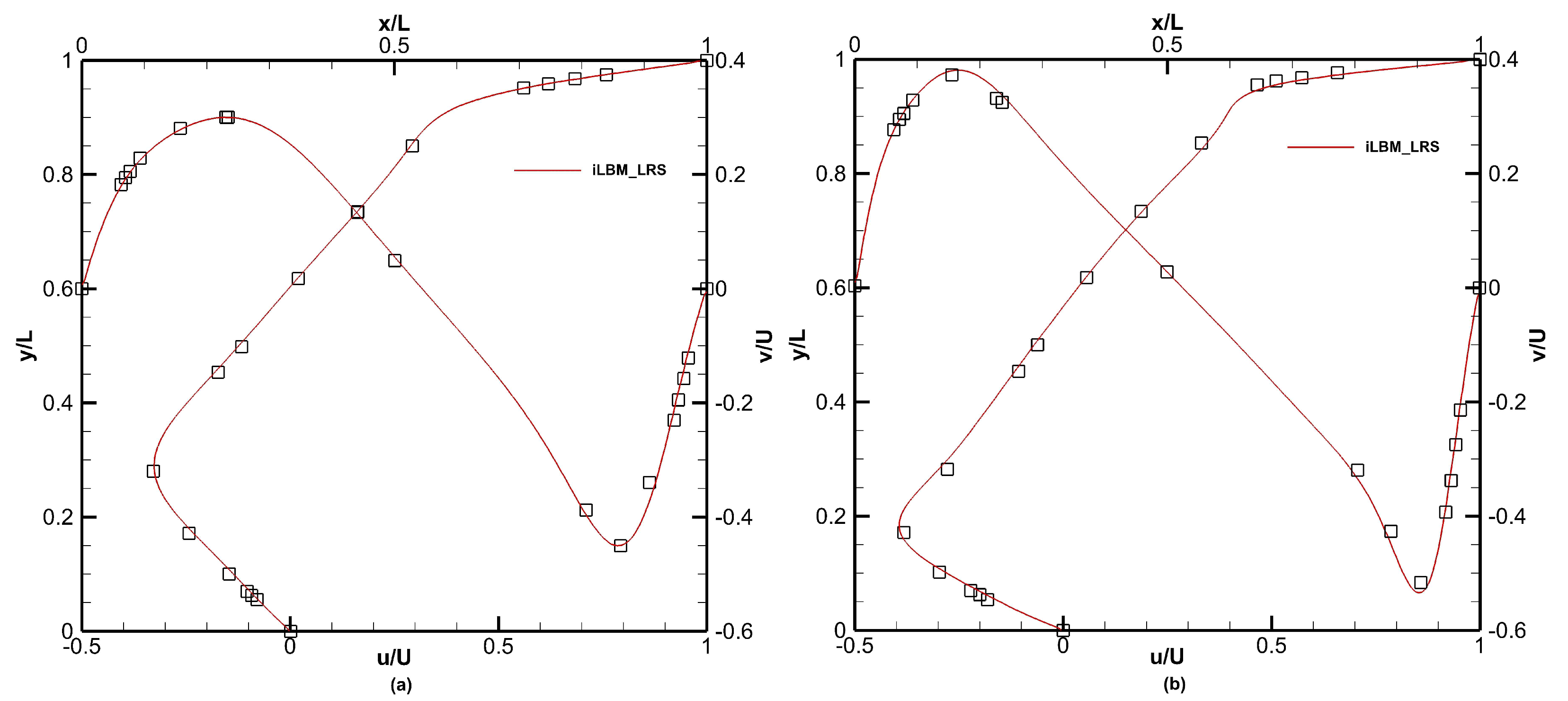
Figure 13 shows the normalized axial and radial velocity profiles for lid-driven flow with
and
, respectively. The FV-iLBM model in conjunction with the LR scheme captures the velocity profiles accurately.
Table 4 lists the x- and y-coordinates of primary, bottom right, and bottom left vortices for lid-driven flow with
and
. As the Reynolds number increases, the locations of the vortices produced by the FV-iLBM model agree well with the benchmark solution. Comparing the vortex locations produced by the FV-iLBM model with those obtained by Patil et al. [
15] and Wang et al. [
31], FV-iLBM predictions are competitive with both of these studies for
. For
, the FV-iLBM predictions are better than those obtained by Patil et al. [
15] and are competitive with those obtained by Wang et al. [
31].
For
, we have also compared the width and height of the corner vortices obtained using the FV-iLBM model with benchmark [
57] and those obtained by Patil et al. [
15] and Wang et al. [
31]. As shown in
Table 5, the size of the bottom left vortex obtained using the FV-iLBM model agrees adequately with the benchmark solution and is competitive with those obtained by Patil et al. [
15] and Wang et al. [
31]. For the bottom right vortex, the FV-iLBM model produces superior results than both of these studies and agrees well with the benchmark solution.
Figure 14 shows the normalized axial and radial velocity profiles obtained for
using the FV-iLBM model in conjunction with the LR model. The FV-iLBM predictions are compared with those obtained by Patil et al. [
15] to make a qualitative comparison. The FV-iLBM predictions agree well with the benchmark solution and are superior to those obtained by Patil et al. [
15]. As pointed out by Wang et al. [
31], the numerical predictions obtained by Patil et al. [
15] suffer from non-physical numerical diffusion, resulting in disagreement between their numerical predictions and the benchmark solution.
Table 6 lists the x- and y-coordinates of the centers of primary, bottom right, bottom left, and top left vortices, whereas
Table 7 details the width and height of the corner vortices obtained for the lid-driven flow with
. The locations of corner vortices obtained using the FV-iLBM model in conjunction with the LR scheme agree well with the benchmark solution and are superior to those obtained by Patil et al. [
15]. Barring the size of the top left vortex, the width and height of the bottom left and bottom right vortices produced by the FV-iLBM model are very close to the benchmark solution, are superior to those obtained by Patil et al. [
15], and are close to those obtained by Wang et al. [
31].
3.6. Flow over NACA0012 Airfoil
External flow over the NACA0012 airfoil with a Reynolds number of 1000 has been simulated using the FV-iLBM model. Reynolds number, in this case, is defined as
. Here,
is the free-stream velocity,
is the chord length, and
is the kinematic viscosity.
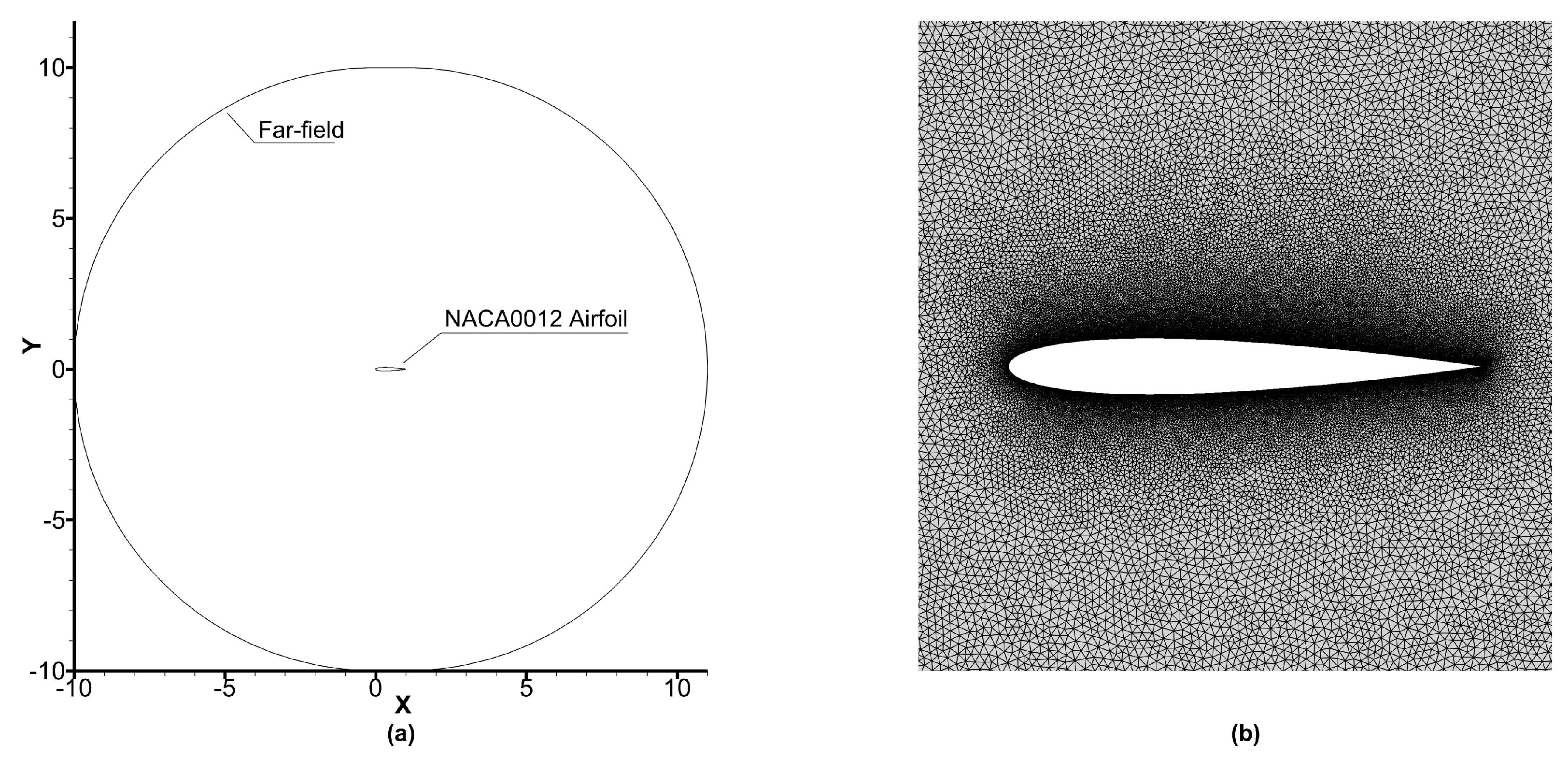
Figure 15a shows the schematic of the domain used for simulating flow over the NACA0012 airfoil. The far-field boundary is located at 10L in all directions from the stagnation point, which is large enough to eliminate the influence of far-field boundary on the flow.
The far-field boundary condition is applied using ghost cells in conjunction with Equations (
40), (
59), and (
60) as
where
is the free-stream pressure in LBM units. On the airfoil surface, the no-slip boundary condition has been imposed for velocity and the normal gradient of pressure has been taken as zero. The time step has been taken as
.
We have studied four different angles of attack:
,
,
, and
. The velocity components corresponding to different angles of attack are set as
where
represents the angle of attack in degrees.
We have used three different unstructured grids with cell counts of 38,710 (grid 1), 75,868 (grid 2), and 120,520 cells (grid 3) having 198, 398, and 798 cells on the airfoil surface, respectively. The grid is refined by refining the cell count surrounding the airfoil as shown in
Figure 15b.
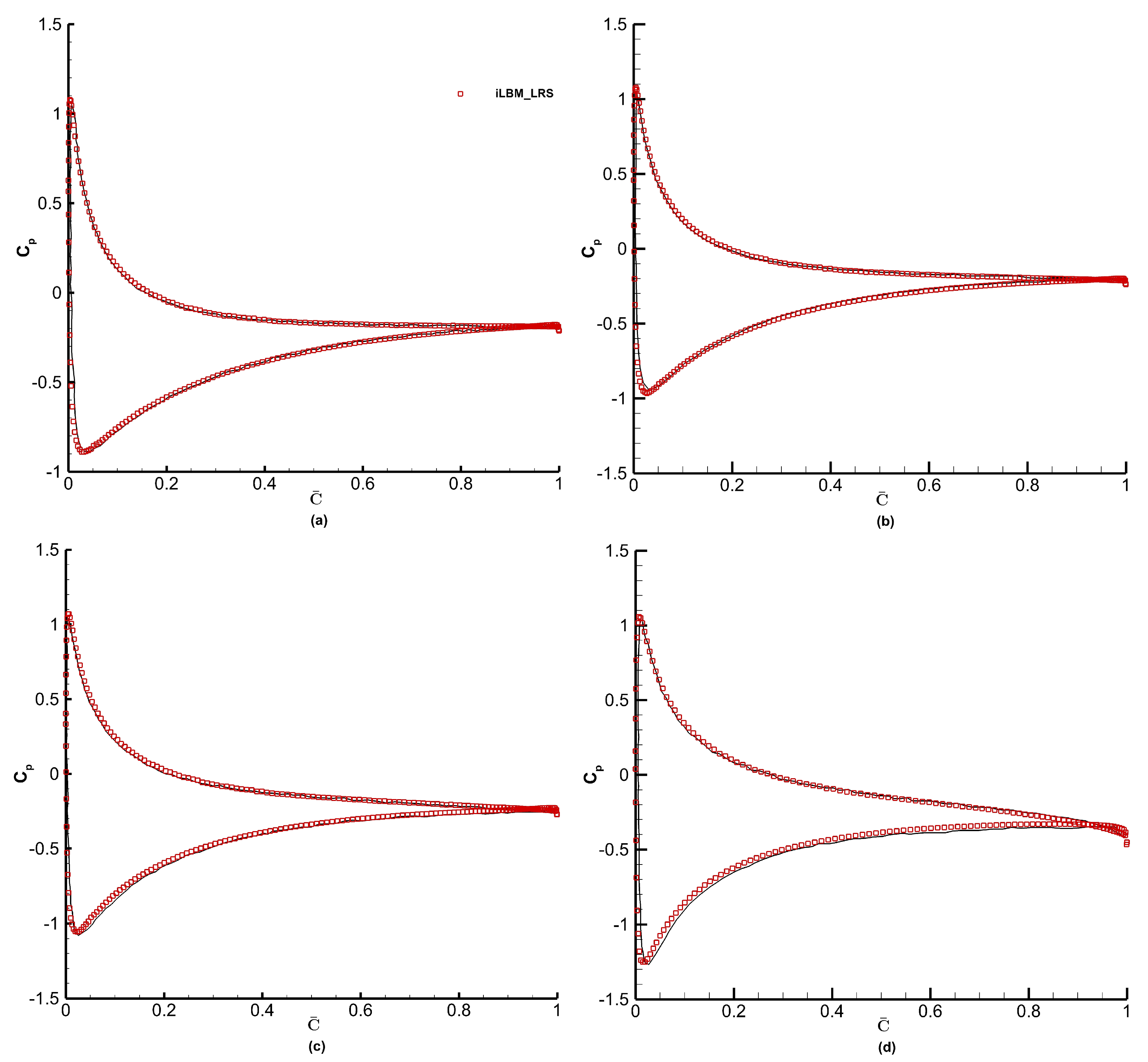
To understand the effect of grid refinement on numerical predictions, we have simulated the flow over the NACA0012 airfoil at an angle of attack of
and plotted the distribution of pressure coefficient
on the airfoil surface. The NSE solution of Kurtulus [
58] has been used as the benchmark solution in the present study. The distribution of
on the airfoil surface is computed as
Figure 16a shows the distribution of mean pressure coefficient for angle of attack
obtained using the FV-iLBM model using the three grids mentioned above. The
profile obtained using grid 1 captures the distribution adequately on the bottom surface; however, it also shows disagreement with the benchmark solution on the top surface. Grid 2 and grid 3 accurately capture the
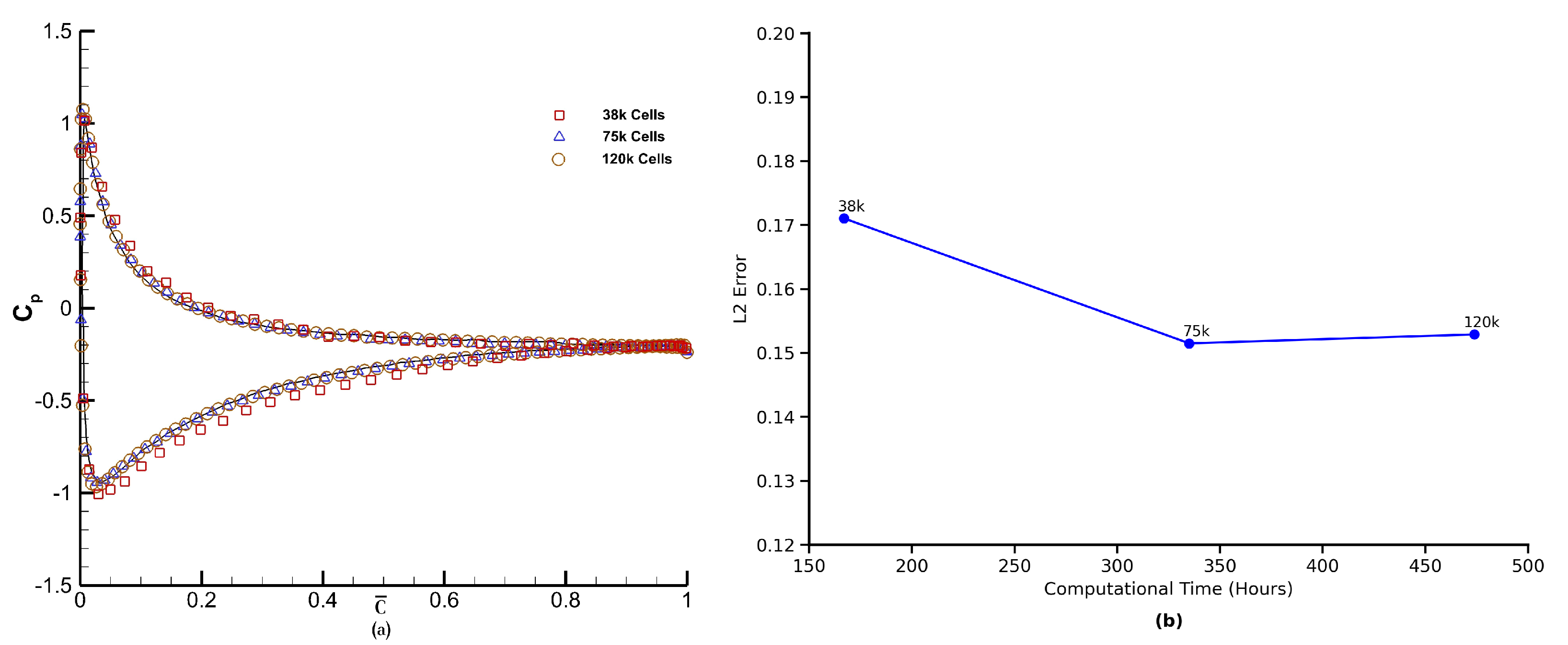
distribution on both the top and bottom surfaces of the airfoil. Based on this analysis, we have used grid 2 for the remaining analysis. Furthermore, to evaluate the trade-off between accuracy and computational efficiency, we examined the L2 error as a function of total runtime for three successively refined grids (38 k, 75 k, and 120 k cells). As shown in
Figure 16b, the error decreases significantly when increasing resolution from 38 k to 75 k cells, reducing from 0.171 to 0.151. However, further refinement to 120 k cells yields minimal improvement (L2 error = 0.1529) while increasing runtime from 335 to 473.8 h—an increase of ∼41%. This trend illustrates a classic diminishing return with respect to grid refinement and motivates the choice of the 75 k cell grid for the bulk of our simulations as the optimal compromise between accuracy and computational cost.
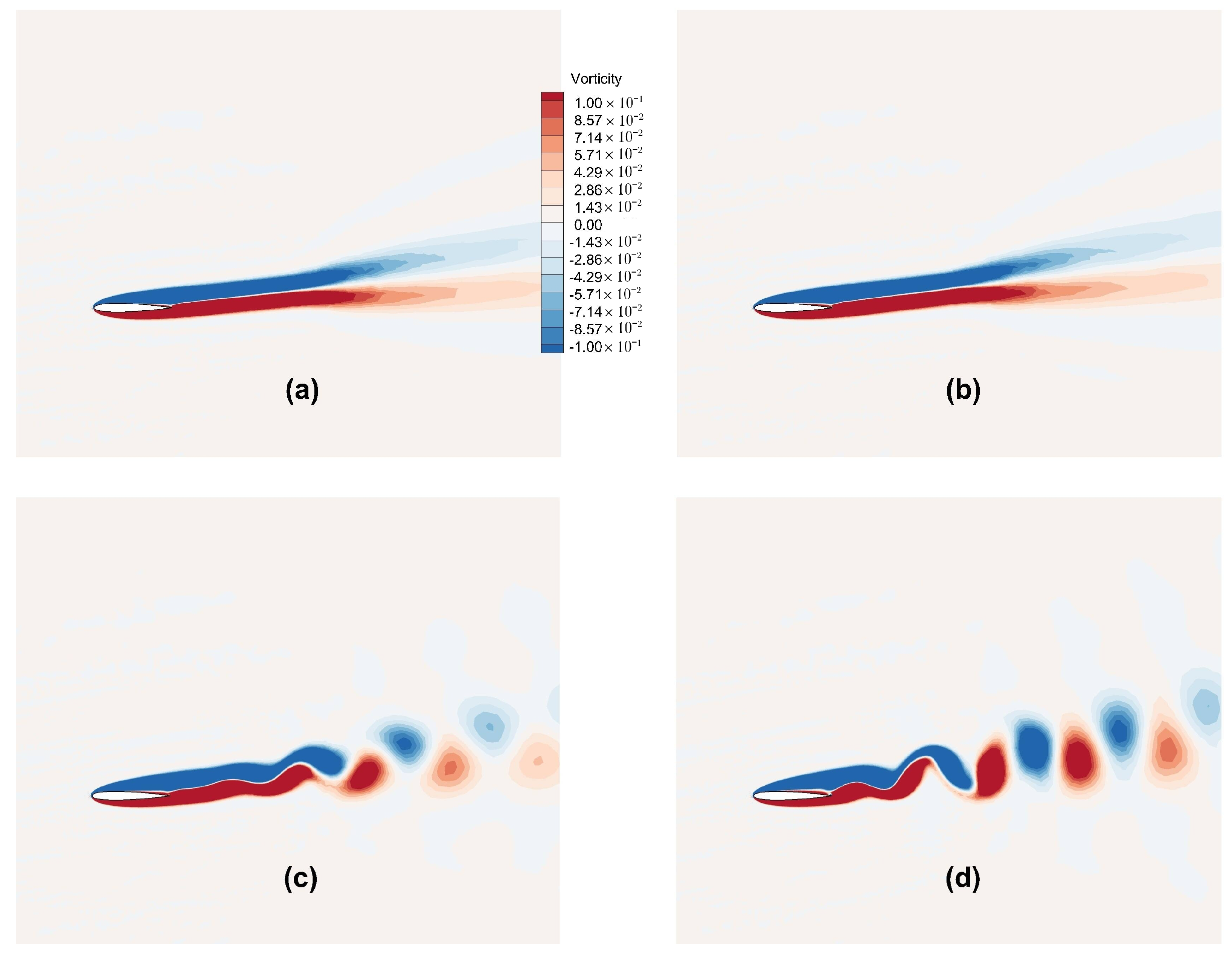
The Karman vortex street theory, studying the flow around bluff bodies, describes the occurrence of periodic vortex shedding, which in the case of a flow over an airfoil depends on the angle of attack.
Figure 17 depicts the instantaneous streamlines for flow over the NACA0012 airfoil for
and angles of attack,
and
. The initiation of unsteady vortex shedding has been observed at angle of attack
, and as the angle of attack is increased further, an enlargement of flow instabilities at the rear of the airfoil has been observed. These observations are in line with those pointed out in the literature [
58,
59].
The distribution of mean pressure coefficient along the non-dimensional chord
for angles of attack
and
is shown in
Figure 18. The
profiles obtained using the FV-iLBM model are compared with those available in ref. [
58]. The
distribution over both lower and upper airfoil surfaces obtained using the FV-iLBM model agrees well with those from the literature. As stated above, as
increases, the flow instabilities at the rear of the airfoil grow in size along the trailing edge, where a very minor disagreement between FV-iLBM predictions and the benchmark [
58] has been observed for
.
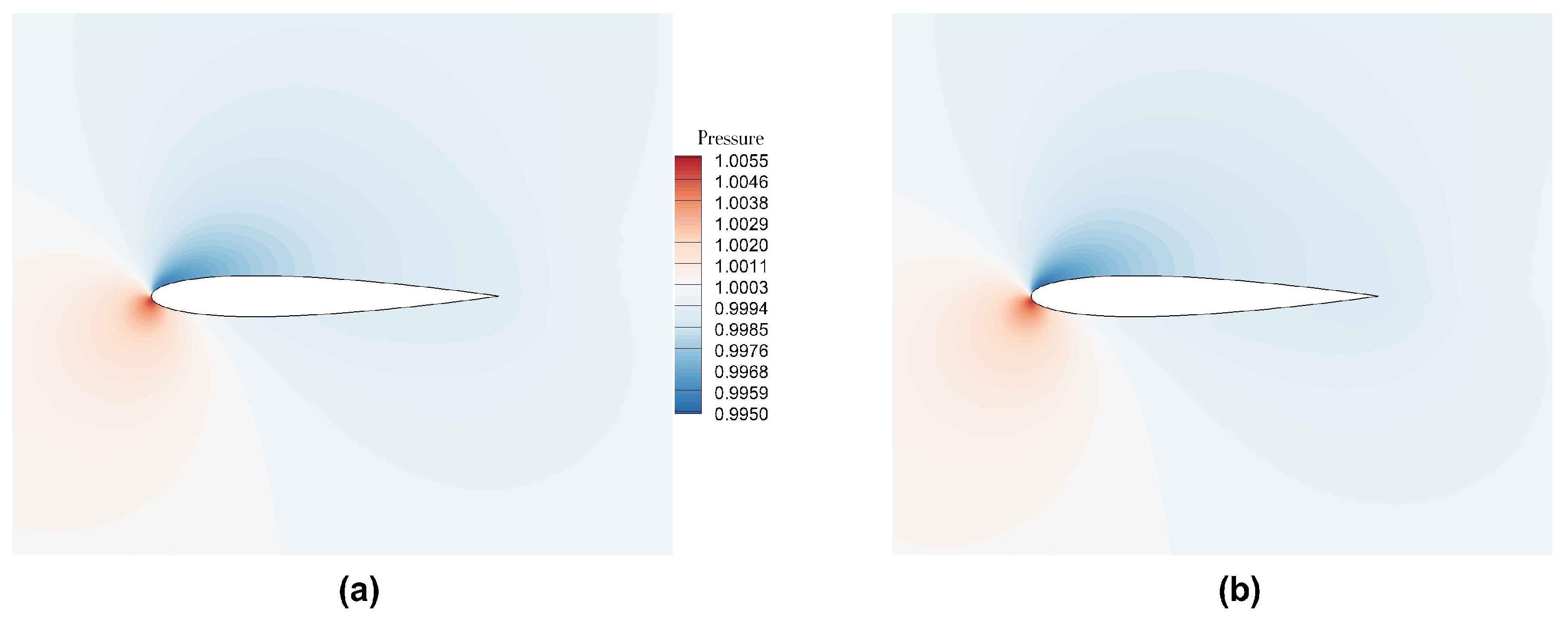
Figure 19 shows the instantaneous pressure field.
Figure 20 shows the instantaneous vorticity contours. As the angle of attack is increased, we observe the onset of lift generation and an observation of onset of unsteady vortex shedding at
. Vortex shedding becomes apparent at
, giving rise to the formation of two counter-rotating vortices. As the angle of attack increases further, there is an augmentation in the strength of vortices, which is in line with the observation in
Figure 17.
Overall, a good agreement is observed between the FV-iLBM predictions and those from the ref. [
58] with the FV-iLBM model producing consistent and encouraging results. As the FV-iLBM predictions transition from the symmetric flow at lower angles of attack to the onset of lift generation, flow separation, and increased pressure drag at higher angles, these findings highlight the airfoil’s sensitivity to the changes in angle of attack and the capability of the present FV-iLBM solver to accurately capture the flow physics of the unsteady aerodynamic applications.
3.7. Validation of FV-iLBM Solver for Non-Newtonian Fluid Flow
The FV-iLBM model has been validated for non-Newtonian fluid flow by simulating the flow through a straight as well as a stenosed channel and comparing the results with those provided by Janela et al. [
50]. The Carreau model [
41] has been used to simulate the non-Newtonian blood viscosity.
As explained in
Section 2.7, the Carreau model [
41] simulates the effective viscosity using Equation (
32). In the Carreau model, the parameter
governs the transition region where the viscosity drop occurs and has the unit of time (
s). The Carreau model is most sensitive to
and
, which can have substantial impact on numerical predictions. They are essentially derived from extrapolated data due to technical challenges associated with measuring viscosity at low shear rates [
50]. The FV-iLBM model has been validated by modeling the non-Newtonian flow through a 2D straight channel with different values of
. The schematic of the 2D straight channel is shown in
Figure 21.
The selected geometry and flow conditions are suitable for simulating blood flow in an arteriole. The flow conditions [
50] are as follows:
Using Equation (
33), the Reynolds number is found to be 0.157. Considering the physiological range [
50], two different
values, namely 0.01 and 0.1 s, have been simulated using the FV-iLBM model in conjunction with the LR scheme.
The flow domain shown in
Figure 21 has been normalized to a rectangle of dimension [−3, 3] × [−1, 1] and the flow conditions mentioned in Equation (
62) are scaled from physical units to LB units using Equation (
35), Equation (
36), and Equation (
37). Following Janela et al. [
50], the scaling parameter,
, from Equation (
35) has been taken as
. After scaling into the LB units, we have the following flow conditions:
The power-law index (
n) in the Carreau model (Equation (
32)) has been taken as 0.3568 and
has been carefully scaled in LB units using Equation (
37). The iLBM model [
17] is a pressure-based LBM scheme derived from a constant density (
), and density plays no further part in the iLBM’s formulation.
A parabolic velocity profile with a maximum velocity
is enforced at inlet, whereas at the outlet, the pressure is fixed (
) and the velocity is linearly extrapolated from the interior domain. No-slip condition is applied at the top and bottom walls using the NEE scheme [
35] and the normal gradient of pressure is taken as zero.
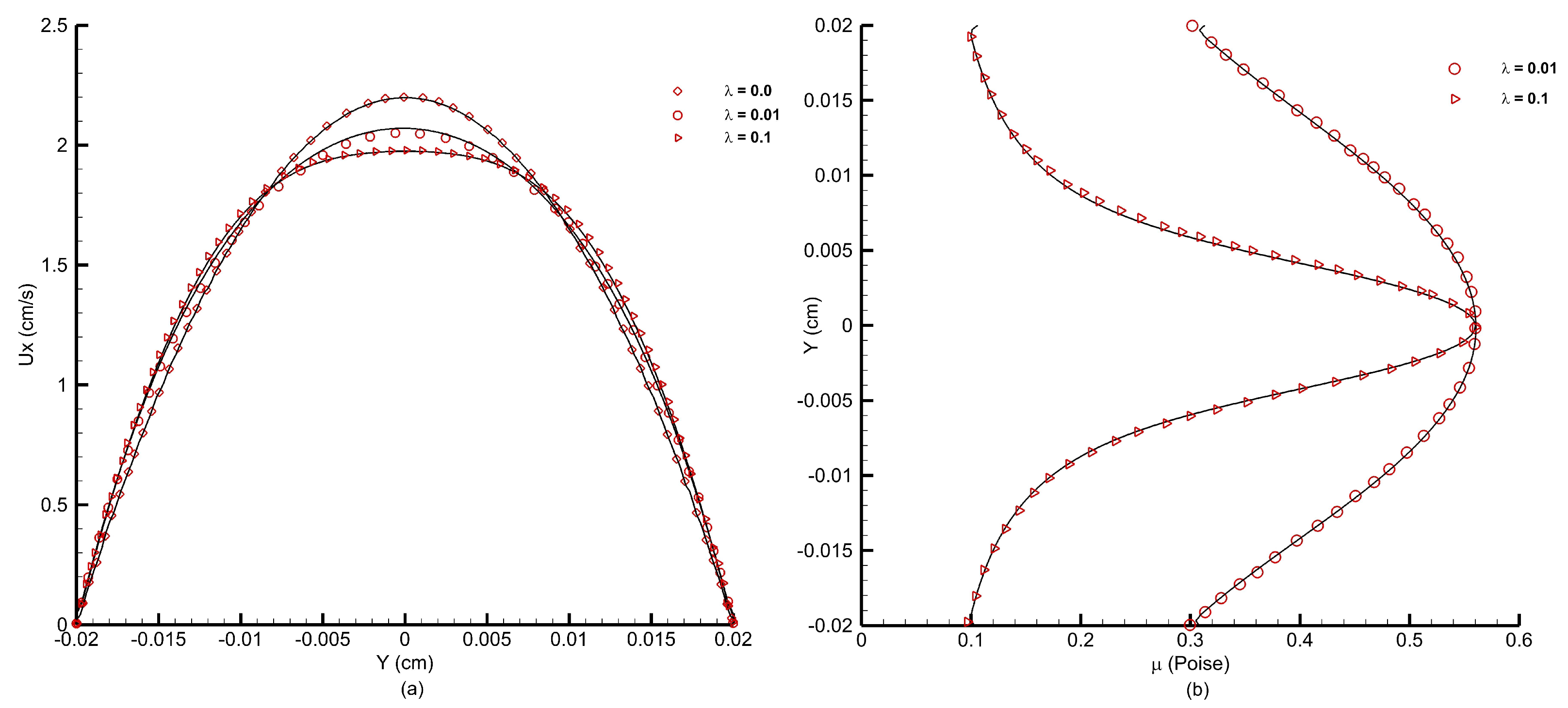
Figure 22a shows the velocity profiles obtained using the FV-iLBM model for different
values. A Newtonian case with
s has also been simulated for comparison. As the
value increases, the velocity profile flattens at the core of the channel due to a larger value of viscosity at low shear rates, and the FV-iLBM model has accurately captured this trend.
Figure 22b shows the viscosity profiles for
and
s. As the
value increases, the viscosity also increases along the core of the channel changing from almost a parabolic profile at a lower
value to a more refined profile at a higher
value, which is in line with the flattening of the velocity profile at the core of the channel observed in
Figure 22a. As observed from
Figure 22b, the FV-iLBM model accurately captures the effect of different
values on viscosity.
The FV-iLBM model has also been validated for modeling non-Newtonian flow through a stenosed channel. For this purpose, a 2D channel with a longitudinal span of
and a stenosis centered at
as shown in
Figure 23 has been considered.
Figure 23 shows the schematic of the 2D channel used to simulate the flow of blood through arterial stenosis. The stenosis or constriction is obtained using the following equation [
50]:
where
is the height of the channel,
is the degree of stenosis, and
is the length of the stenosis. To maintain a slowly varying profile of stenosis, a sufficiently small
should be chosen. Different degrees of stenosis (
), in a circular cross-section, can be calculated as follows [
60]:
A flow obstruction of 50%, as shown in
Figure 23, has been obtained using the following parameters [
50]:
In the present study, following Janela et al. [
50] and Cho and Kinsey [
61], the following parameters are used for the Carreau model:
The flow is driven by a pressure gradient. Following Janela et al. [
50], this gradient matches that of flow in a straight channel with
s and
. A no-slip condition is applied at the top and bottom walls using the NEE scheme [
35], with a zero normal gradient of pressure. At the outlet, the pressure is fixed at
, and the velocity is linearly extrapolated from the interior domain. With
= 0.02 cm,
L = 0.1 cm, and
, the selected geometry and flow conditions are consistent with blood flow in an arteriole or small artery.
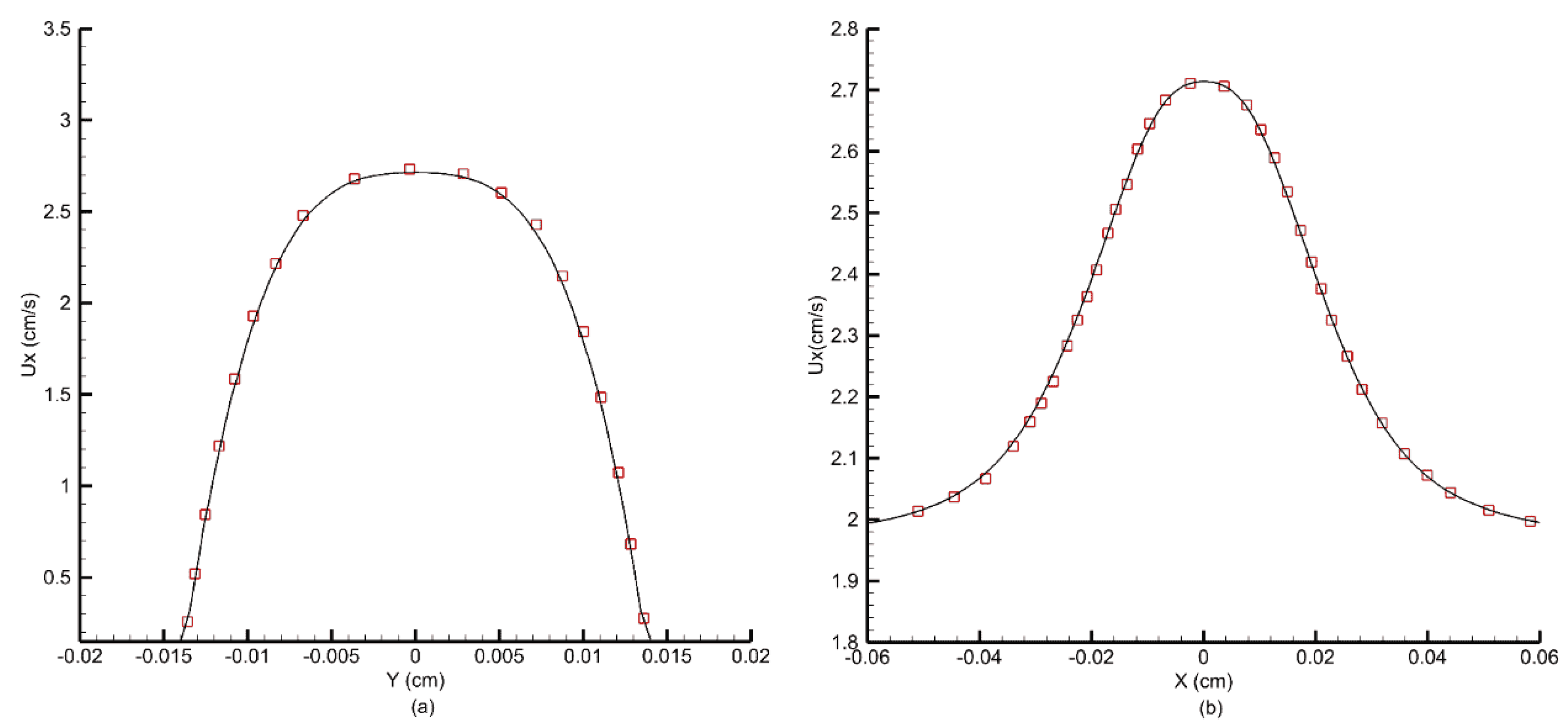
Figure 24 shows the velocity profiles obtained at the throat of the stenosis and at the centerline along the length of the channel.
Figure 25 shows the viscosity profile at the throat of the channel. For the Carreau model, when
s, the viscosity shows a prominent peak along the centerline due to low shear stress [
50]. The FV-iLBM model accurately captures this effect and predicts both velocity and viscosity profiles accurately.
3.8. Blood Flow Through an Artery Afflicted with Multiple Stenoses
Based on the results above, the finite volume iLBM approach can be used to simulate cardiovascular fluid dynamics effectively. The effects of different cardiovascular flow conditions on the well-being of arterial health can be quantified using parameters such as time-averaged wall pressure (TAWP), time-averaged wall shear stress (TAWSS), and oscillatory shear index (OSI). These indices are computed by time-averaged wall shear stress over one cardiac cycle. TAWSS, which is a measure of wall shear stress for one cardiac cycle, is computed as
OSI—which is used to identify regions where the flow deviates most from its average direction and wall shear stress fluctuates the most—is computed as
Time-averaged wall pressure (TAWP) is computed as
Using these indices, regions of low and oscillatory wall shear stress can be identified, which can be used to understand the factors that contribute to the progression of cardiovascular diseases such as atherosclerosis.
The coronary artery, a vital vessel supplying oxygen-rich blood to the myocardium, has a complex geometry often affected by multiple stenoses [
62]. Simplifying its structure while preserving key characteristics is essential for studying arterial hemodynamics.
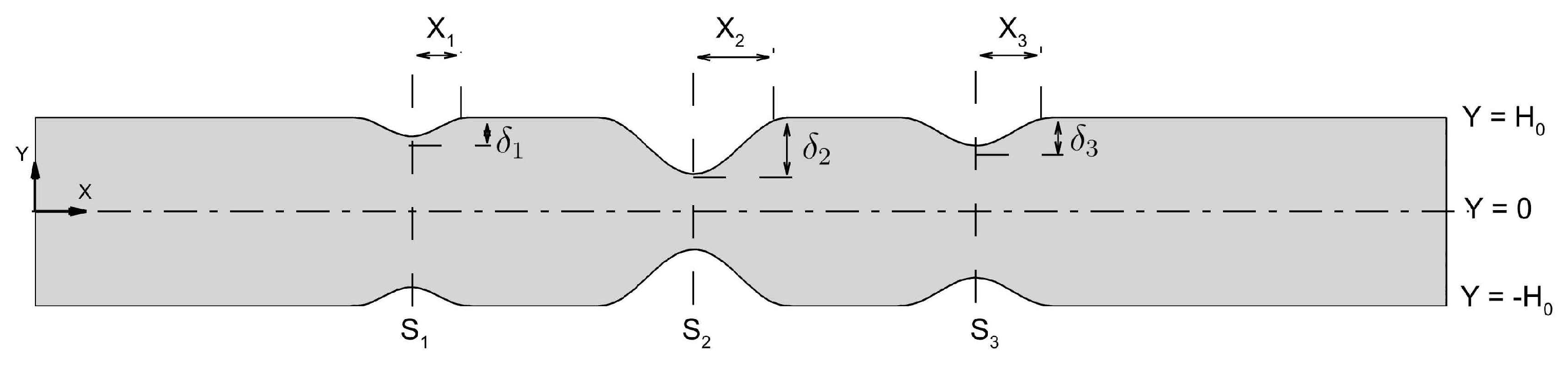
Figure 26 illustrates the schematic of an artery with triple stenoses, each of varying severity. In the figure,
denotes the stenosis location,
i is the stenosis index,
X represents the half-length,
the severity, and
the channel half-width.
The triple stenoses shown in
Figure 26 has been obtained using Equation (
69) as
Table 8 shows different degrees of stenosis for corresponding
values that have been considered in the present study. The third column in
Table 8, titled designation, facilitates reference for terminology in the discussion. Here,
S,
M, and
L denote ‘small’, ‘medium’, and ‘large’ stenosis severity, respectively. The configuration shown in
Figure 26 is termed ‘S-M-L’ configuration.
The human coronary artery diameter typically ranges from 3 to 5 mm. In this study, for a 2D planar domain (
Figure 26), an artery height (
) of 5 mm is considered. The model parameters representing blood flow in human arteries have been considered as per Abbasian et al. [
63]. They are as follows:
The problem is scaled to LB units by setting the scaling parameter to
. In
Figure 26, the locations of stenoses are as follows:
Considering the pulsatile nature of blood flow through the arteries, a pulsatile velocity has been set at the inlet of the artery using
where
A is the cross-sectional area of the inlet surface and
is the pulsatile flow rate. Considering a 2D planar domain, the cross-sectional area is calculated considering unit width. Referring to
Figure 26, if
is the artery height and
W is the width of the artery, then the cross-sectional area is calculated as
.
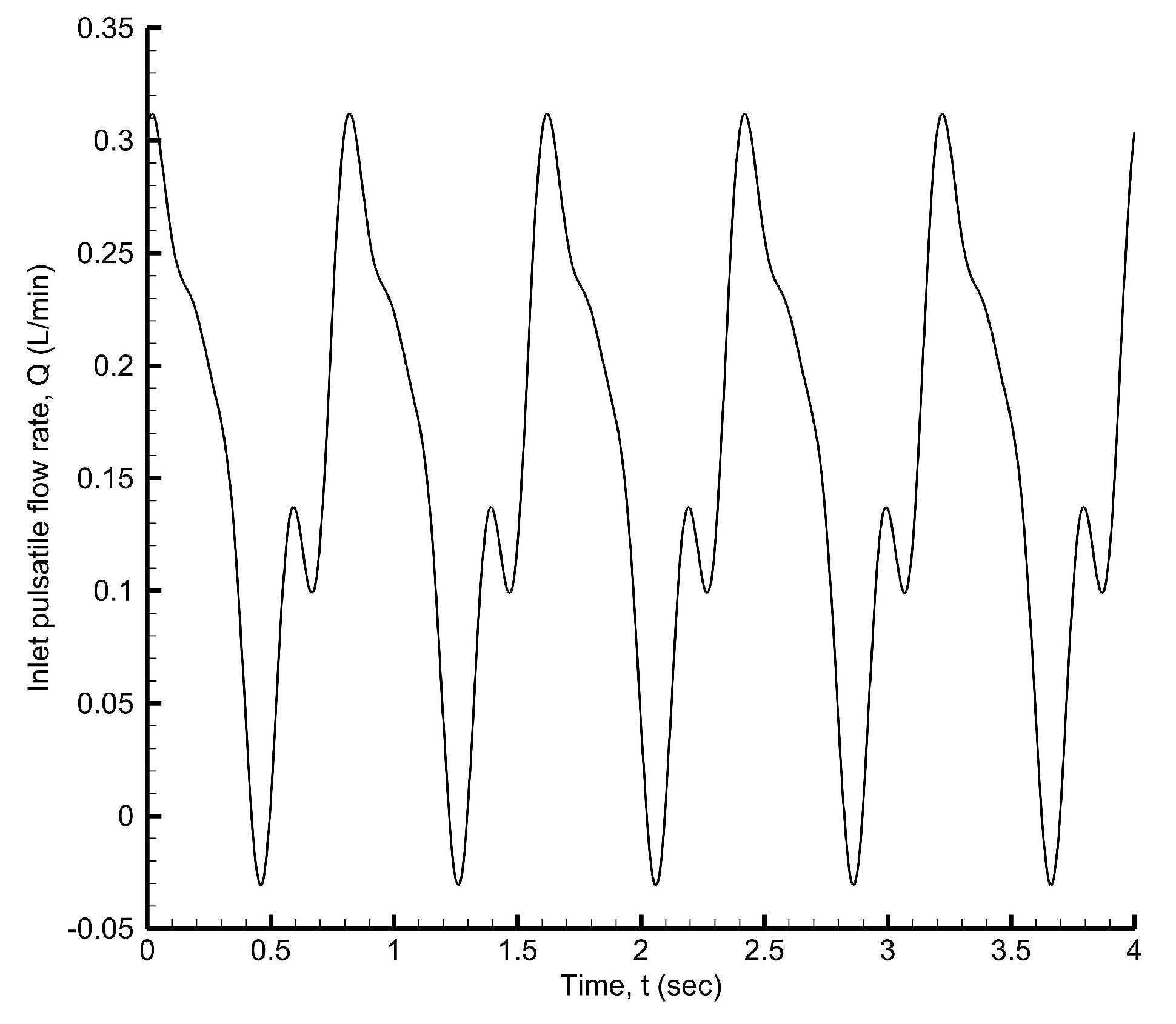
The inlet pulsatile velocity is derived from the pulsatile flow rate waveform [
64] shown in
Figure 27, which is expressed using the Fourier series as
The coefficients
and
are obtained using the MATLAB 2021b curve fitting tool. The coefficients are obtained using the [
65] curve fitting tool and are listed in
Table 9.
The time-averaged volumetric flow rate obtained using the MATLAB curve fitting tool is
L/min. The pulsatile inlet velocity is scaled to LB units using Equation (
36). In Equation (
71), the angular velocity
relates to the cardiac cycle as
. The waveform [
64] shown in
Figure 27 has a time period of
s, corresponding to an average pulse rate of 75 beats/min. Given the coronary artery diameter, the Womersley number is
and the Reynolds number is
. Three cardiac cycles are simulated with a time step of
, as shown in
Figure 27.
Figure 28 illustrates the unstructured triangular mesh used for the S-L-M configuration, with a highly refined grid near the artery walls to accurately capture flow physics and a progressively coarser mesh toward the core to optimize cell count. A grid independence study was conducted using three meshes: grid 1 (coarse) with 519 wall cells and 41,772 elements, grid 2 (medium) with 802 wall cells and 72,600 elements, and grid 3 (fine) with 1209 wall cells and 99,892 elements. As shown in
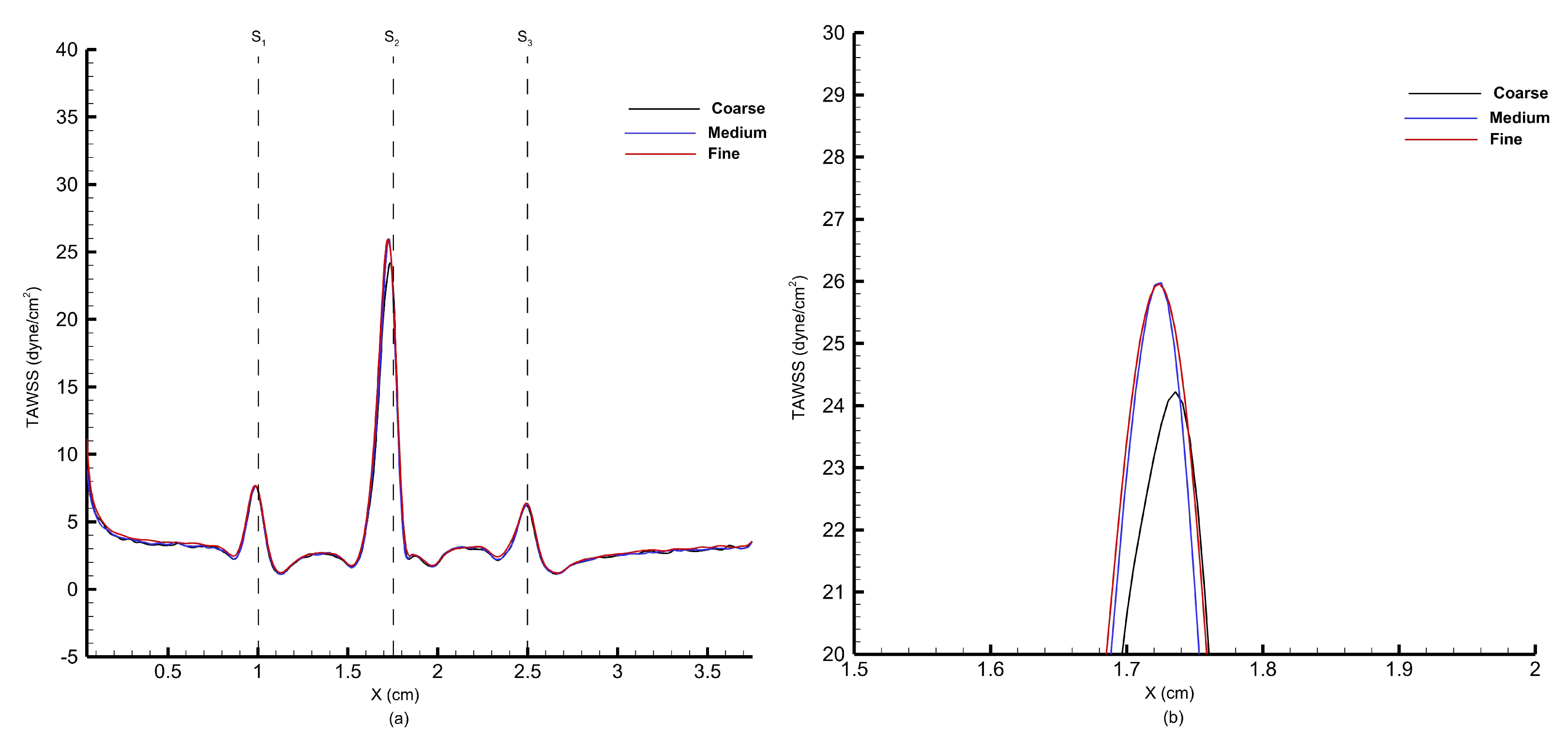
Figure 29, the wall shear stress profiles from grid 2 and grid 3 closely match, and therefore, grid 2 has been selected for the blood flow analysis through multiple stenoses.
The pressure along the arterial wall plays a critical role in the development of arterial diseases.
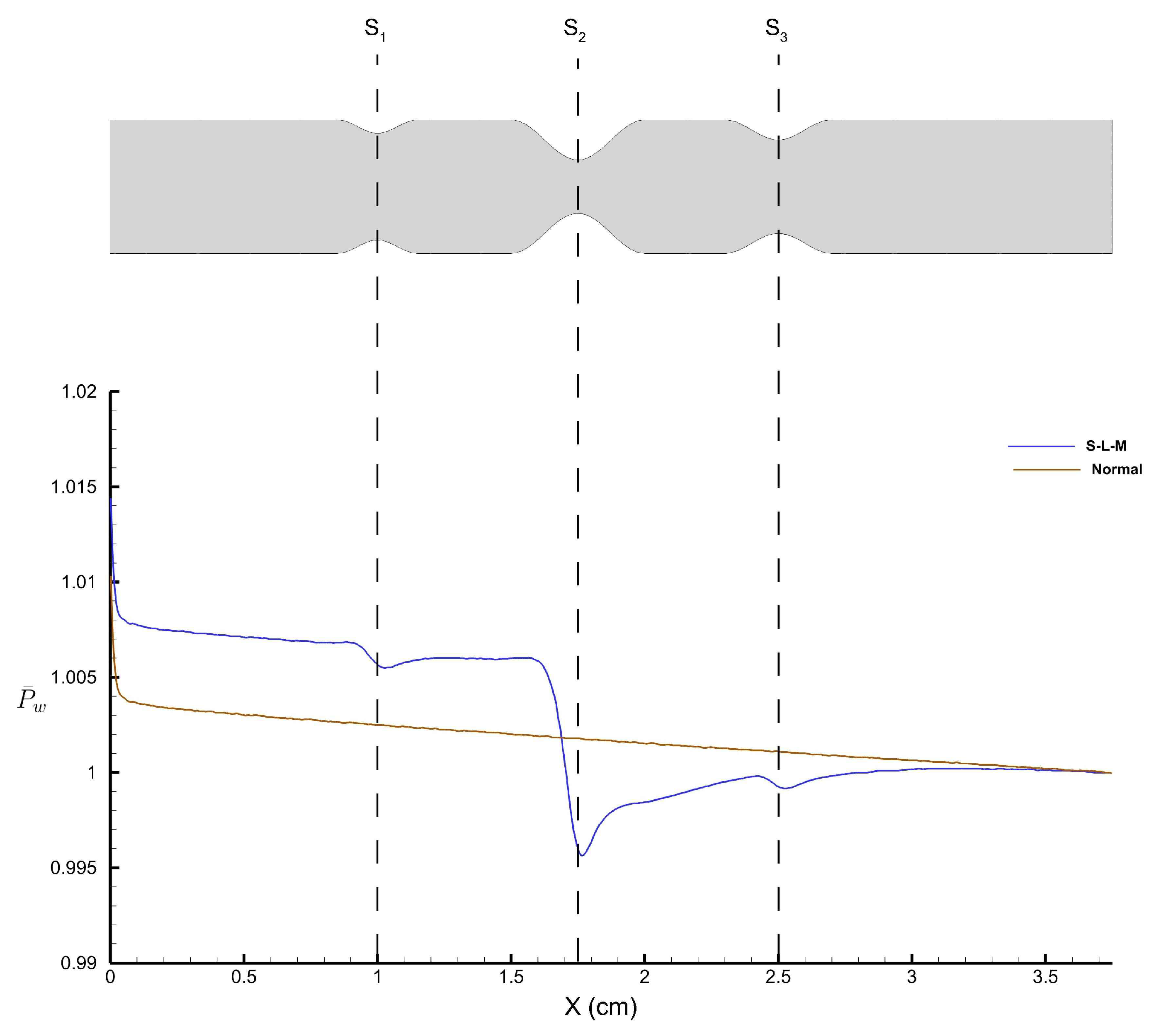
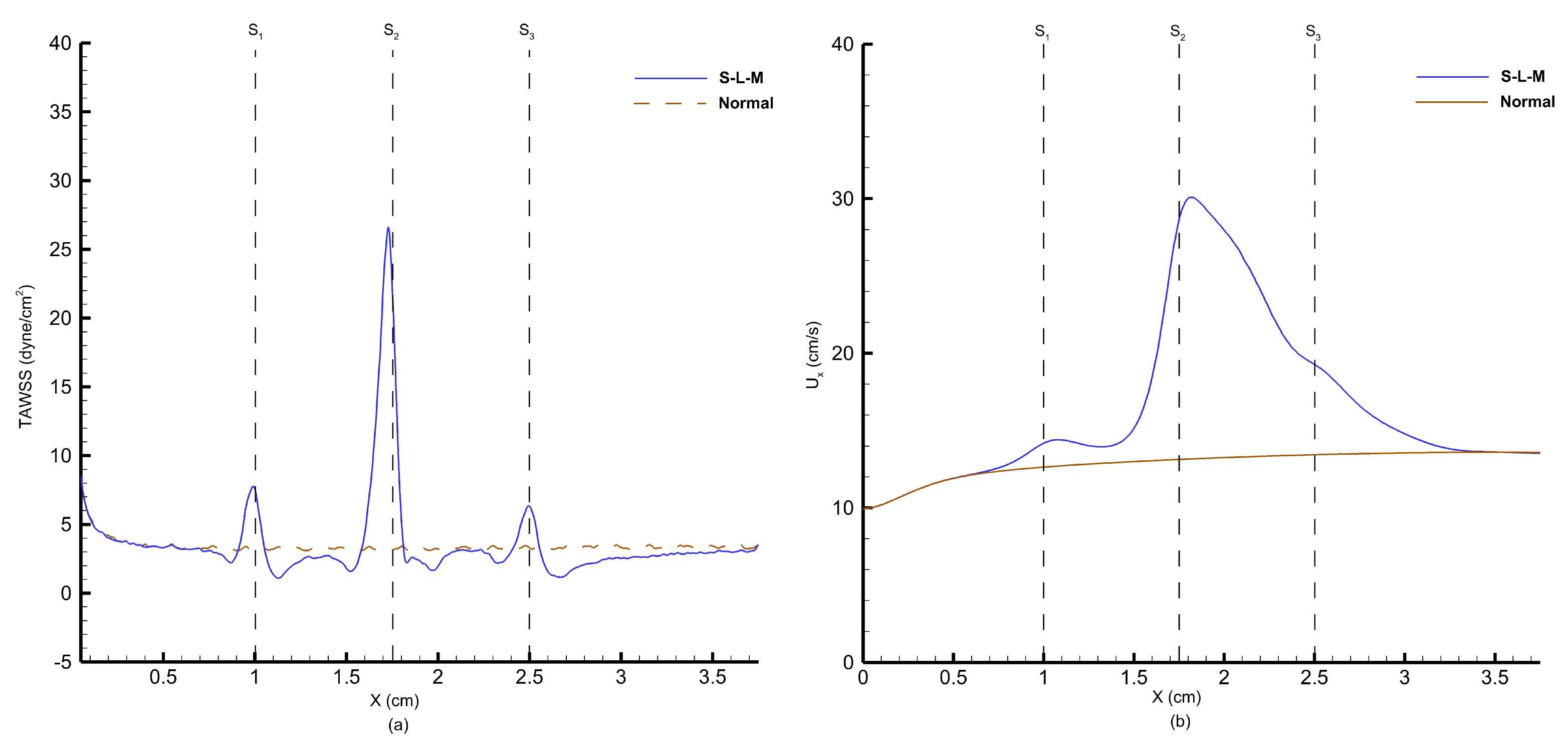
Figure 30 shows the time-averaged wall pressure along the top wall of the stenosed artery obtained using the FV-iLBM model.
refers to the wall pressure obtained along the top wall of the stenosed artery with the S-L-M stenosis configuration, whereas ‘
’ refers to the wall pressure obtained for the normal artery without any stenosis.
As the flow encounters the small stenosis, we observe a drop in pressure and attempts to recover post the stenosis. However, the flow encounters the large stenosis and we observe the largest pressure drop, after which we observe pressure recovery. A final dip in pressure distribution is observed after the the medium stenosis; however, it does not affect the wall pressure substantially. On the other hand, a linearly decreasing pressure profile is observed for the normal artery, which is the normal tendency of the fluid pressure due to friction along the length of the artery wall.
As fluid flow approaches the narrowing (stenosis) in the artery, there is a sudden drop in pressure with the lowest wall pressure corresponding to the highest average velocity of fluid flow. The cross-sectional area for flow decreases as it approaches the stenosis, reaching its minimum at the narrowest point (throat), then increases again as it moves toward the exit of the stenosis. According to the principle of continuity for incompressible fluids, the average velocity of flow is inversely proportional to the cross-sectional area. Therefore, the maximum average velocity at the stenosis throat corresponds to the minimum pressure at that point. Following a sharp pressure drop at the stenosis, there is a partial recovery in wall pressure downstream due to the conversion of kinetic energy into pressure energy in the diverging section of the stenosis. However, the pressure does not return to its original value due to irreversible losses caused by viscous effects.
As mentioned above, the average flow velocity is inversely proportional to the cross-sectional area and as the flow approaches the stenosis, the cross-sectional area for the flow decreases, resulting in a rise in flow velocity.
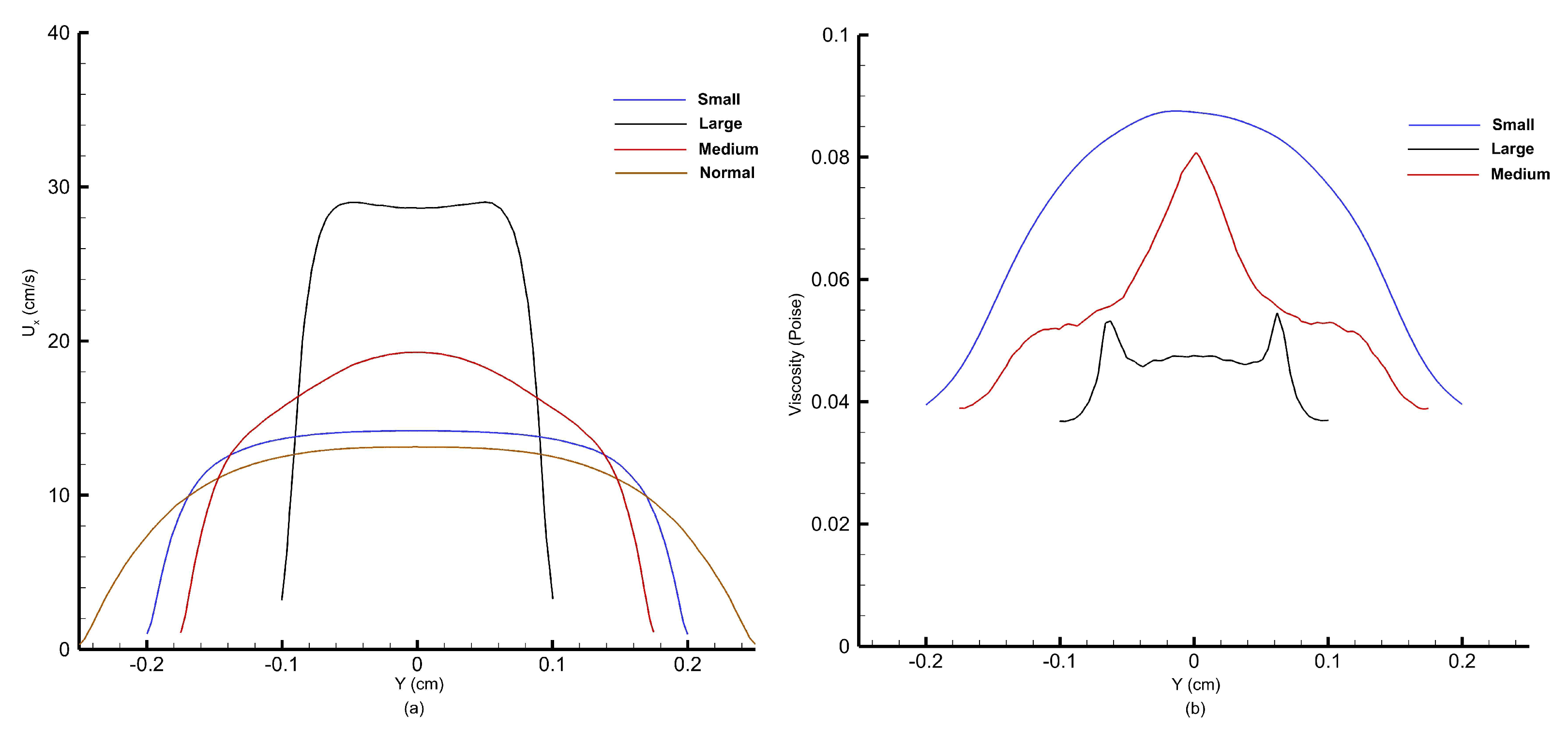
Figure 31 shows the velocity and viscosity profiles obtained at the peak of the cardiac cycle corresponding to systolic pressure.
Figure 31a shows the velocity profiles, whereas
Figure 31b shows the viscosity profiles obtained along the throat at three stenoses.
As observed from
Figure 31a, as the percentage of stenosis increases, the peak velocity at the stenosis throat increases. At the peak of the cardiac cycle, blood flow velocity reaches 14 cm/s at the small stenosis, 19 cm/s at the medium stenosis, and almost 30 cm/s at the large stenosis, which is more than twice of that obtained for the normal artery without any stenosis. From
Figure 31b, we obtain viscosity profiles that correspond to velocity profiles observed in
Figure 31a. Viscosity is a measure of a fluid’s resistance to flow. It determines how easily a fluid deforms under shear stress. In the context of fluid flow, viscosity affects the velocity gradient within the fluid. This means that in regions where there is a high velocity gradient (such as near walls of the artery), viscosity resists the flow, resulting in viscous drag. Therefore, as the blood accelerates after encountering small, large, and medium stenoses, we observe a corresponding reduction in magnitude of viscosity. The viscosity, which depends on shear, decreases notably in the central region where shear is minimal. Consequently, the typically parabolic shape of the velocity profile becomes flattened at the center of the channel, which is what we have observed for velocity profiles at the throat of small and large stenoses.
The shear stress experienced by the arterial wall near a stenosis is significant, as it can provide insights into the mechanisms behind post-stenotic dilatation and contribute to understanding the development and progression of arterial stenosis.
Figure 32a shows the time-averaged wall shear stress at the top wall. Changes in blood flow velocity occur within both the stenotic and post-stenotic zones due to alterations in the flow pressure gradient. Since wall shear stress is a function of velocity gradient and the highest velocity gradient occurs at the stenosis throat, we observe shear stress peaks as the flow accelerates across the stenosis. Referring to
Figure 32a, as the flow encounters the small stenosis, we observe the first rise in wall shear stress that is proportional to the degree of small stenosis, following which shear stress reduces in the post-stenotic region. The largest peak in wall shear stress is observed for the large stenosis, where the velocity gradient is at its peak. Downstream of the large stenosis, the wall shear stress reduces to the normal value, and upon encountering the medium stenosis does in fact result in its rise again; however, this rise is not commensurate with the degree of medium stenosis, which was observed for small stenosis. From this observation, it is evident that large stenosis has affected the downstream flow dynamics. From
Figure 32b, we can observe the rise in instantaneous centerline velocity at the peak of the cardiac cycle as the flow encounters multiple stenoses. Following the large stenosis, we observe a fall in centerline velocity, and after the medium stenosis, the velocity behavior is not altered.substantially.
Endothelial cells are the cells that form the inner lining of blood vessels, including arteries. They play a crucial role in maintaining the health and function of blood vessels. One of the factors that influence endothelial cell function is shear stress [
66]. When blood flows smoothly and evenly along the vessel wall, shear stress is relatively constant. However, in case of a stenosed artery, high peak wall shear stress can lead to damage in the vessel wall, resulting in the thickening of the intima (the innermost layer of an artery), aggregation of platelets, and ultimately leading to thrombus (a blood clot) formation. Conversely, low shear stress in the region of flow separation is linked to mass transportation across the arterial wall and prolonged interaction time between platelets and endothelium. This increases the likelihood of atherosclerosis formation as the percentage of restriction in the artery increases.
In areas where blood flow is disturbed or chaotic, such as bends or downstream of stenosis, shear stress becomes oscillatory, meaning that it fluctuates in magnitude and direction rapidly over time. Endothelial cells respond differently to oscillatory shear stress compared to steady shear stress [
67]. This behavior can be quantified with the help of the oscillatory shear index. OSI varies from 0 to 0.5 with 0 representing no oscillations or flow reversal, whereas 0.5 represents highly oscillatory flow behavior. A high OSI value is associated with areas of disturbed flow, which can result in endothelial cell dysfunction, leading to a higher risk of atherosclerotic plaque formation [
67]. This is because the oscillatory nature of the shear stress can cause endothelial cells to promote inflammation within the artery walls and, subsequently, plaque formation. This plaque is made up of cholesterol, fatty substances, cellular waste products, calcium, and fibrin, and it can restrict blood flow through the arteries, leading to various cardiovascular problems like heart attacks and strokes.
Figure 33 shows the oscillatory shear index obtained along the top wall at small, large, and medium stenoses for the S-L-M configuration. As the blood flow follows the cardiac cycle depicted in
Figure 27 and reaches the bottom of the cycle, corresponding to diastolic pressure, we observe a flow reversal. On top of that, the presence of multiple stenoses also induces recirculation regions in the immediate downstream of stenoses. This affects the instantaneous wall shear stress, which can be quantified using OSI. As shown in the figure above, before encountering the small stenosis, a normal flow is observed; however, as the small stenosis is encountered, OSI reduces in the converging section of the stenosis, whereas it rises sharply in the diverging section of the stenosis and in the downstream region of the stenosis. It is in the downstream of stenosis that a peak in OSI is observed where major flow reversal and recirculation is observed for large and medium stenoses. Major oscillations in wall shear stress are observed pre- and post-medium stenosis, indicating key regions of atherosclerosis progression.
Based on the above results, it is evident that downstream of stenoses, blood flow experiences flow recirculation and oscillatory wall shear stress, which prompts further plaque deposition aggravating the degree of blockage further. Another aspect that affects the flow dynamics is the presence of multiple stenoses such that one or more stenoses are present downstream of each other. This arrangement can affect blood flow behavior across each stenosis, either aggravating or suppressing plaque formation.
Figure 33 indicates that the peak OSI value following the large stenosis is smaller than those obtained for medium stenosis. This phenomenon is likely due to the fact that, after a small stenosis, as the flow encounters a large stenosis, the velocity gradient changes abruptly, while the oscillations in wall shear stress do not change proportionally. On the other hand, high OSI coupled with low wall shear stress, at medium stenosis, is a crucial factor in the development, formation, and localization of atherosclerosis.
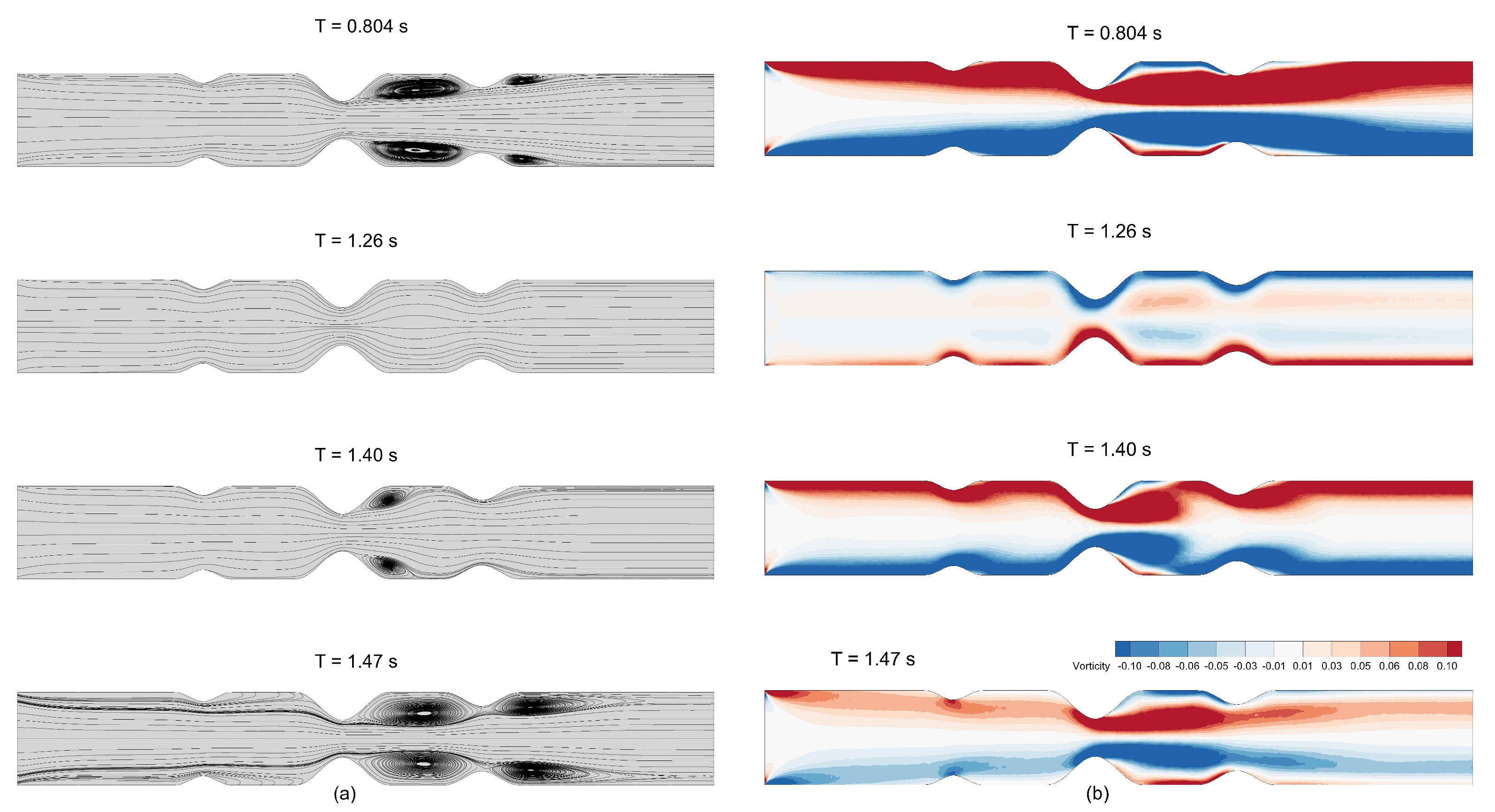
Figure 34a shows the streamlines obtained for blood flow through stenosed artery with S-L-M configuration at different time instances of the cardiac cycle, whereas
Figure 34b shows the vorticity contours at the same time instances. At
s, which represents the peak of the cardiac cycle, we observe major flow recirculation zones downstream of large and medium stenoses. At
s, which represents the trough of the cardiac cycle, we observe a reversal in flow direction. Once the flow exceeds the trough of the cardiac cycle, it regains its normal flow direction and vortex strength, and vorticity magnitude increases as the cycle marches toward the peak of the cardiac cycle again. Major vortices are observed on both sides of the throat of the medium stenosis during the cardiac cycle. This signifies that this is the region of high intensity and fluctuations in wall shear stress and, thus, we observe two OSI peaks on both sides of the throat of the medium stenosis in
Figure 33c.
The FV-iLBM solver presented in this study demonstrates significant potential for accurately simulating blood flow rheology in complex arterial vasculature. By combining the advantages of finite volume discretization with the incompressible lattice Boltzmann method, our approach effectively captures the non-Newtonian behavior of blood, particularly in regions of low shear rates and complex geometries. The solver’s ability to handle unstructured meshes and curved boundaries makes it particularly suitable for patient-specific simulations of arterial stenoses and aneurysms. Future studies will explore the impact of varying parameters in the Carreau model, specifically the relaxation time , on blood flow dynamics. Additionally, investigations into the effects of different Reynolds and Womersley numbers on blood flow in arteries afflicted with multiple stenoses and aneurysms will provide valuable insights into disease progression. These studies will also examine how various stenosis configurations and aneurysm sizes influence blood flow dynamics, contributing to a broader understanding of arterial disease development and progression.