Comprehensive Integrated Analyses of Proteins and Metabolites in Equine Seminal Plasma (Horses and Donkeys)
Abstract
1. Introduction
2. Materials and Methods
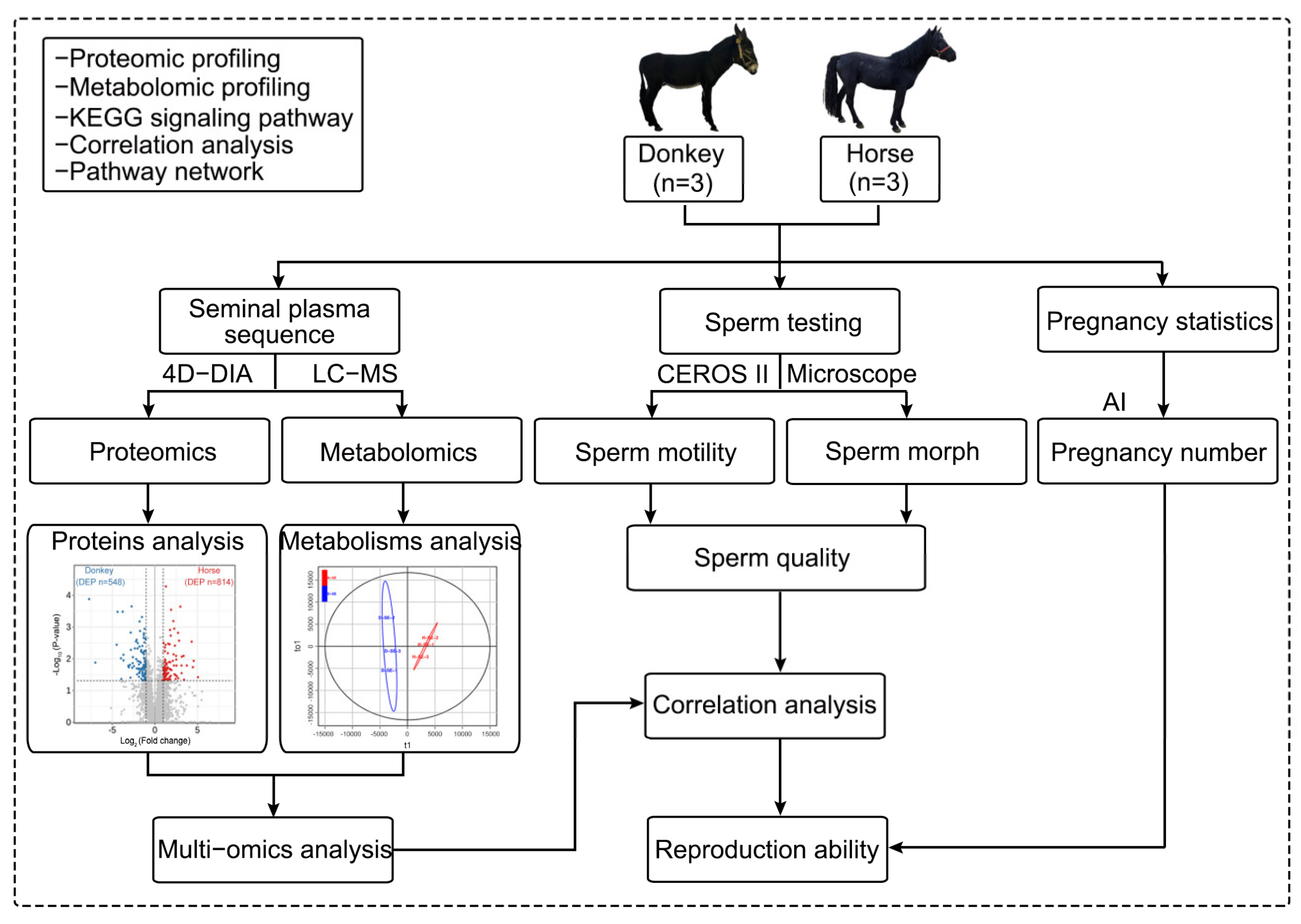
2.1. Experimental Design
2.2. Experimental Animals
2.3. Seminal Plasma Collection
2.4. Sperm Assessment
2.5. Artificial Insemination of Female Livestock
2.6. Proteomics and LC-MS/MS
2.7. Proteomic Data Analysis
2.8. Metabolomics and LC-MS
2.9. Metabolomic Data Analysis
2.10. Combined Proteomics and Metabolomic Analysis
2.11. PPI and Correlation Matrix
2.12. Statistical Analysis
3. Results
3.1. Testing of Horse and Donkey Sperm Quality
3.2. Testing of Horse and Donkey Fertility
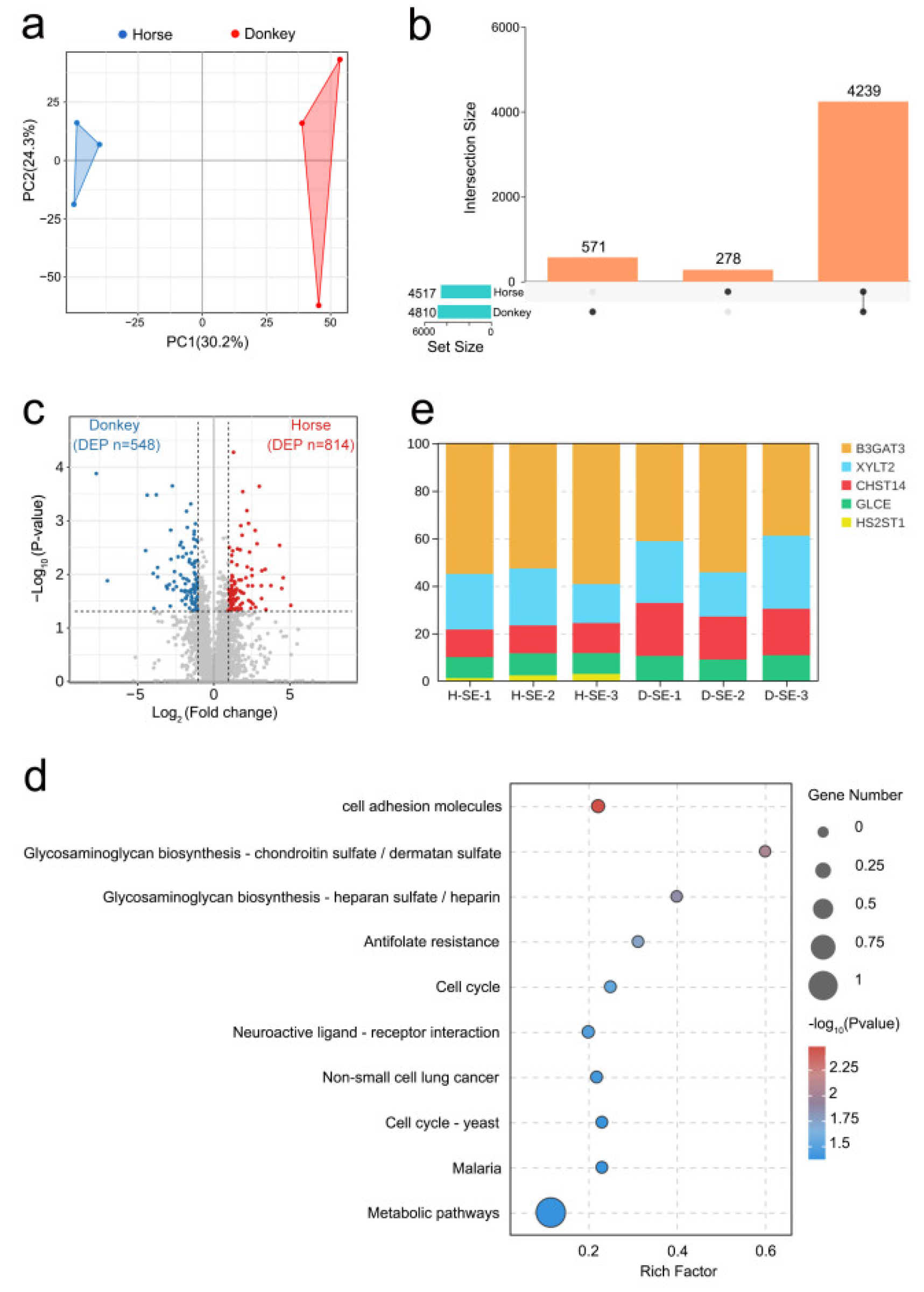
3.3. Proteomics Analysis of Horse and Donkey Seminal Plasma
3.4. Metabolomics Analysis of Horse and Donkey Seminal Plasma
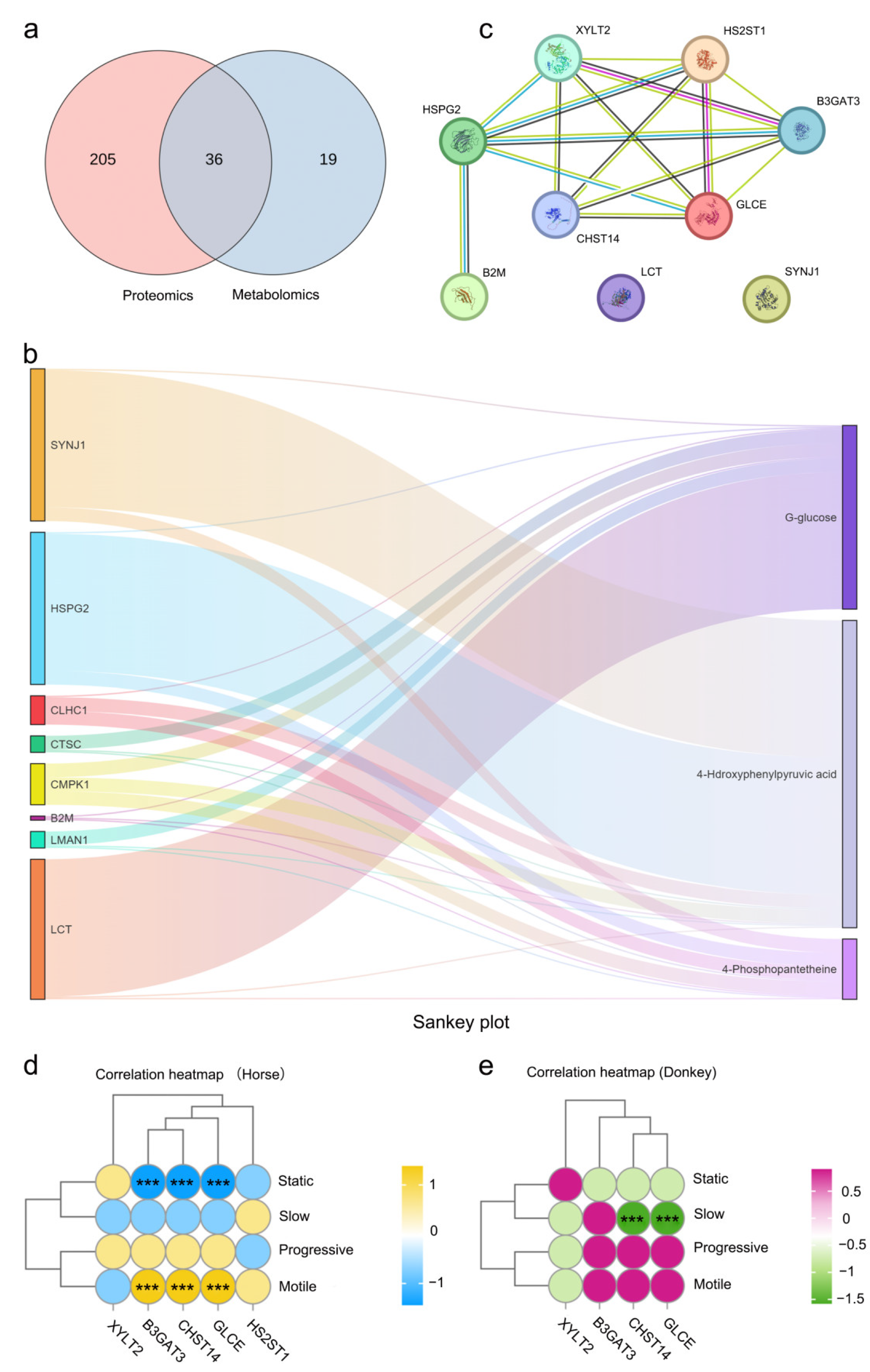
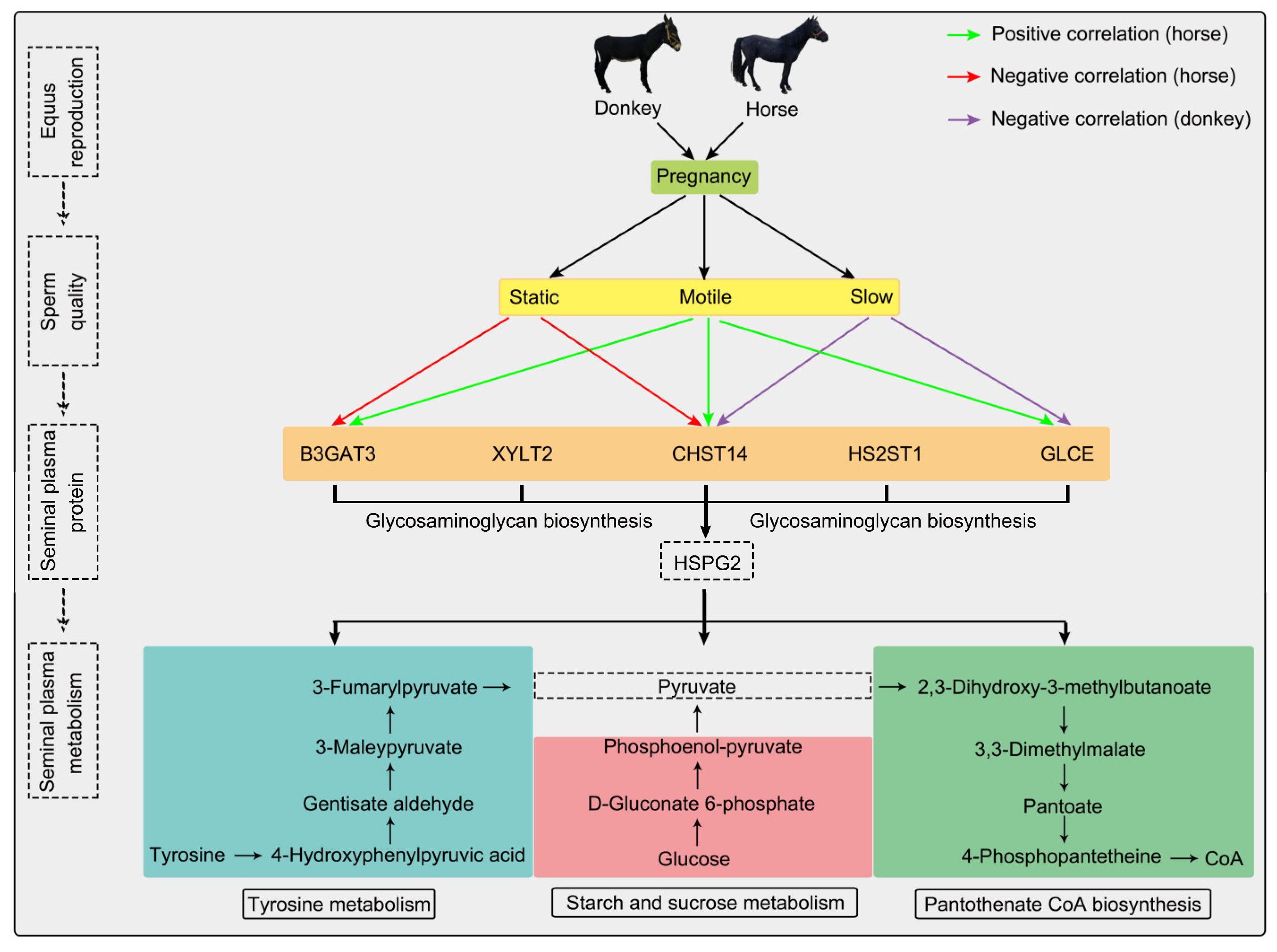
3.5. Integrated Proteomics and Metabolomics Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, G.H.; Makarewicz, C.A. Horse Paleogenomes and Human-Animal Interactions in Prehistory. Trends Genet. TIG 2019, 35, 473–475. [Google Scholar] [CrossRef]
- Parsad, R.; Bagiyal, M.; Ahlawat, S.; Arora, R.; Gera, R.; Chhabra, P.; Sharma, U. Unraveling the genetic and physiological potential of donkeys: Insights from genomics, proteomics, and metabolomics approaches. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2024, 36, 10–24. [Google Scholar] [CrossRef]
- Marlin, D.J.; Schroter, R.C.; White, S.L.; Maykuth, P.; Matthesen, G.; Mills, P.C.; Waran, N.; Harris, P. Recovery from transport and acclimatisation of competition horses in a hot humid environment. Equine Vet. J. 2001, 33, 371–379. [Google Scholar] [CrossRef]
- Rzekęć, A.; Vial, C.; Bigot, G. Green Assets of Equines in the European Context of the Ecological Transition of Agriculture. Animals 2020, 10, 106. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, M.; Ma, Z.; Zhang, Y.; Cao, H. Species-specific identification of donkey-hide gelatin and its adulterants using marker peptides. PLoS ONE 2022, 17, e0273021. [Google Scholar] [CrossRef]
- Prasinou, P.; De Amicis, I.; Fusaro, I.; Bucci, R.; Cavallini, D.; Parrillo, S.; Caputo, M.; Gramenzi, A.; Carluccio, A. The Lipidomics of Spermatozoa and Red Blood Cells Membrane Profile of Martina Franca Donkey: Preliminary Evaluation. Animals 2022, 13, 8. [Google Scholar] [CrossRef]
- Hernández-Avilés, C.; Ramírez-Agámez, L.; Varner, D.D.; Love, C.C. The stallion sperm acrosome: Considerations from a research and clinical perspective. Theriogenology 2023, 196, 121–149. [Google Scholar] [CrossRef]
- Fusaro, I.; Parrillo, S.; Buonaiuto, G.; Prasinou, P.; Gramenzi, A.; Bucci, R.; Cavallini, D.; Carosi, A.; Carluccio, A.; De Amicis, I. Effects of hemp-based polyunsaturated fatty acid supplementation on membrane lipid profiles and reproductive performance in Martina Franca jacks. Front. Vet. Sci. 2025, 12, 1553218. [Google Scholar] [CrossRef]
- Ren, H.; Wen, X.; He, Q.; Yi, M.; Dugarjaviin, M.; Bou, G. Comparative Study on the Sperm Proteomes of Horses and Donkeys. Animals 2024, 14, 2237. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef]
- Wang, F.; Yang, W.; Ouyang, S.; Yuan, S. The Vehicle Determines the Destination: The Significance of Seminal Plasma Factors for Male Fertility. Int. J. Mol. Sci. 2020, 21, 8499. [Google Scholar] [CrossRef]
- Juyena, N.S.; Stelletta, C. Seminal plasma: An essential attribute to spermatozoa. J. Androl. 2012, 33, 536–551. [Google Scholar] [CrossRef]
- Colenbrander, B.; Gadella, B.M.; Stout, T.A. The predictive value of semen analysis in the evaluation of stallion fertility. Reprod. Domest. Anim. = Zuchthyg. 2003, 38, 305–311. [Google Scholar] [CrossRef]
- Samper, J.C.; Hernandez Aviles, J.C.; Ramirez-Agamez, L.F.; Love, C.C.; Gonzalez-Marin, C.; Fleury, P.; Dini, P.; De La Fuente, A.; Foss, R.; Campos, F.L.; et al. The use of sex-sorted semen in horses. J. Equine Vet. Sci. 2024, 145, 105251. [Google Scholar] [CrossRef]
- Villa-Duque, N.; Agudelo-Flórez, J.J.; Terraza-Martinez, R.; Romero-Cárdenas, E.; Gómez, G.; Valencia, J. NPC2, a seminal plasma protein with membrane cholesterol-binding ability, influences the cryotolerance and functionality of spermatozoa from Chino Santandereano bulls. Anim. Reprod. Sci. 2025, 274, 107758. [Google Scholar] [CrossRef]
- Giaretta, E.; Damato, A.; Zennaro, L.; Bonfatti, V.; Mislei, B.; Vigolo, V.; Falomo, M.E.; Bertuzzo, F.; Gabai, G.; Bucci, D. Metabolome and oxidative stress markers in the seminal plasma of Holstein bulls and their relationship with the characteristics of fresh and frozen/thawed sperm. Theriogenology 2025, 235, 262–274. [Google Scholar] [CrossRef]
- Calderón-Celis, F.; Encinar, J.R.; Sanz-Medel, A. Standardization approaches in absolute quantitative proteomics with mass spectrometry. Mass Spectrom. Rev. 2018, 37, 715–737. [Google Scholar] [CrossRef]
- Gomes, F.P.; Yates, J.R., 3rd. Recent trends of capillary electrophoresis-mass spectrometry in proteomics research. Mass Spectrom. Rev. 2019, 38, 445–460. [Google Scholar] [CrossRef]
- Pflieger, D.; Gonnet, F.; de la Fuente van Bentem, S.; Hirt, H.; de la Fuente, A. Linking the proteins—Elucidation of proteome-scale networks using mass spectrometry. Mass Spectrom. Rev. 2011, 30, 268–297. [Google Scholar] [CrossRef]
- Wang, D.; Song, J.; Wang, J.; Quan, R. Quantitative proteomic analysis of PK-15 cells infected with porcine circovirus type 3 using 4D-DIA approach. Vet. Res. Commun. 2024, 48, 3593–3603. [Google Scholar] [CrossRef]
- Moura, A.A.; Memili, E.; Portela, A.M.R.; Viana, A.G.; Velho, A.L.C.; Bezerra, M.J.B.; Vasconselos, F.R. Seminal plasma proteins and metabolites: Effects on sperm function and potential as fertility markers. Anim. Reprod. 2018, 15 (Suppl. S1), 691–702. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, Y.; Guo, C.; Li, C.; Wei, H.; Li, L.; Meng, L.; Zhang, S. Analysis of differentially abundant proteins related to boar fertility in seminal plasma using iTRAQ-based quantitative proteomics. J. Proteom. 2021, 236, 104120. [Google Scholar] [CrossRef]
- Mandal, V.; Ajabiya, J.; Khan, N.; Tekade, R.K.; Sengupta, P. Advances and challenges in non-targeted analysis: An insight into sample preparation and detection by liquid chromatography-mass spectrometry. J. Chromatogr. A 2024, 1737, 465459. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Carpena, M.; Garcia-Oliveira, P.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Analytical Metabolomics and Applications in Health, Environmental and Food Science. Crit. Rev. Anal. Chem. 2022, 52, 712–734. [Google Scholar] [CrossRef]
- Ashrafian, H.; Sounderajah, V.; Glen, R.; Ebbels, T.; Blaise, B.J.; Kalra, D.; Kultima, K.; Spjuth, O.; Tenori, L.; Salek, R.M.; et al. Metabolomics: The Stethoscope for the Twenty-First Century. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2021, 30, 301–310. [Google Scholar] [CrossRef]
- Michalek, K.; Oberska, P.; Malkowska, P.; Bartkiene, E. In seaarch of new potential markers for male fertility and semen quality control. Aquaporins in reproductive system and metabolomic profiling of semen. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2021, 72, 309–319. [Google Scholar]
- Mateo-Otero, Y.; Fernández-López, P.; Delgado-Bermúdez, A.; Nolis, P.; Roca, J.; Miró, J.; Barranco, I.; Yeste, M. Metabolomic fingerprinting of pig seminal plasma identifies in vivo fertility biomarkers. J. Anim. Sci. Biotechnol. 2021, 12, 113. [Google Scholar] [CrossRef]
- Catalán, J.; Yánez-Ortiz, I.; Martínez-Rodero, I.; Mateo-Otero, Y.; Nolis, P.; Yeste, M.; Miró, J. Comparison of the metabolite profile of donkey and horse seminal plasma and its relationship with sperm viability and motility. Res. Vet. Sci. 2023, 165, 105046. [Google Scholar] [CrossRef]
- Canisso, I.F.; Carvalho, G.R.; Morel, M.C.; Guimarães, J.D.; McDonnell, S.M. Sexual behavior and ejaculate characteristics in Pêga donkeys (Equus asinus) mounting estrous horse mares (Equus caballus). Theriogenology 2010, 73, 56–63. [Google Scholar] [CrossRef]
- Gobato, M.L.M.; Segabinazzi, L.; Scheeren, V.F.C.; Bandeira, R.S.; Freitas-Dell’Aqua, C.P.; Dell’Aqua, J.A., Jr.; Papa, F.O. Ability of donkey sperm to tolerate cooling: Effect of extender base and removal of seminal plasma on sperm parameters and fertility rates in mares. Front. Vet. Sci. 2022, 9, 1011899. [Google Scholar] [CrossRef]
- Medica, A.J.; Aitken, R.J.; Nicolson, G.L.; Sheridan, A.R.; Swegen, A.; De Iuliis, G.N.; Gibb, Z. Glycerophospholipids protect stallion spermatozoa from oxidative damage in vitro. Reprod. Fertil. 2021, 2, 199–209. [Google Scholar] [CrossRef]
- Gacem, S.; Valverde, A.; Catalán, J.; Yánez Ortiz, I.; Soler, C.; Miró, J. A New Approach of Sperm Motility Subpopulation Structure in Donkey and Horse. Front. Vet. Sci. 2021, 8, 651477. [Google Scholar] [CrossRef]
- Gacem, S.; Catalán, J.; Yánez-Ortiz, I.; Soler, C.; Miró, J. New Sperm Morphology Analysis in Equids: Trumorph® Vs Eosin-Nigrosin Stain. Vet. Sci. 2021, 8, 79. [Google Scholar] [CrossRef]
- Aitken, R.J.; Lambourne, S.; Medica, A.J. Predicting the outcome of Thoroughbred stallion matings on the basis of dismount semen sample analyses. Reproduction 2023, 165, 281–288. [Google Scholar] [CrossRef]
- Fanelli, D.; Moroni, R.; Bocci, C.; Camillo, F.; Rota, A.; Panzani, D. Interspecific and Intraspecific Artificial Insemination in Domestic Equids. Animals 2023, 13, 582. [Google Scholar] [CrossRef]
- Mahé, C.; Pranomphon, T.; Reynaud, K.; Laffont, L.; Meylheuc, T.; Schoen, J.; Mermillod, P.; Saint-Dizier, M. Sperm-fluid-cell interplays in the bovine oviduct: Glycosaminoglycans modulate sperm binding to the isthmic reservoir. Sci. Rep. 2023, 13, 10311. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Ali, M.A.; Qin, Z.; Guo, Z.; Zhang, Y.; Zhang, M.; Zhou, G.; Yang, J.; Chen, L.; et al. Odorant Receptor OR2C1 Is an Essential Modulator of Boar Sperm Capacitation by Binding with Heparin. Int. J. Mol. Sci. 2023, 24, 1664. [Google Scholar] [CrossRef]
- do Nascimento, C.C.; Aguiar, O.; Viana, G.M.; Vânia, D.A. Evidence that glycosaminoglycan storage and collagen deposition in the cauda epididymidis does not impair sperm viability in the Mucopolysaccharidosis type I mouse model. Reprod. Fertil. Dev. 2020, 32, 304–312. [Google Scholar] [CrossRef]
- Mor, V.; Das, T.; Bhattacharjee, M.; Chatterjee, T. Protein tyrosine phosphorylation of a heparin-binding sperm membrane mitogen (HBSM) is associated with capacitation and acrosome reaction. Biochem. Biophys. Res. Commun. 2007, 352, 404–409. [Google Scholar] [CrossRef]
- Dapino, D.; Teijeiro, J.; Cane, F.; Marini, P.E. Heparin binding analysis of boar sperm and its relation with farrowing capacity. Acta Vet. Hung. 2014, 62, 96–105. [Google Scholar] [CrossRef]
- Wu, A.; Anupriwan, A.; Iamsaard, S.; Chakrabandhu, K.; Santos, D.C.; Rupar, T.; Tsang, B.K.; Carmona, E.; Tanphaichitr, N. Sperm surface arylsulfatase A can disperse the cumulus matrix of cumulus oocyte complexes. J. Cell. Physiol. 2007, 213, 201–211. [Google Scholar] [CrossRef]
- Mogielnicka-Brzozowska, M.; Cichowska, A.W. Molecular Biomarkers of Canine Reproductive Functions. Curr. Issues Mol. Biol. 2024, 46, 6139–6168. [Google Scholar] [CrossRef]
- Metzler-Guillemain, C.; Victorero, G.; Lepoivre, C.; Bergon, A.; Yammine, M.; Perrin, J.; Sari-Minodier, I.; Boulanger, N.; Rihet, P.; Nguyen, C. Sperm mRNAs and microRNAs as candidate markers for the impact of toxicants on human spermatogenesis: An application to tobacco smoking. Syst. Biol. Reprod. Med. 2015, 61, 139–149. [Google Scholar] [CrossRef]
- Habicher, J.; Haitina, T.; Eriksson, I.; Holmborn, K.; Dierker, T.; Ahlberg, P.E.; Ledin, J. Chondroitin/dermatan sulfate modification enzymes in zebrafish development. PLoS ONE 2015, 10, e0121957. [Google Scholar] [CrossRef]
- Eriksen, G.V.; Malmström, A.; Uldbjerg, N.; Huszar, G. A follicular fluid chondroitin sulfate proteoglycan improves the retention of motility and velocity of human spermatozoa. Fertil. Steril. 1994, 62, 618–623. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Sedo, C.A.; Julianelli, V.L.; Romanato, M.; Calvo, L.; Calvo, J.C.; Fontana, V.A. Dermatan sulfate synergizes with heparin in murine sperm chromatin decondensation. Syst. Biol. Reprod. Med. 2013, 59, 82–90. [Google Scholar] [CrossRef]
- Qin, Y.; Ke, J.; Gu, X.; Fang, J.; Wang, W.; Cong, Q.; Li, J.; Tan, J.; Brunzelle, J.S.; Zhang, C.; et al. Structural and functional study of D-glucuronyl C5-epimerase. J. Biol. Chem. 2015, 290, 4620–4630. [Google Scholar] [CrossRef]
- Bazzano, M.; Zhu, C.; Laus, F.; Giambattista, A.D.; Laghi, L. Exploring the metabolome of seminal plasma in two different horse types: Light versus draft stallions. Reprod. Domest. Anim. = Zuchthyg. 2023, 58, 109–116. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Xin, S.; Ouyang, Q.; Li, J.; Zhu, L.; Hu, J.; He, H.; Liu, H.; Li, L.; et al. Genome sequencing of drake semen micobiome with correlation with their compositions, sources and potential mechanisms affecting semen quality. Poult. Sci. 2024, 103, 103533. [Google Scholar] [CrossRef]
- Calvert, S.J.; Reynolds, S.; Paley, M.N.; Walters, S.J.; Pacey, A.A. Probing human sperm metabolism using 13C-magnetic resonance spectroscopy. Mol. Hum. Reprod. 2019, 25, 30–41. [Google Scholar] [CrossRef]
- Wang, K.; Jiao, H.; Cheng, X.; Zhang, L.; Zhang, S.; Liu, G.; Meng, F.; Zhan, F.; Yang, F. Proteomic Analysis of Differences in the Freezability of Porcine Sperm Identifies α-Amylase As a Key Protein. J. Proteome Res. 2024, 23, 2641–2650. [Google Scholar] [CrossRef]
- Sousa, S.D.; Lucini, L.; Ajmone-Marsan, P.; van Tilburg, M.F.; Moura, A.A. Untargeted metabolomic profiling of accessory sex gland fluid from Morada Nova rams. Mol. Reprod. Dev. 2020, 87, 409–418. [Google Scholar] [CrossRef]
- Rambhatla, A.; Strug, M.R.; De Paredes, J.G.; Cordoba Munoz, M.I.; Thakur, M. Fertility considerations in targeted biologic therapy with tyrosine kinase inhibitors: A review. J. Assist. Reprod. Genet. 2021, 38, 1897–1908. [Google Scholar] [CrossRef]
- Dahan, T.; Breitbart, H. Involvement of metabolic pathway in the sperm spontaneous acrosome reaction. Theriogenology 2022, 192, 38–44. [Google Scholar] [CrossRef]
- Barranco, I.; Spinaci, M.; Nesci, S.; Mateo-Otero, Y.; Baldassarro, V.A.; Algieri, C.; Bucci, D.; Roca, J. Seminal extracellular vesicles alter porcine in vitro fertilization outcome by modulating sperm metabolism. Theriogenology 2024, 219, 167–179. [Google Scholar] [CrossRef]
- de Andrade, A.F.; Zaffalon, F.G.; Celeghini, E.C.; Nascimento, J.; Bressan, F.F.; Martins, S.M.; de Arruda, R.P. Post-thaw addition of seminal plasma reduces tyrosine phosphorylation on the surface of cryopreserved equine sperm, but does not reduce lipid peroxidation. Theriogenology 2012, 77, e1861–e1863. [Google Scholar] [CrossRef]
- Griffin, R.A.; Swegen, A.; Baker, M.A.; Ogle, R.A.; Smith, N.; Aitken, R.J.; Skerrett-Byrne, D.A.; Fair, S.; Gibb, Z. Proteomic analysis of spermatozoa reveals caseins play a pivotal role in preventing short-term periods of subfertility in stallions†. Biol. Reprod. 2022, 106, 741–755. [Google Scholar] [CrossRef]
- Kalaiarasan, P.; Subbarao, N.; Bamezai, R.N. Molecular simulation of Tyr105 phosphorylated pyruvate kinase M2 to understand its structure and dynamics. J. Mol. Model. 2014, 20, 2447. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Glycolysis: A multifaceted metabolic pathway and signaling hub. J. Biol. Chem. 2024, 300, 107906. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Bioenergetics of mammalian sperm capacitation. BioMed Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef]
- Ramírez-Agámez, L.; Hernández-Avilés, C.; Ortíz, I.; Love, C.C.; Varner, D.D.; Hinrichs, K. Lactate as the sole energy substrate induces spontaneous acrosome reaction in viable stallion spermatozoa. Andrology 2024, 12, 459–471. [Google Scholar] [CrossRef]
- Wen, X.; Ren, H.; He, Q.; Yi, M.; Ulaangerel, T.; Bou, G. Comparative Analysis of Proteomic Characteristics in Seminal Plasma Between Horses and Donkeys. Animals 2025, 15, 1532. [Google Scholar] [CrossRef]

| Project | Parameter | Horse | Donkey |
|---|---|---|---|
| Sperm motility | Static | 31.64 ± 6.62 | 9.90 ± 0.63 ** |
| Progressive | 35.07 ± 1.76 | 71.57 ± 6.21 ** | |
| Motile | 68.37 ± 6.62 | 88.77 ± 2.92 ** | |
| Slow | 12.17 ± 4.68 | 1.77 ± 0.71 * | |
| Sperm morph | Normal morph | 77.14 ± 15.76 | 91.87 ± 7.70 |
| Bent tail | 4.47 ± 2.25 | 2.64 ± 0.93 | |
| Coiled tail | 0.90 ± 0.63 | 0.74 ± 0.71 |
| Project | Species | Number of Matings | Number of Pregnancies | Pregnancy Rate |
|---|---|---|---|---|
| Reproductive ability | Horse | 37 | 25 | 67.57% |
| 29 | 21 | 72.42% | ||
| 35 | 23 | 65.72% | ||
| Donkey | 6 | 4 | 66.67% | |
| 40 | 30 | 75.00% | ||
| 30 | 23 | 76.67% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Bou, G.; He, Q.; Liu, Q.; Yi, M.; Ren, H. Comprehensive Integrated Analyses of Proteins and Metabolites in Equine Seminal Plasma (Horses and Donkeys). Proteomes 2025, 13, 33. https://doi.org/10.3390/proteomes13030033
Wen X, Bou G, He Q, Liu Q, Yi M, Ren H. Comprehensive Integrated Analyses of Proteins and Metabolites in Equine Seminal Plasma (Horses and Donkeys). Proteomes. 2025; 13(3):33. https://doi.org/10.3390/proteomes13030033
Chicago/Turabian StyleWen, Xin, Gerelchimeg Bou, Qianqian He, Qi Liu, Minna Yi, and Hong Ren. 2025. "Comprehensive Integrated Analyses of Proteins and Metabolites in Equine Seminal Plasma (Horses and Donkeys)" Proteomes 13, no. 3: 33. https://doi.org/10.3390/proteomes13030033
APA StyleWen, X., Bou, G., He, Q., Liu, Q., Yi, M., & Ren, H. (2025). Comprehensive Integrated Analyses of Proteins and Metabolites in Equine Seminal Plasma (Horses and Donkeys). Proteomes, 13(3), 33. https://doi.org/10.3390/proteomes13030033








