The Low-Abundance Plasma Proteome Reveals Differentially Abundant Proteins Associated with Breast Implant Capsular Contracture: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
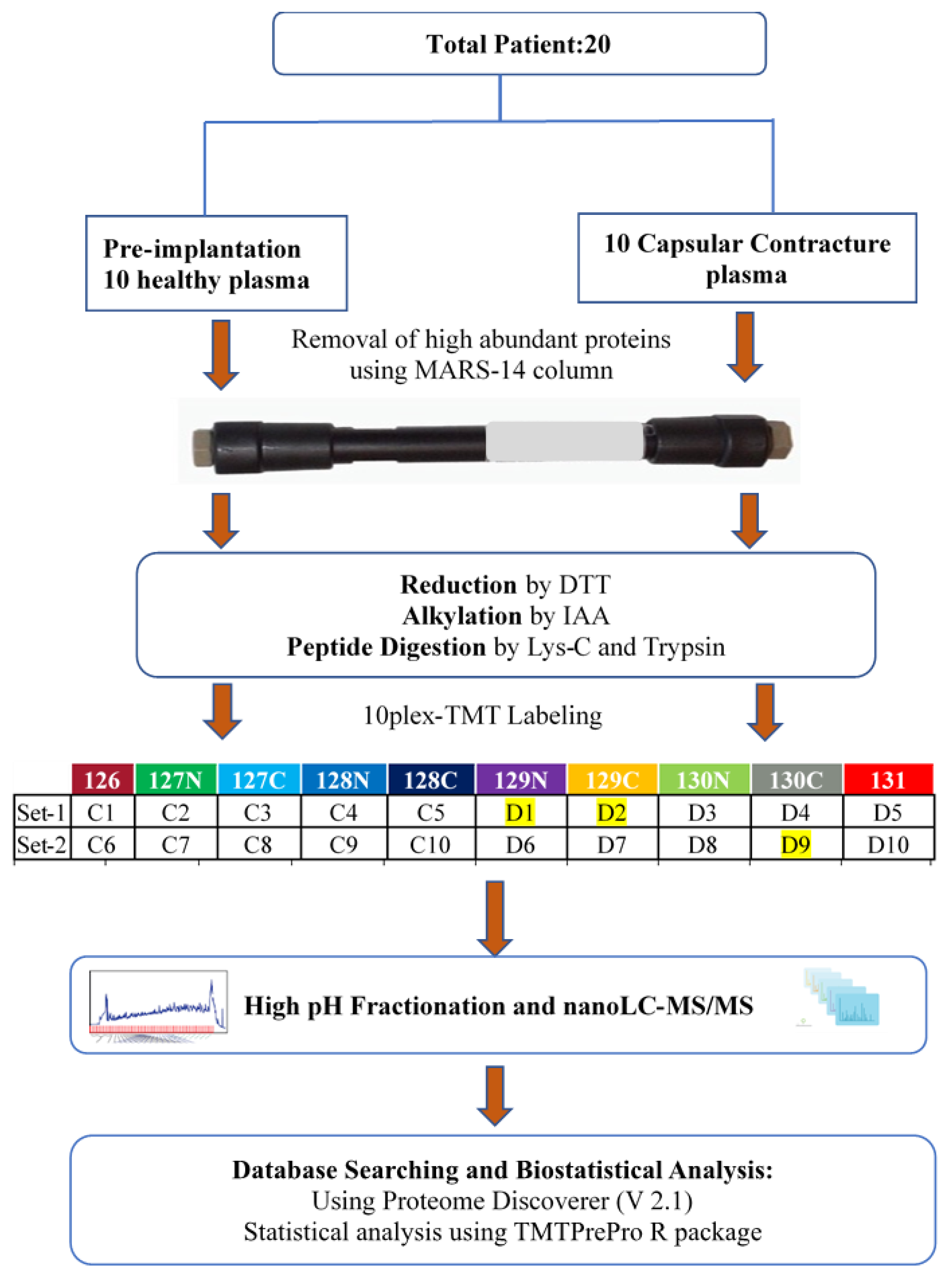
2.1. Patients and Ethics
2.2. Collection and Processing of Human Plasma Samples
2.3. Depletion of High-Abundance Human Plasma Proteins
2.4. Protein Reduction, Alkylation, and Digestion
2.5. TMT Labelling and High pH Fractionation
2.6. Nanoflow LC-ESI-MS/MS Using Q Exactive
2.7. Database Search, Statistical Analysis, and Bioinformatics
3. Results
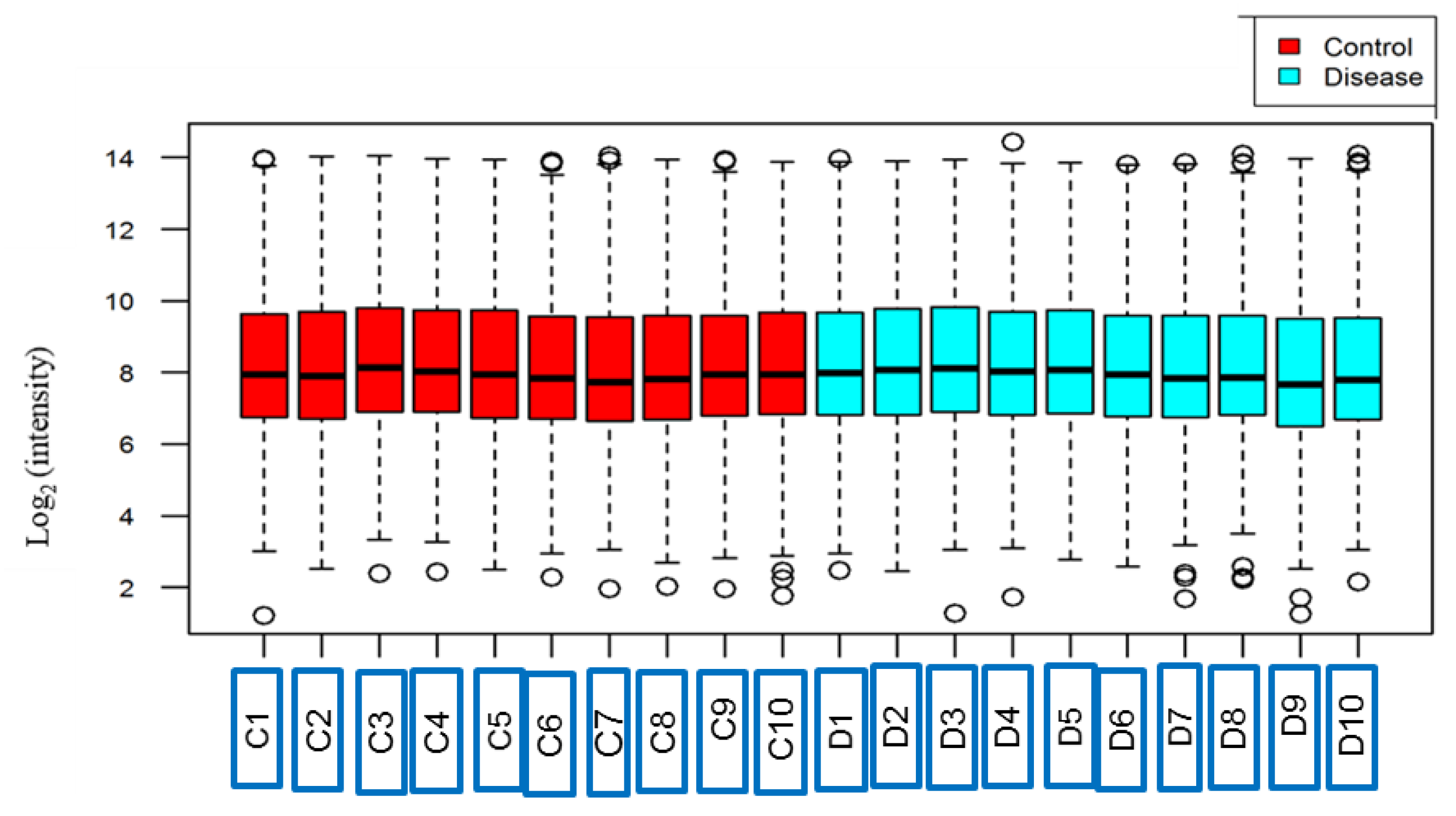
3.1. Protein Identification
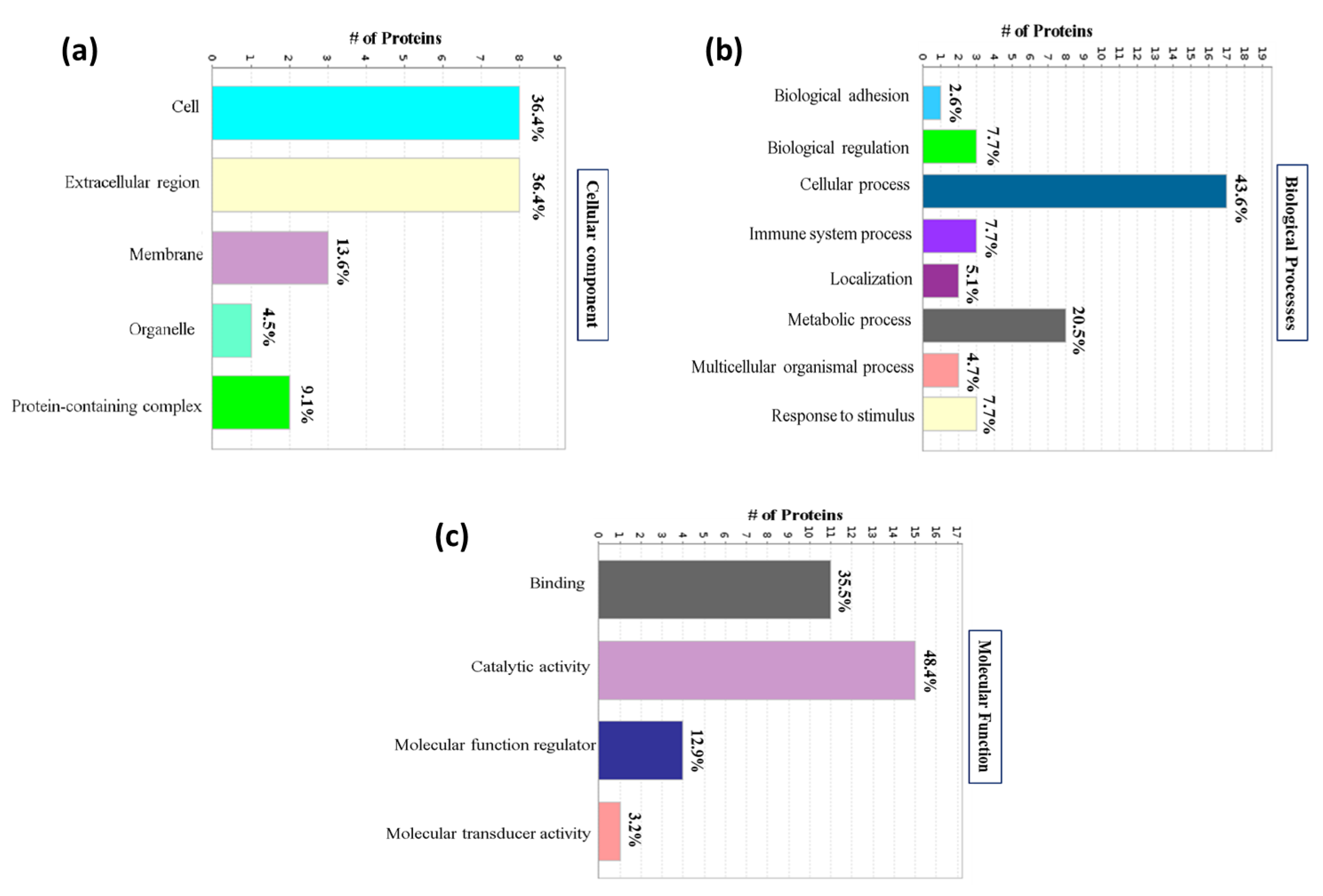
3.2. Gene Ontology Analysis of Identified Proteins
3.3. KEGG Pathway and Protein–Protein Interaction Analysis of the Identified Proteins
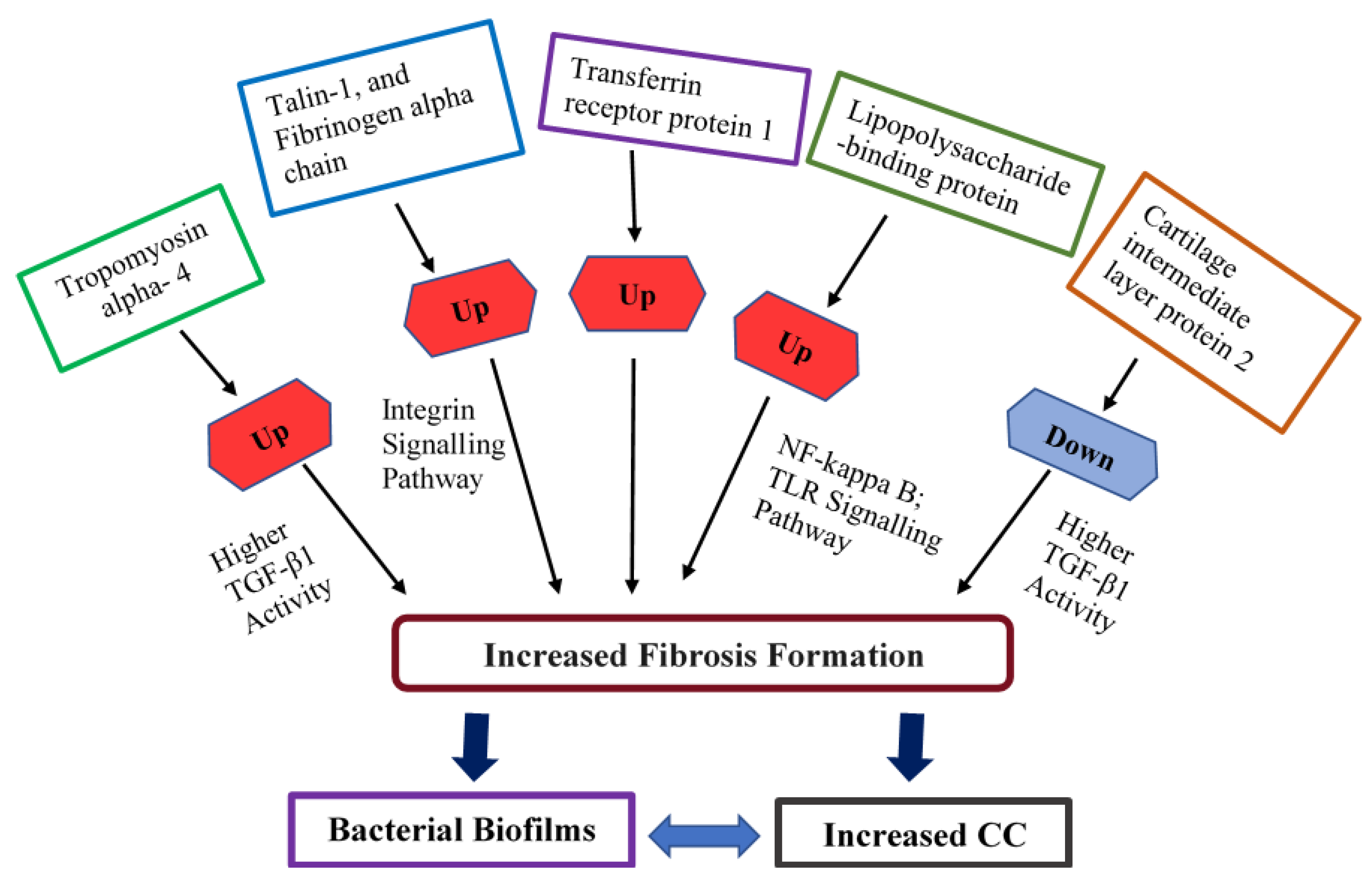
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conte, M.P.; Superti, F.; Moio, M.; Ammendolia, M.G.; Longhi, C.; Aleandri, M.; Marazzato, M.; Goldoni, P.; Parisi, P.; Borab, Z. Bacterial biofilm associated with a case of capsular contracture. New Microbiol. 2018, 41, 238–241. [Google Scholar] [PubMed]
- Ajdic, D.; Zoghbi, Y.; Gerth, D.; Panthaki, Z.J.; Thaller, S. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthetic Surg. J. 2016, 36, 297–309. [Google Scholar]
- McCurdy, J.A. Capsular contracture following augmentation mammaplasty: Etiology and pathogenesis. In Breast Augmentation; Springer: Berlin/Heidelberg, Germany, 2009; pp. 525–540. [Google Scholar]
- Araco, A.; Caruso, R.; Araco, F.; Overton, J.; Gravante, G. Capsular contractures: A systematic review. Plast. Reconstr. Surg. 2009, 124, 1808–1819. [Google Scholar] [PubMed]
- Tran, N.V.; Del Pozo, J.L.; Petty, P.M.; Johnson, C.H.; Walsh, M.F.; Bite, U.; Clay, R.P.; Mandrekar, J.N.; Piper, K.E.; Steckelberg, J.M. Bacteria on breast implants are associated with capsular contracture. Plast. Reconstr. Surg. 2009, 124, 38–39. [Google Scholar]
- Snell, L.; Brown, M. Breast implant capsules and subclinical infection. Plast. Reconstr. Surg. 2009, 124, 38. [Google Scholar]
- Handel, N.; Cordray, T.; Gutierrez, J.; Jensen, J.A. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast. Reconstr. Surg. 2006, 117, 757–767. [Google Scholar] [PubMed]
- Tamboto, H.; Vickery, K.; Deva, A.K. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast. Reconstr. Surg. 2010, 126, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Rieger, U.; Mesina, J.; Kalbermatten, D.; Haug, M.; Frey, H.; Pico, R.; Frei, R.; Pierer, G.; Lüscher, N.; Trampuz, A. Bacterial biofilms and capsular contracture in patients with breast implants. Br. J. Surg. 2013, 100, 768–774. [Google Scholar] [PubMed]
- Rieger, U.M.; Pierer, G.; Luscher, N.J.; Trampuz, A. Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plast. Surg. 2009, 33, 404–408. [Google Scholar] [CrossRef]
- Hu, H.; Jacombs, A.; Vickery, K.; Merten, S.L.; Pennington, D.G.; Deva, A.K. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: Implications for breast implant–associated lymphoma. Plast. Reconstr. Surg. 2015, 135, 319–329. [Google Scholar]
- Chessa, D.; Ganau, G.; Spiga, L.; Bulla, A.; Mazzarello, V.; Campus, G.V.; Rubino, S. Staphylococcus aureus and Staphylococcus epidermidis Virulence Strains as Causative Agents of Persistent Infections in Breast Implants. PLoS ONE 2016, 11, e0146668. [Google Scholar] [CrossRef] [PubMed]
- Rehman, I.; Evans, C.A.; Glen, A.; Cross, S.S.; Eaton, C.L.; Down, J.; Pesce, G.; Phillips, J.T.; Yen, O.S.; Thalmann, G.N. iTRAQ identification of candidate serum biomarkers associated with metastatic progression of human prostate cancer. PLoS ONE 2012, 7, e30885. [Google Scholar] [CrossRef]
- Ray, S.; Patel, S.K.; Kumar, V.; Damahe, J.; Srivastava, S. Differential expression of serum/plasma proteins in various infectious diseases: Specific or nonspecific signatures. PROTEOMICS–Clin. Appl. 2014, 8, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Gouthamchandra, K.; Kumar, A.; Shwetha, S.; Mukherjee, A.; Chandra, M.; Ravishankar, B.; Khaja, M.; Sadhukhan, P.C.; Das, S. Serum proteomics of hepatitis C virus infection reveals retinol-binding protein 4 as a novel regulator. J. Gen. Virol. 2014, 95, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.M.; Bei, J.; Amigo, N.; Valacco, P.; Amadio, A.F.; Zhang, Q.; Wu, X.; Larzábal, M.; Chen, Z.; Cataldi, A. Quantification of Enterohemorrhagic Escherichia coli O157: H7 proteome using TMT-Based Analysis. bioRxiv 2018, 312652. [Google Scholar] [CrossRef]
- Paulo, J.A.; O’Connell, J.D.; Everley, R.A.; O’Brien, J.; Gygi, M.A.; Gygi, S.P. Quantitative mass spectrometry-based multiplexing compares the abundance of 5000 S. cerevisiae proteins across 10 carbon sources. J. Proteom. 2016, 148, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.J.; Mirzaei, M.; Vuong, D.; Pascovici, D.; Chick, J.M.; Lacey, E.; Haynes, P.A. Induction of virulence factors in Giardia duodenalis independent of host attachment. Sci. Rep. 2016, 6, 20765. [Google Scholar] [CrossRef] [PubMed]
- Köcher, T.; Pichler, P.; Schutzbier, M.; Stingl, C.; Kaul, A.; Teucher, N.; Hasenfuss, G.; Penninger, J.M.; Mechtler, K. High precision quantitative proteomics using iTRAQ on an LTQ Orbitrap: A new mass spectrometric method combining the benefits of all. J. Proteome Res. 2009, 8, 4743–4752. [Google Scholar] [CrossRef] [PubMed]
- Pichler, P.; Köcher, T.; Holzmann, J.; Möhring, T.; Ammerer, G.; Mechtler, K. Improved precision of iTRAQ and TMT quantification by an axial extraction field in an Orbitrap HCD cell. Anal. Chem. 2011, 83, 1469–1474. [Google Scholar] [CrossRef]
- Li, Z.; Adams, R.M.; Chourey, K.; Hurst, G.B.; Hettich, R.L.; Pan, C. Systematic comparison of label-free, metabolic labeling, and isobaric chemical labeling for quantitative proteomics on LTQ Orbitrap Velos. J. Proteome Res. 2012, 11, 1582–1590. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, K.; Fang, S.; Zhao, X.; Huang, T.; Chen, H.; Yan, F.; Tang, Y.; Zhou, H.; Zhu, J. Discovery of a potential plasma protein biomarker panel for acute-on-chronic liver failure induced by Hepatitis B Virus. Front. Physiol. 2017, 8, 1009. [Google Scholar] [CrossRef] [PubMed]
- Kuusela, P.; Saraswat, M.; Joenväärä, S.; Kaartinen, J.; Järvinen, A.; Renkonen, R. Changes in plasma protein levels as an early indication of a bloodstream infection. PLoS ONE 2017, 12, e0172987. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, L.; Xu, F.; Xiao, J.; Jiao, W.; Qi, H.; Shen, C.; Shen, A. Characterization of plasma proteins in children of different Mycobacterium tuberculosis infection status using label-free quantitative proteomics. Oncotarget 2017, 8, 103290. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Ahirwar, S.K.; Nath, G. Proteomics-based identification of plasma proteins and their association with the host–pathogen interaction in chronic typhoid carriers. Int. J. Infect. Dis. 2014, 19, 59–66. [Google Scholar] [CrossRef]
- Mölleken, C.; Sitek, B.; Henkel, C.; Poschmann, G.; Sipos, B.; Wiese, S.; Warscheid, B.; Broelsch, C.; Reiser, M.; Friedman, S.L. Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology 2009, 49, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Amirkhani, A.; Chowdhury, D.; Mempin, M.; Molloy, M.P.; Deva, A.K.; Vickery, K.; Hu, H. Proteome of Staphylococcus aureus Biofilm Changes Significantly with Aging. Int. J. Mol. Sci. 2022, 23, 6415. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Pascovici, D.; Wu, J.X.; Chick, J.; Wu, Y.; Cooke, B.; Haynes, P.; Molloy, M.P. TMT One-Stop Shop: From Reliable Sample Preparation to Computational Analysis Platform. In Proteome Bioinformatics; Keerthikumar, S., Mathivanan, S., Eds.; Springer: New York, NY, USA, 2017; pp. 45–66. [Google Scholar]
- Mempin, M.; Hu, H.; Chowdhury, D.; Deva, A.; Vickery, K. The A, B and C’s of Silicone Breast Implants: Anaplastic Large Cell Lymphoma, Biofilm and Capsular Contracture. Materials 2018, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Moulder, R.; Bhosale, S.D.; Goodlett, D.R.; Lahesmaa, R. Analysis of the plasma proteome using iTRAQ and TMT-based Isobaric labeling. Mass Spectrom. Rev. 2018, 37, 583–606. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, A.; Ao, W.; Wang, Z.; Yuan, J.; Song, Q.; Wei, D.; Ye, H. Proteomic analysis of serum proteins from HIV/AIDS patients with Talaromyces marneffei infection by TMT labeling-based quantitative proteomics. Clin. Proteom. 2018, 15, 40. [Google Scholar] [CrossRef]
- Geyer, P.E.; Holdt, L.M.; Teupser, D.; Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 2017, 13, 942. [Google Scholar] [CrossRef]
- Pimienta, G.; Heithoff, D.M.; Rosa-Campos, A.; Tran, M.; Esko, J.D.; Mahan, M.J.; Marth, J.D.; Smith, J.W. Plasma Proteome Signature of Sepsis: A Functionally Connected Protein Network. Proteomics 2019, 19, 1800389. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, O.; Haubitz, M.; Brunkhorst, F.; Kliem, V.; Koch, K.; Brunkhorst, R. Usefulness of procalcitonin for differentiation between activity of systemic autoimmune disease (systemic lupus erythematosus/systemic antineutrophil cytoplasmic antibody-associated vasculitis) and invasive bacterial infection. Arthritis Rheum. 1997, 40, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Nikfardjam, M.; Müllner, M.; Schreiber, W.; Oschatz, E.; Exner, M.; Domanovits, H.; Laggner, A.; Huber, K. The association between C-reactive protein on admission and mortality in patients with acute myocardial infarction. J. Intern. Med. 2000, 247, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, S.; Li, Z.; Buggage, R.; Mor, F.; Cohen, I.; Chew, E.; Nussenblatt, R. Alpha tropomyosin as a self-antigen in patients with Behçet’s disease. Clin. Exp. Immunol. 2005, 140, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kuramitsu, Y.; Makino, A.; Fujimoto, M.; Iizuka, N.; Hoshii, Y.; Takashima, M.; Tamesa, M.; Nishimura, T.; Takeda, S. Expression of tropomyosin alpha 4 chain is increased in esophageal squamous cell carcinoma as evidenced by proteomic profiling by two-dimensional electrophoresis and liquid chromatography-mass spectrometry/mass spectrometry. PROTEOMICS–Clin. Appl. 2007, 1, 215–223. [Google Scholar] [CrossRef]
- Lam, C.Y.; Yip, C.W.; Poon, T.C.; Cheng, C.K.; Ng, E.W.; Wong, N.C.; Cheung, P.F.; Lai, P.B.; Ng, I.O.; Fan, S.T. Identification and characterization of tropomyosin 3 associated with granulin-epithelin precursor in human hepatocellular carcinoma. PLoS ONE 2012, 7, e40324. [Google Scholar] [CrossRef] [PubMed]
- Biancone, L.; Monteleone, G.; Marasco, R.; Pallone, F. Autoimmunity to tropomyosin isoforms in ulcerative colitis (UC) patients and unaffected relatives. Clin. Exp. Immunol. 1998, 113, 198. [Google Scholar] [CrossRef] [PubMed]
- Galloway, P.; Mulvihill, P.; Siedlak, S.; Mijares, M.; Kawai, M.; Padget, H.; Kim, R.; Perry, G. Immunochemical demonstration of tropomyosin in the neurofibrillary pathology of Alzheimer’s disease. Am. J. Pathol. 1990, 137, 291. [Google Scholar]
- Dighiero, G.; Lymberi, P.; Monot, C.; Abuaf, N. Sera with high levels of anti-smooth muscle and anti-mitochondrial antibodies frequently bind to cytoskeleton proteins. Clin. Exp. Immunol. 1990, 82, 52–56. [Google Scholar] [CrossRef]
- Van De Water, L.; Varney, S.; Tomasek, J.J. Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: Opportunities for new therapeutic intervention. Adv. Wound Care 2013, 2, 122–141. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348, aaa2151. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, K.; Gunawardana, M.; Webster, P.; Hochstim, C.; Koempel, J.; Kokot, N.; Sinha, U.; Rice, D.; Baum, M. Bacterial biofilms and increased bacterial counts are associated with airway stenosis. Otolaryngol. Head Neck Surg. 2014, 150, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Vedrenne, N.; Coulomb, B.; Danigo, A.; Bonté, F.; Desmoulière, A. The complex dialogue between (myo) fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathol. Biol. 2012, 60, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Sim, H.B.; Huan, F.; Kim, D.J. Myofibroblasts and capsular tissue tension in breast capsular contracture. Aesthetic Plast. Surg. 2010, 34, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Switalski, L.M.; Patti, J.M.; Butcher, W.; Gristina, A.G.; Speziale, P.; Höök, M. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol. Microbiol. 1993, 7, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kreikemeyer, B.; Nakata, M.; Oehmcke, S.; Gschwendtner, C.; Normann, J.; Podbielski, A. Streptococcus pyogenes Collagen Type I-binding Cpa Surface Protein Expression profile, binding characteristics, biological functions, and potential clinical impact. J. Biol. Chem. 2005, 280, 33228–33239. [Google Scholar] [CrossRef] [PubMed]
- Menzies, B.E. The role of fibronectin binding proteins in the pathogenesis of Staphylococcus aureus infections. Curr. Opin. Infect. Dis. 2003, 16, 225–229. [Google Scholar] [CrossRef]
- Talay, S.R.; Valentin-Weigand, P.; Jerlström, P.; Timmis, K.; Chhatwal, G. Fibronectin-binding protein of Streptococcus pyogenes: Sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect. Immun. 1992, 60, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Tsujioka, M.; Yoshida, K.; Inouye, K. Talin B is required for force transmission in morphogenesis of Dictyostelium. EMBO J. 2004, 23, 2216–2225. [Google Scholar] [CrossRef]
- Xu, N.; Chen, H.-J.; Chen, S.-H.; Xue, X.-Y.; Chen, H.; Zheng, Q.-S.; Wei, Y.; Li, X.-D.; Huang, J.-B.; Cai, H. Upregulation of Talin-1 expression associates with advanced pathological features and predicts lymph node metastases and biochemical recurrence of prostate cancer. Medicine 2016, 95, e4326. [Google Scholar] [CrossRef]
- Senetar, M.A.; McCann, R.O. Gene duplication and functional divergence during evolution of the cytoskeletal linker protein talin. Gene 2005, 362, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zheng, B.; Huang, Z.; Lu, H.; Lin, Q.; Liao, Z.; Lin, Z.; Zhao, L.; Wang, X.; Gu, J. Over-expression of talin 1 and integrin-linked kinase in PBMCs of patients with ankylosing spondylitis: A proteomic study. Clin. Exp. Rheumatol. Incl. Suppl. 2010, 28, 828. [Google Scholar]
- Khoontawad, J.; Pairojkul, C.; Rucksaken, R.; Pinlaor, P.; Wongkham, C.; Yongvanit, P.; Pugkhem, A.; Jones, A.; Plieskatt, J.; Potriquet, J. Differential protein expression marks the transition from infection with Opisthorchis viverrini to cholangiocarcinoma. Mol. Cell. Proteom. 2017, 16, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Klim, S.; Amerstorfer, F.; Gruber, G.; Bernhardt, G.; Radl, R.; Leitner, L.; Leithner, A.; Glehr, M. Fibrinogen–A Practical and Cost Efficient Biomarker for Detecting Periprosthetic Joint Infection. Sci. Rep. 2018, 8, 8802. [Google Scholar] [CrossRef] [PubMed]
- Marfà, S.; Jimenez, W. Fibrinogen α-Chain as a Serum Marker of Liver Disease. Biomark. Liver Dis. 2016, 493–511. [Google Scholar] [CrossRef]
- Ko, Y.-P.; Flick, M.J. Fibrinogen is at the interface of host defense and pathogen virulence in Staphylococcus aureus infection. Semin. Thromb. Hemost. 2016, 42, 408–421. [Google Scholar] [CrossRef]
- Barr, S.P.; Hill, E.W.; Bayat, A. Novel proteomic assay of breast implants reveals proteins with significant binding differences: Implications for surface coating and biocompatibility. Aesthetic Surg. J. 2018, 38, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, N.; Ryan, E.J.; Widaa, A.; Sexton, G.; Fennell, J.; O’Rourke, S.; Cahill, K.C.; Kearney, C.J.; O’Brien, F.J.; Kerrigan, S.W. Staphylococcal osteomyelitis: Disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev. 2018, 31, e00084-00017. [Google Scholar] [CrossRef]
- Martin, D.N.; Uprichard, S.L. Identification of transferrin receptor 1 as a hepatitis C virus entry factor. Proc. Natl. Acad. Sci. USA 2013, 110, 10777–10782. [Google Scholar] [CrossRef]
- Pan, X.; Tamilselvam, B.; Hansen, E.J.; Daefler, S. Modulation of iron homeostasis in macrophages by bacterial intracellular pathogens. BMC Microbiol. 2010, 10, 64. [Google Scholar] [CrossRef]
- Andriopoulos, B.; Hegedüsch, S.; Mangin, J.; Riedel, H.-D.; Hebling, U.; Wang, J.; Pantopoulos, K.; Mueller, S. Sustained hydrogen peroxide induces iron uptake by transferrin receptor-1 independent of the iron regulatory protein/iron-responsive element network. J. Biol. Chem. 2007, 282, 20301–20308. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Kohgo, Y.; Kondo, H.; Shintani, N.; Fujikawa, K.; Sasaki, K.; Kato, J.; Nhtsu, Y. Regulation of iron metabolism in HepG2 cells: A possible role for cytokines in the hepatic deposition of iron. Hepatology 1993, 18, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Upton, R.; Chen, Y.; Mumby, S.; Gutteridge, J.; Anning, P.; Nicholson, A.; Evans, T.; Quinlan, G. Variable tissue expression of transferrin receptors: Relevance to acute respiratory distress syndrome. Eur. Respir. J. 2003, 22, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Afdhal, N.H.; Schuppan, D. Iron, HCV, and liver cancer: Hard metal setting the pace? Gastroenterology 2006, 130, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J. Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): Structure, function and regulation in host defence against Gram-negative bacteria. Biochem. Soc. Trans. 2003, 31, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, I.L.; Bjartell, A.; Egesten, A.; Riesbeck, K. Lipopolysaccharide-Binding Protein Increases Toll-like Receptor 4–Dependent Activation by Nontypeable Haemophilus influenzae. J. Infect. Dis. 2001, 184, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Prucha, M.; Herold, I.; Zazula, R.; Dubska, L.; Dostal, M.; Hildebrand, T.; Hyanek, J. Significance of lipopolysaccharide-binding protein (an acute phase protein) in monitoring critically ill patients. Crit. Care 2003, 7, R154. [Google Scholar] [CrossRef] [PubMed]
- Zweigner, J.; Gramm, H.-J.; Singer, O.C.; Wegscheider, K.; Schumann, R.R. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood 2001, 98, 3800–3808. [Google Scholar] [CrossRef]
- Nien, H.-C.; Hsu, S.-J.; Su, T.-H.; Yang, P.-J.; Sheu, J.-C.; Wang, J.-T.; Chow, L.-P.; Chen, C.-L.; Kao, J.-H.; Yang, W.-S. High serum lipopolysaccharide-binding protein level in chronic hepatitis C viral infection is reduced by anti-viral treatments. PLoS ONE 2017, 12, e0170028. [Google Scholar] [CrossRef]
- Opal, S.M.; Scannon, P.J.; Vincent, J.-L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Brănescu, C.; Şerban, D.; Şavlovschi, C.; Dascălu, A.; Kraft, A. Lipopolysaccharide binding protein (LBP)–an inflammatory marker of prognosis in the acute appendicitis. J. Med. Life 2012, 5, 342. [Google Scholar] [PubMed]
- Lehr, S.; Hartwig, S.; Lamers, D.; Famulla, S.; Müller, S.; Hanisch, F.-G.; Cuvelier, C.; Ruige, J.; Eckardt, K.; Ouwens, D.M. Identification and validation of novel adipokines released from primary human adipocytes. Mol. Cell. Proteom. 2012, 11, M111.010504. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]

| SN | Patient ID | Age (Years) | |
| 1 | C1 | 33 | |
| 2 | C2 | 34 | |
| 3 | C3 | 25 | |
| 4 | C4 | 31 | |
| 5 | C5 | 29 | |
| 6 | C6 | 39 | |
| 7 | C7 | 36 | |
| 8 | C8 | 35 | |
| 9 | C9 | 29 | |
| 10 | C10 | 32 | |
| SN | Patient ID | Age at Diagnosis (Years) | Bacteria Isolated |
| 1 | D1 | 42 | Enterococcus spp., S. epidermidis |
| 2 | D2 | 58 | S. epidermidis |
| 3 | D3 | 62 | N/A |
| 4 | D4 | 39 | Micrococcus luteus |
| 5 | D5 | 49 | N/A |
| 6 | D6 | 64 | S. epidermidis |
| 7 | D7 | 49 | N/A |
| 8 | D8 | 37 | S. aureus |
| 9 | D9 | 44 | S. epidermidis |
| 10 | D10 | 52 | N/A |
| Accession | Description | Fold Change | Subcellular Location | KEGG Pathway |
|---|---|---|---|---|
| P15636 | Protease 1 OS = Achromobacter lyticus | 1.0 | Secreted | N/A |
| P06654 | Immunoglobulin G-binding protein G OS = Streptococcus sp. group G, GN = spg | 1.1 | Cell Wall | N/A |
| Q890U2 | Glutamine--fructose-6-phosphate aminotransferase [isomerizing] OS = Clostridium tetani (strain Massachusetts/E88), GN = glmS | 1.3 | Cytoplasm | Alanine, aspartate and glutamate metabolism, Biosynthesis of antibiotics, Metabolic pathways, Amino sugar and nucleotide sugar metabolism |
| A9MPX1 | Zinc transporter ZupT OS = Salmonella arizonae (strain ATCC BAA-731/CDC346-86/RSK2980), GN = zupT | 1.0 | N/A |
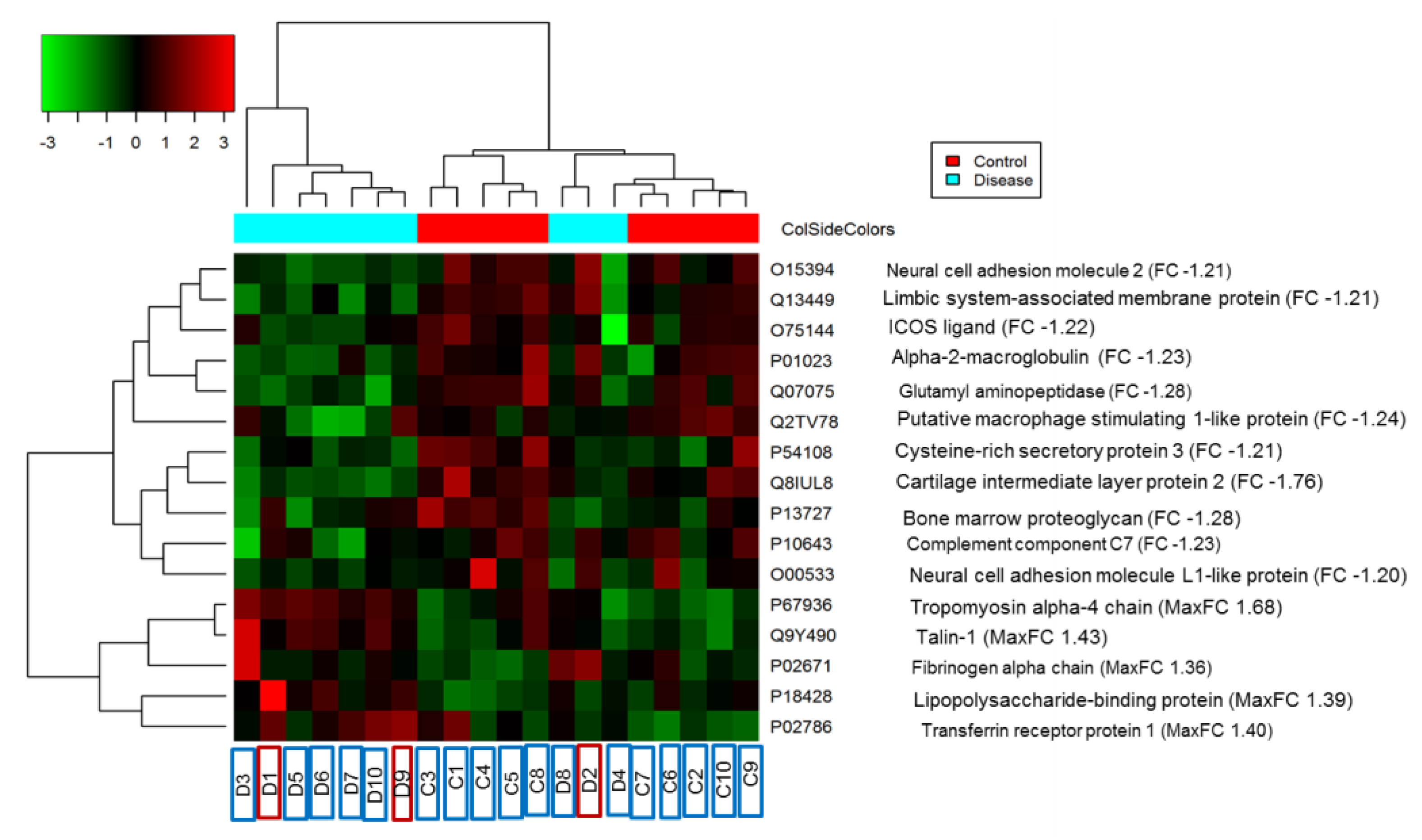
| Accession ID | Protein Name | Mean Disease /Mean Control | Fold Change | Protein Pathways | Subcellular Localization |
|---|---|---|---|---|---|
| Q8IUL8 | Cartilage intermediate layer protein 2 | 0.579 | −1.7 | Secreted | |
| P67936 | Tropomyosin alpha-4 chain | 1.687 | 1.7 | Hypertrophic cardiomyopathy, Adrenergic signaling in cardiomyocytes, Dilated cardiomyopathy (DCM), Cardiac muscle contraction | Cytoskeleton /Cytoplasm |
| Q9Y490 | Talin-1 OS = Homo sapiens | 1.440 | 1.4 | Rap1 signaling pathway, Human T-cell leukemia virus 1 infection, Platelet activation, Focal adhesion | Cytoskeleton /plasma membrane |
| P02786 | Transferrin receptor protein 1 | 1.399 | 1.4 | Hematopoietic cell lineage, Endocytosis, HIF-1 signaling pathway, Phagosome, Ferroptosis | Plasma membrane /secreted |
| P18428 | Lipopolysaccharide-binding protein | 1.389 | 1.4 | NF-kappa B signaling pathway, Tuberculosis, Toll-like receptor signaling pathway, Salmonella infection | Secreted |
| P02671 | Fibrinogen alpha chain | 1.360 | 1.4 | Platelet activation Complement and coagulation cascades | Secreted |
| P01023 | Alpha-2-macroglobulin | 0.758 | −1.3 | Complement and coagulation cascades | Secreted |
| P13727 | Bone marrow proteoglycan | 0.780 | −1.3 | Asthma | Secreted |
| Q07075 | Glutamyl aminopeptidase | 0.781 | −1.3 | Renin–angiotensin system | Plasma membrane |
| Q2TV78 | Putative macrophage stimulating 1-like protein | 0.802 | −1.2 | Secreted | |
| P10643 | Complement component C7 | 0.813 | −1.2 | Prion diseases, Complement and coagulation cascades, Systemic lupus erythematosus | Secreted |
| O75144 | ICOS ligand | 0.814 | −1.2 | Cell adhesion molecules (CAMs), Intestinal immune network for IgA production | Plasma membrane |
| O15394 | Neural cell adhesion molecule 2 | 0.821 | −1.2 | Cell adhesion molecules (CAMs), Prion diseases | Plasma membrane |
| Q13449 | Limbic system-associated membrane protein | 0.823 | −1.2 | Plasma membrane | |
| P54108 | Cysteine-rich secretory protein 3 | 0.824 | −1.2 | Secreted | |
| O00533 | Neural cell adhesion molecule L1-like protein | 0.827 | −1.2 | Plasma membrane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Amirkhani, A.; Mempin, M.; Ahn, S.B.; Deva, A.K.; Baker, M.S.; Vickery, K.; Hu, H. The Low-Abundance Plasma Proteome Reveals Differentially Abundant Proteins Associated with Breast Implant Capsular Contracture: A Pilot Study. Proteomes 2024, 12, 22. https://doi.org/10.3390/proteomes12030022
Rahman MA, Amirkhani A, Mempin M, Ahn SB, Deva AK, Baker MS, Vickery K, Hu H. The Low-Abundance Plasma Proteome Reveals Differentially Abundant Proteins Associated with Breast Implant Capsular Contracture: A Pilot Study. Proteomes. 2024; 12(3):22. https://doi.org/10.3390/proteomes12030022
Chicago/Turabian StyleRahman, Md. Arifur, Ardeshir Amirkhani, Maria Mempin, Seong Beom Ahn, Anand K. Deva, Mark S. Baker, Karen Vickery, and Honghua Hu. 2024. "The Low-Abundance Plasma Proteome Reveals Differentially Abundant Proteins Associated with Breast Implant Capsular Contracture: A Pilot Study" Proteomes 12, no. 3: 22. https://doi.org/10.3390/proteomes12030022
APA StyleRahman, M. A., Amirkhani, A., Mempin, M., Ahn, S. B., Deva, A. K., Baker, M. S., Vickery, K., & Hu, H. (2024). The Low-Abundance Plasma Proteome Reveals Differentially Abundant Proteins Associated with Breast Implant Capsular Contracture: A Pilot Study. Proteomes, 12(3), 22. https://doi.org/10.3390/proteomes12030022








