Influence of the Effective Reproduction Number on the SIR Model with a Dynamic Transmission Rate
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Analytical Results
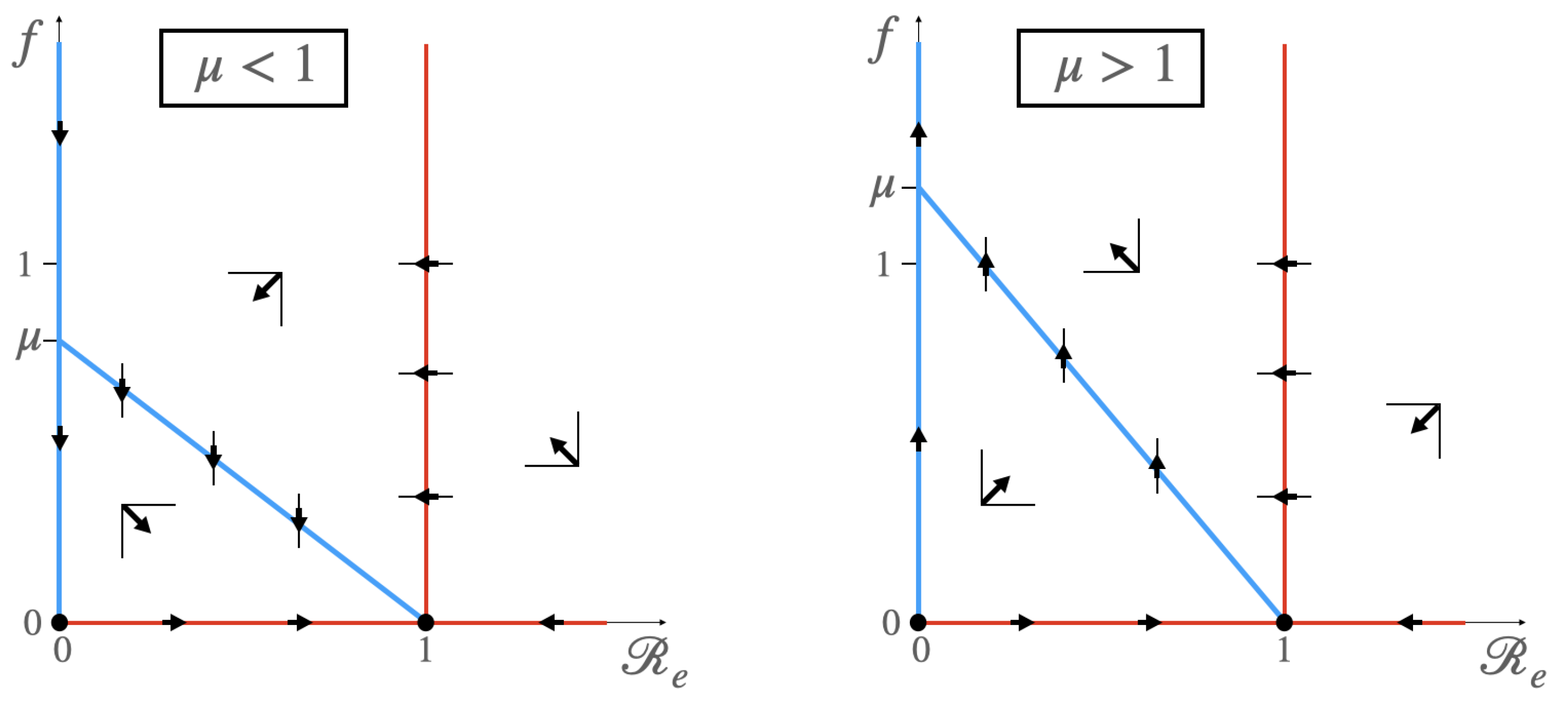
3.1.1. Case
- (a)
- If , then is a saddle point, and is an attractor node.
- (b)
- If , then is a repulsor, and is a saddle point.
3.1.2. Case
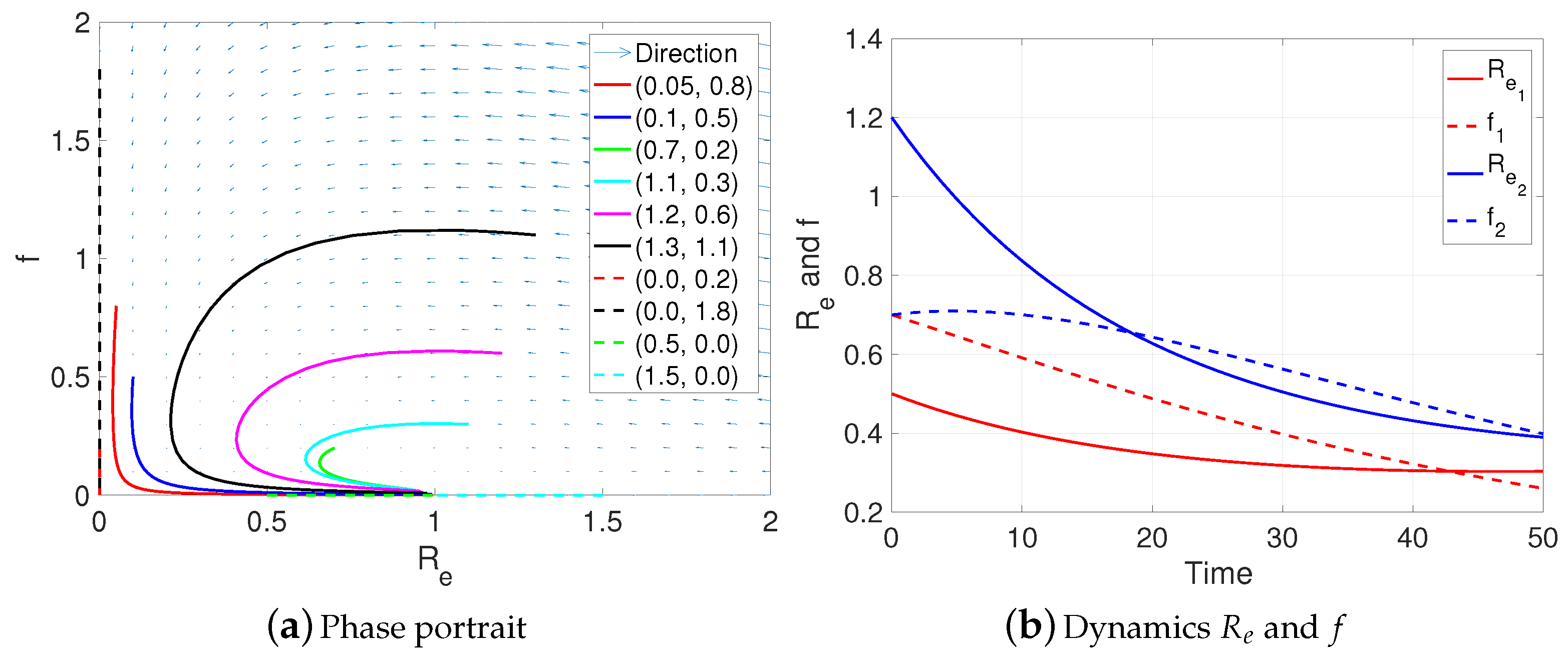
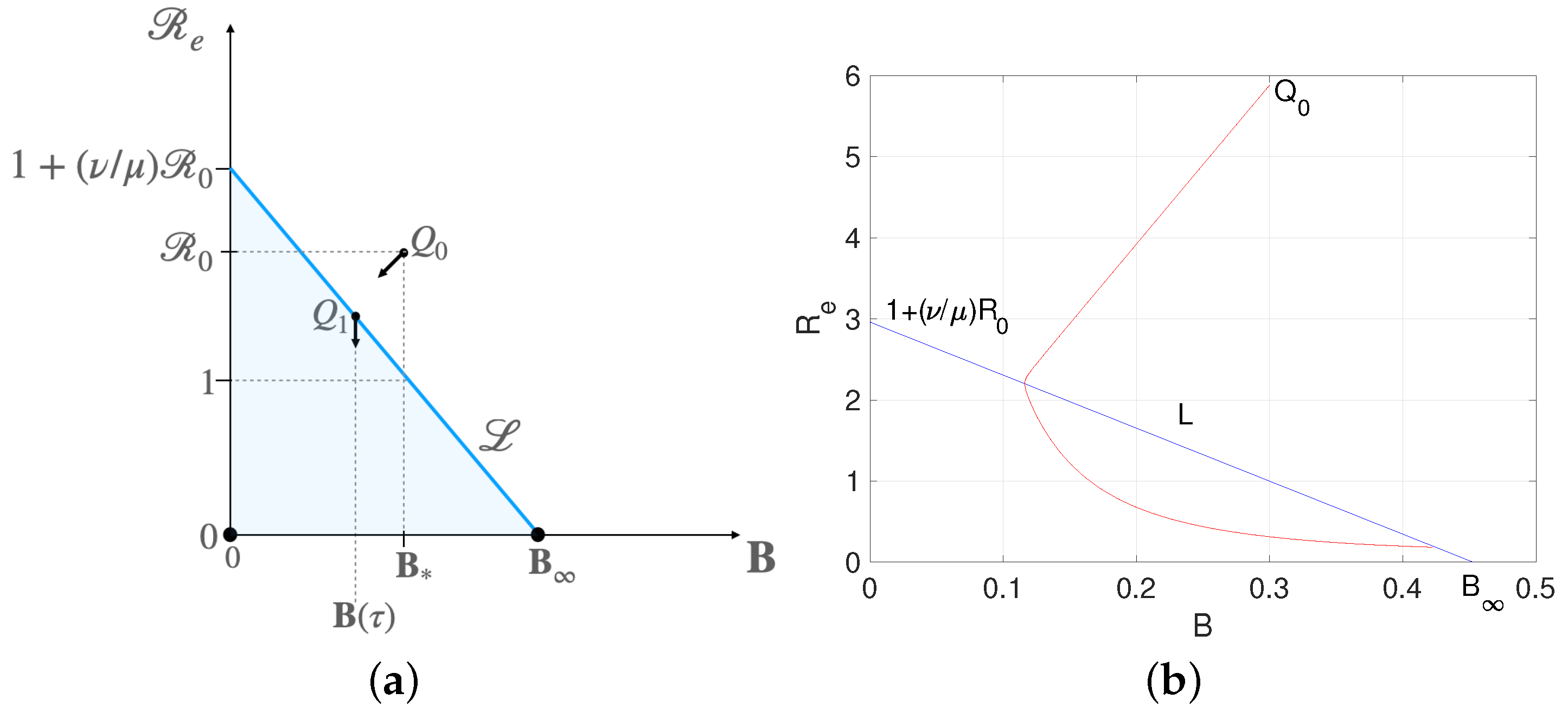
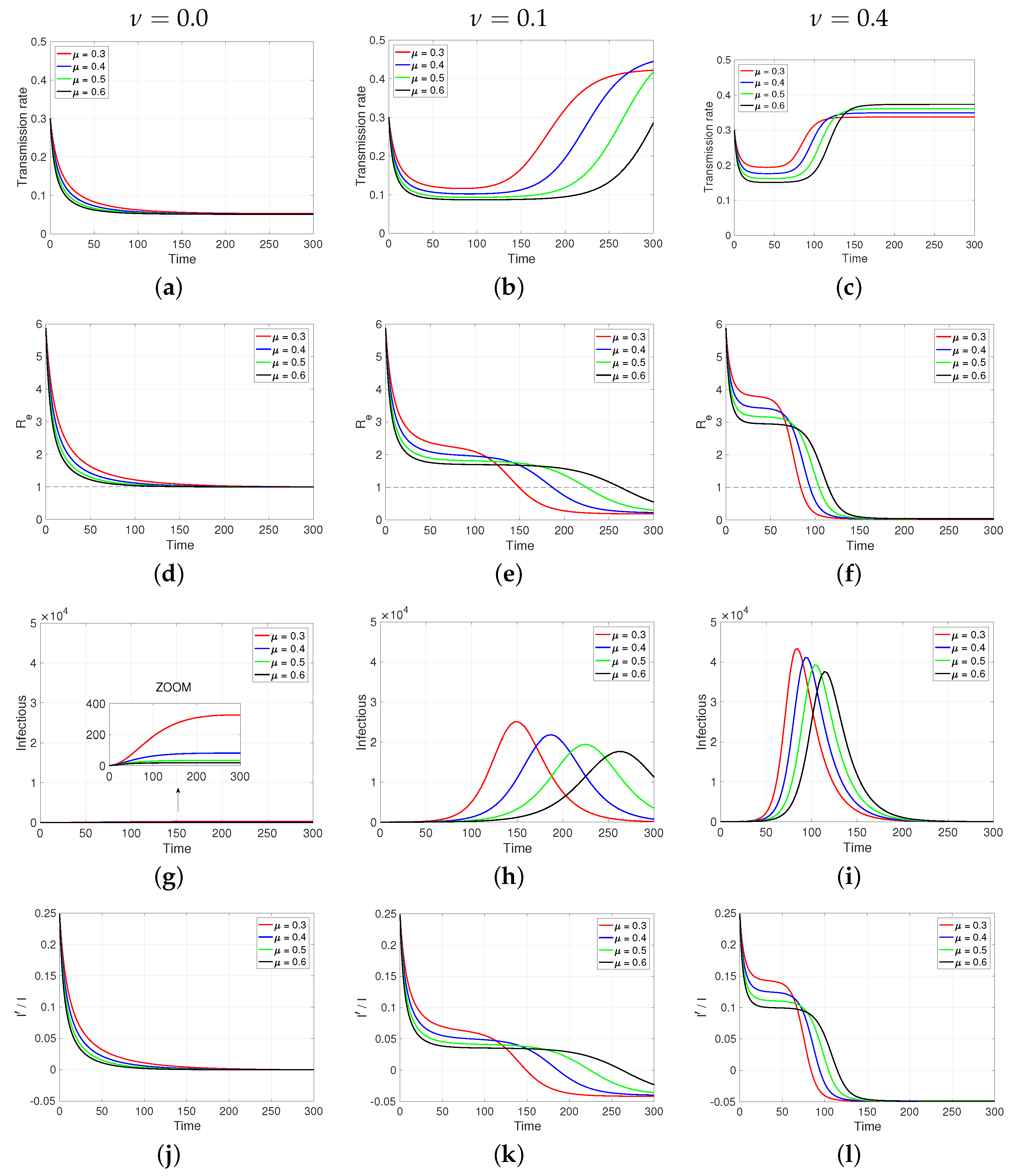
3.2. Numerical Results
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilardino, R.E. Does “flattening the curve” affect critical care services delivery for COVID-19? A global health perspective. Int. J. Health Policy Manag. 2020, 9, 503. [Google Scholar] [CrossRef]
- Palomo, S.; Pender, J.J.; Massey, W.A.; Hampshire, R.C. Flattening the curve: Insights from queueing theory. PLoS ONE 2023, 18, e0286501. [Google Scholar] [CrossRef]
- Tashiro, A.; Shaw, R. COVID-19 pandemic response in Japan: What is behind the initial flattening of the curve? Sustainability 2020, 12, 5250. [Google Scholar] [CrossRef]
- Di Lauro, F.; Kiss, I.Z.; Rus, D.; Della Santina, C. COVID-19 and flattening the curve: A feedback control perspective. IEEE Control Syst. Lett. 2020, 5, 1435–1440. [Google Scholar] [CrossRef]
- Giannakeas, V.; Bhatia, D.; Warkentin, M.T.; Bogoch, I.I.; Stall, N.M. Estimating the maximum capacity of COVID-19 cases manageable per day given a health care system’s constrained resources. Ann. Intern. Med. 2020, 173, 407–410. [Google Scholar] [CrossRef]
- Bustan, S.; Nacoti, M.; Botbol-Baum, M.; Fischkoff, K.; Charon, R.; Madé, L.; Simon, J.R.; Kritzinger, M. COVID-19: Ethical dilemmas in human lives. J. Eval. Clin. Pract. 2021, 27, 716–732. [Google Scholar] [CrossRef]
- Neves, N.M.; Bitencourt, F.B.; Bitencourt, A.G. Ethical dilemmas in COVID-19 times: How to decide who lives and who dies? Rev. Assoc. Med. Bras. 2020, 66, 106–111. [Google Scholar] [CrossRef]
- Holleran, S. Dying Apart, Buried Together: COVID-19, Cemeteries and Fears of Collective Burial. In Death’s Social and Material Meaning Beyond the Human; Bristol University Press: Bristol, UK, 2024; pp. 100–113. [Google Scholar]
- Hamid, W.; Jahangir, M.S. Dying, death and mourning amid COVID-19 pandemic in Kashmir: A qualitative study. OMEGA-J. Death Dying 2022, 85, 690–715. [Google Scholar] [CrossRef]
- Raffaetà, R. Another Day in Dystopia. Italy in the Time of COVID-19. Med. Anthropol. 2020, 39, 371–373. [Google Scholar] [CrossRef]
- Calmon, M. Considerations of coronavirus (COVID-19) impact and the management of the dead in Brazil. Forensic Sci. Int. Rep. 2020, 2, 100110. [Google Scholar] [CrossRef]
- Çakan, S. Dynamic analysis of a mathematical model with health care capacity for COVID-19 pandemic. Chaos Solitons Fractals 2020, 139, 110033. [Google Scholar] [CrossRef]
- Gutiérrez-Aguilar, R.; Córdova-Lepe, F.; Muñoz-Quezada, M.T.; Gutiérrez-Jara, J.P. Model for a threshold of daily rate reduction of COVID-19 cases to avoid hospital collapse in Chile. Medwave 2020, 20, e7871. [Google Scholar] [CrossRef]
- Jiliberto, R.R. Deja a la Estructura Hablar: Modelización y Análisis de Sistemas Naturales, Sociales y Socioecológicos; Ediciones UM: Murcia, Spain, 2020. [Google Scholar]
- Canals, M.; Cuadrado, C.; Canals, A. COVID-19 in Chile: The usefulness of simple epidemic models in practice. Medwave 2021, 21, e8119. [Google Scholar] [CrossRef]
- Adiga, A.; Dubhashi, D.; Lewis, B.; Marathe, M.; Venkatramanan, S.; Vullikanti, A. Mathematical models for COVID-19 pandemic: A comparative analysis. J. Indian Inst. Sci. 2020, 100, 793–807. [Google Scholar] [CrossRef]
- Rojas-Vallejos, J. Strengths and limitations of mathematical models in pandemics—The case of COVID-19 in Chile. Medwave 2020, 20, e7876. [Google Scholar] [CrossRef]
- Manca, D.; Caldiroli, D.; Storti, E. A simplified math approach to predict ICU beds and mortality rate for hospital emergency planning under COVID-19 pandemic. Comput. Chem. Eng. 2020, 140, 106945. [Google Scholar] [CrossRef]
- Biggerstaff, M.; Cowling, B.J.; Cucunubá, Z.M.; Dinh, L.; Ferguson, N.M.; Gao, H.; Hill, V.; Imai, N.; Johansson, M.A.; Kada, S.; et al. Early insights from statistical and mathematical modeling of key epidemiologic parameters of COVID-19. Emerg. Infect. Dis. 2020, 26, e1–e14. [Google Scholar] [CrossRef]
- Thompson, R.N.; Hollingsworth, T.D.; Isham, V.; Arribas-Bel, D.; Ashby, B.; Britton, T.; Challenor, P.; Chappell, L.H.; Clapham, H.; Cunniffe, N.J.; et al. Key questions for modelling COVID-19 exit strategies. Proc. R. Soc. B 2020, 287, 20201405. [Google Scholar] [CrossRef]
- Gostic, K.M.; McGough, L.; Baskerville, E.B.; Abbott, S.; Joshi, K.; Tedijanto, C.; Kahn, R.; Niehus, R.; Hay, J.A.; De Salazar, P.M.; et al. Practical considerations for measuring the effective reproductive number, Rt. PLoS Comput. Biol. 2020, 16, e1008409. [Google Scholar] [CrossRef]
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef]
- Córdova-Lepe, F.; Vogt-Geisse, K. Adding a reaction-restoration type transmission rate dynamic-law to the basic SEIR COVID-19 model. PLoS ONE 2022, 17, e0269843. [Google Scholar] [CrossRef]
- Córdova-Lepe, F.; Gutiérrez-Jara, J.P. A dynamic reaction-restore-type transmission-rate model for COVID-19. WSEAS Trans. Biol. Biomed. 2024, 21, 118–130. [Google Scholar] [CrossRef]
- Huisman, J.S.; Scire, J.; Angst, D.C.; Li, J.; Neher, R.A.; Maathuis, M.H.; Bonhoeffer, S.; Stadler, T. Estimation and worldwide monitoring of the effective reproductive number of SARS-CoV-2. eLife 2022, 11, e71345. [Google Scholar] [CrossRef]
- Bsat, R.; Chemaitelly, H.; Coyle, P.; Tang, P.; Hasan, M.R.; Al Kanaani, Z.; Al Kuwari, E.; Butt, A.A.; Jeremijenko, A.; Kaleeckal, A.H.; et al. Characterizing the effective reproduction number during the COVID-19 pandemic: Insights from Qatar’s experience. J. Glob. Health 2022, 12, 05004. [Google Scholar] [CrossRef]
- Bokányi, E.; Vizi, Z.; Koltai, J.; Röst, G.; Karsai, M. Real-time estimation of the effective reproduction number of COVID-19 from behavioral data. Sci. Rep. 2023, 13, 21452. [Google Scholar] [CrossRef]
- Hridoy, A.E.E.; Tipo, I.H.; Sami, M.S.; Babu, M.R.; Ahmed, M.S.; Rahman, S.M.; Tusher, S.M.S.H.; Rashid, K.J.; Naim, M. Spatio-temporal estimation of basic and effective reproduction number of COVID-19 and post-lockdown transmissibility in Bangladesh. Spat. Inf. Res. 2022, 30, 23–35. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdova-Lepe, F.; Gutiérrez-Jara, J.P.; Chowell, G. Influence of the Effective Reproduction Number on the SIR Model with a Dynamic Transmission Rate. Mathematics 2024, 12, 1793. https://doi.org/10.3390/math12121793
Córdova-Lepe F, Gutiérrez-Jara JP, Chowell G. Influence of the Effective Reproduction Number on the SIR Model with a Dynamic Transmission Rate. Mathematics. 2024; 12(12):1793. https://doi.org/10.3390/math12121793
Chicago/Turabian StyleCórdova-Lepe, Fernando, Juan Pablo Gutiérrez-Jara, and Gerardo Chowell. 2024. "Influence of the Effective Reproduction Number on the SIR Model with a Dynamic Transmission Rate" Mathematics 12, no. 12: 1793. https://doi.org/10.3390/math12121793
APA StyleCórdova-Lepe, F., Gutiérrez-Jara, J. P., & Chowell, G. (2024). Influence of the Effective Reproduction Number on the SIR Model with a Dynamic Transmission Rate. Mathematics, 12(12), 1793. https://doi.org/10.3390/math12121793






