1. Introduction
Brain–computer interfaces (BCI) based on EEG signals have flooded scientific research and applications in recent years [
1]. These BCI systems typically allow direct communication between a subject and a surrounding environment without muscle synergy movement and provide specific applications in various research fields. For example, BCIs have been used to diagnose cerebral diseases [
2] and to propose patient treatment [
3]. In addition, BCIs promise to improve the quality of life for many people [
4]. Among BCI paradigms based on EEG signals, Motor Imagery (MI) signals take advantage of having direct social and medical impacts [
5] by improving conditions of people who have lost motor skills, facilitating their independent communications with their surrounding environment [
6]. Brain activity depends on the specific stimulus to which the subject under test is exposed. In particular, Electroencephalography records, invasively or non-invasively, the brain’s electromagnetic activity, i.e., neurons’ activity belonging to a specific area. Moreover, for visual stimulation, Bihan et al. [
7] concluded that the visual cortex is activated in the same way as the mental representation of the same stimulus.
Innovative approaches have been proposed to solve the spatial resolution problem from non-invasive electroencephalography, increasing the number of active electrodes. These approaches intend to cover a larger cortical area obtaining relevant brain activity information [
8]. However, for non-invasive BCI applications based on EEG signals, selecting appropriate electrodes for the targeted brain area is laborious if the equipment does not have built-in options to discriminate channels. For this reason, public EEG databases are developed considering all electrode channels, despite the prior knowledge of the brain zone activated by a specific stimulus.
The following practical challenges have arisen by increasing the number of electrodes for BCI systems:
1. Processing signals from inaccurate electrodes’ location: Basing exclusively on the electrode functions in the 10–20 or 10–10 international system [
9] and neglecting the contribution of non-selected channels for a given task can lead to weak learning of signal features. Thus, there are less efficient results for the application.
2. Learning with noisy samples: Signals from passive electrodes, depending on the specific cognitive activity, are affected by noise, decreasing the performance of the suitable electrode channels. Therefore, signal processing can be computationally expensive and less efficient. In addition, Baig et al. [
10] considered reducing the number of active electrodes between 10 and 30 without losing overall algorithmic performance [
11].
3. Interference between signals from electrodes being too close together: Despite the precautions taken to control the electrode impedance and electromagnetic signal shielding features [
12], another source of interference appears due to uncontrolled electrode closeness between them [
13]. This challenge arises in practice with unconventional or low-tech systems.
Considerable research on EEG signals is still based on the 10–20 system proposed by the international federation for the electrodes placement [
14], despite constructive criticism of its applicability on atypical skulls or specific cases [
15], which also led to the variant 10–10 and 10–5 systems covering all the skull convexity by the electrodes and maximizing the cerebral activity measurements. Recently, motor imagery classification based on EEG channel selection has been developed to deduce the most discriminant channels implied in specific cognitive activities. Methods based on Common Spatial Pattern (CSP) and its variants flood the literature to maximize differences in variance between data labels. Yong et al. [
16] reduced the number of electrodes by 11% on average from 118 electrodes, using a spatial filter based on CSP.
Conversely, Das and Suresh [
11] applied a CSP variant and the effect-size based CSP (E-CSP) to eliminate channels that do not carry useful information, using Cohen’s based effect-size calculation. Likewise, efficient methods for electrode selection were explored using the Genetic Algorithm (GA) [
17], mutual information [
18], improved IterRelCen method built on the relief algorithm [
19], and the modified Sequential Floating Forward Selection (SFFS) [
20]. Additionally, one can gradually increase the number of electrodes to improve classification accuracy [
21].
In summary, two effective ways to select the most discriminant electrode channels for a BCI system based on motor imagery EEG signals are: the measure built on electrode information and the criterion based on the classifier [
10]. The first evaluates data properties like the distance between classes and probabilistic dependency [
22]. The second type uses accuracy metric, error rate, Chi-squared, odds proportion, or probability ratio [
23].
Although
Table 1 specifies the specific brain area activated by a defined stimulus and Deecke’s and Neuper’s works locate imagined and executed limb movements on the somatosensory cortex [
24,
25], various authors experimented on the parallel activation of different brain areas caused by one or more stimuli [
26,
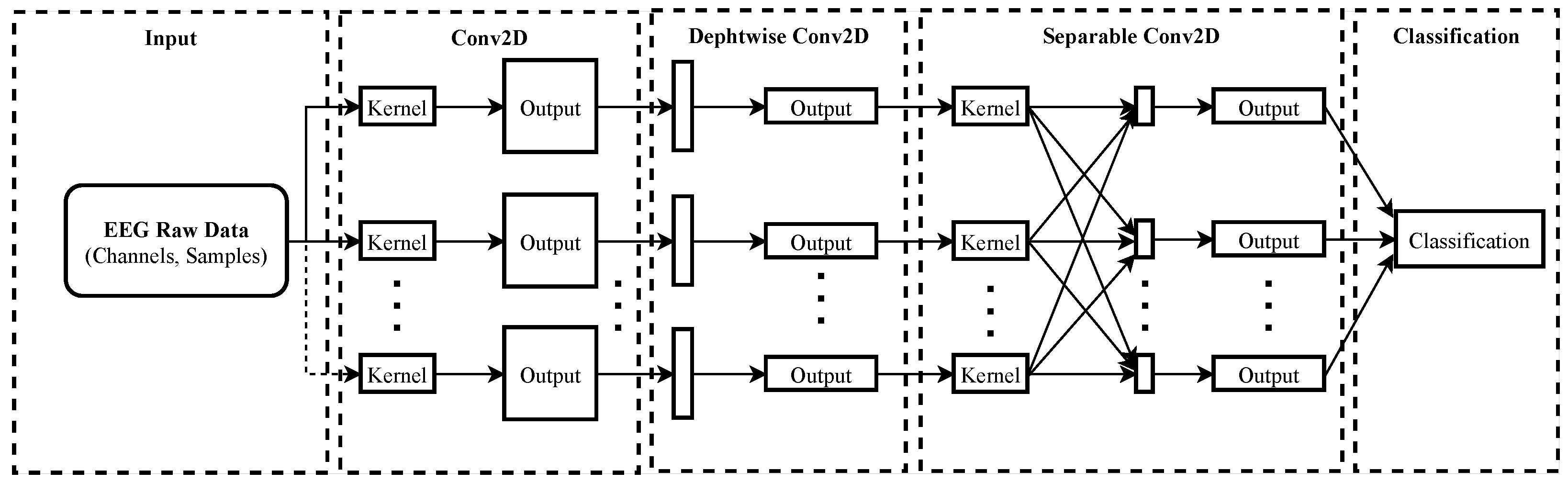
27]. Therefore, the hypothesis that other brain regions than the somatosensory cortex could be activated by fingers’ imagined movements is established in this paper. This work uses a classifier-based evaluation approach to select a discriminant channel subset maximizing the MI-EEG Signals classification using the EEGNet network [
28]. This channel selection strategy is based on a software-level solution using the utility metric [
29] to evaluate the influence of a group of channels on improving classification accuracy. A similar approach was developed by Narayanan and Bertrand in the auditory attention detection with the wireless EEG sensor network (WESN) [
30], using the accuracy as the main outcome metric.
The public dataset proposed in [
32], related to five-fingers MI-EEG signals, was used to analyze the channel contribution before building discriminant channel subsets. Secondly, the channel subset maximizing the classification accuracy using the EEGNet network is compared with channels suggested in the state-of-the-art. The main contributions of this study are summarized as follows:
A subset of discriminant electrode channels is more suitable for individual subject five-finger motor imagery classification.
A practical method to evaluate discriminant channel subsets for BCI systems is provided.
A cyclical learning rate is used in the EEGNet network to process the signal features efficiently and swiftly [
33].
In addition, the classification accuracy achieved by a compact DL technique is used as the BCI channel selection criterion.
This paper is organized as follows:
Section 2 presents the methods used in this work, including the EEG electrodes placement systems, the referred dataset, the proposed algorithm, and the neural network architecture. Then, in
Section 3, the achieved results are discussed and evaluated, and, finally, the conclusions are given in
Section 4.
3. Results and Discussion
The first stage of the proposed algorithm consists of processing each electrode channel independently of the others, to group as many electrodes as possible and, therefore, to maximize the accuracy.
Table 4 presents the results achieved by processing the 19 electrode channels. The top-6 accuracies achieved for each individual subject are indicated in boldface, where the best accuracy is highlighted in blue.
Next, six channels corresponding to the top-6 accuracies were selected to constitute the electrode channel subset for the next algorithm stage. This number of electrodes was chosen accordingly after extensive testing with all channels’ combination, beginning from channels offering two best accuracies per subject and increasing this grouping for three, four, five, and for all best accuracies per subject. Thereby, accuracy curves remained to ascend while more channels were added until the six-channel combination before decreasing.
For
, the highest accuracy was 86.6%, using the F7 channel corresponding to the frontal brain region, curiously dedicated to verbal expression. Therefore, channels Fp1, Fp2, F3, F8, and T5, corresponding to the following top-5 accuracies, are selected to form 2-channel combinations with the F7 channel for the following step. The same procedure was carried out for individual
and
F. For instance, the top-6 accuracies for
correspond to T6, P4, P3, Cz, O1, and T5 channels. Therefore, channels P4, P3, Cz, O1, and T5 are selected to form 2-channel combinations with the T6 channel for the following step.
Table 5 shows the results for the different 2-channel combinations for each subject. It can be observed that the highest classification accuracies are now 90.5%, 81.5%, 82.9%, and 76.6% for
, and
F, respectively.
For , the best results are obtained using channels {F7, Fp2} which correspond to the frontal region. Likewise, for , the best results are obtained using channels {O1, O2} corresponding to the occipital brain region, precisely related to visual processing. Contrariwise, for and C, the best results were obtained for {T6, O1} and {C4, P3}, respectively, corresponding to different brain regions, temporal-occipital for and central-parietal for .
Hence, {F7, Fp2}, {T6, O1}, {C4, P3}, and {O1, O2} combinations are used to form 3-channel combinations with the remaining channels ({Fp1, F8, F3, T5}, {T5, Cz, P3, P4}, {F8, T5, O1, T6} and {T5, P3, C3, P4}), for the next step.
Table 6 presents classification accuracies achieved for each 3-channel combination and subject.
achieved the highest accuracy of 90.8% with {F7, Fp2, T5} channel combination signals,
an accuracy of 83.8% with signals from {T6, O1, Cz} channels combination. With {C4, P3, T6} channel combination signals,
achieved an accuracy of 85.6%, while an accuracy of 79.3% was found with {O1, O2, P3} channel combination signals of
. Therefore, those electrode channel combinations are used to form 4-channel combinations for the next step.
Table 7 presents the results for each 4-channel combination and subject. The highest accuracies are now 91.7%, 85.1%, 88.5%, and 80.1% for
,
B,
C, and
F, respectively.
Table 8 shows the results for each 5-channel combination and subject. The highest accuracies change now to 92.8%, 86.3%, 88.6%, and 80.8% for
,
B,
C and
F, respectively.
Table 9 presents the results for 6-channel combinations. The best accuracies are 93.1%, 87.2%, 90.3%, and 81.0% for subjects A, B, C, and F, respectively.
Figure 4 shows the classification accuracies as a function of the number of channels. Beyond six channels, the curves begin to decrease.
Table 10 presents the gain in classification accuracy by adding selective channels according to Algorithm 1. For
and
F, the best accuracy was obtained for channels {T6, O1, Cz, P4, P3, T5} and {O1, O2, P3, C3, T5, P4}.
For , the best accuracy was achieved using channels {C4, P3, T6, T5, O1, F8}.
By methodically adding channels according to Algorithm 1, the evolution of the classification accuracy reaches a maximum that defines the optimal number of channels.
Figure 5 illustrates the discriminant channel subsets obtained by using the proposed algorithm. As established in the hypothesis, the parietal, temporal, visual, and motor cerebral cortices are stimulated by imaginary finger movements depending on the test subject.
performs a classification accuracy with the frontal and parietal cortices activated. For
and
F, the parietal, motor, and temporal cerebral cortices are activated against the stimulation of all cortices for
. Each increase in the number of channels generates an accuracy gain, see
Table 10.
Table 11 summarizes the optimal channel combinations depending on the desired number of channels.
Table 12 compares the results achieved using the proposed algorithm and other state-of-the-art approaches [
37,
38], where signals from the {C3, Cz, P3, Pz} channel subset or all channels were selected to be processed. In such studies, raw EEG signals were preprocessed using the Empirical Mode Decomposition (EMD) and Common Spatial Pattern (CSP) methods. In [
39], Alomari et al. selected {C3, C4, Cz} EEG channel subset to discriminate right-left imagined and executed fists movements based on Deecke’s and Neuper’s works [
24,
25]. Similarly, O1 and O2 electrodes were evaluated as discriminant by Zhou et al. [
40], in the implementation of a driving car brain–computer interfaces, using EEG signals of visual-motor imagery preprocessed by the Hilbert–Huang Transform. The results obtained in this work satisfactorily prove a classification accuracy improvement compared to the state-of-the-art, which uses wrong or all electrodes, as shown in
Table 12.