Lemon Juice Assisted Green Synthesis of Reduced Graphene Oxide and Its Application for Adsorption of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Lemon Juice
2.2. Materials
2.3. Measurements
2.4. Synthesis of Graphene Oxide (GO)
2.5. Green Synthesis of rGO Using Lemon Juice (Typical Procedure)
2.6. Preparation of Methylene Blue (MB) Solutions
2.7. The Adsorption of MB on rGO
3. Results and Discussion
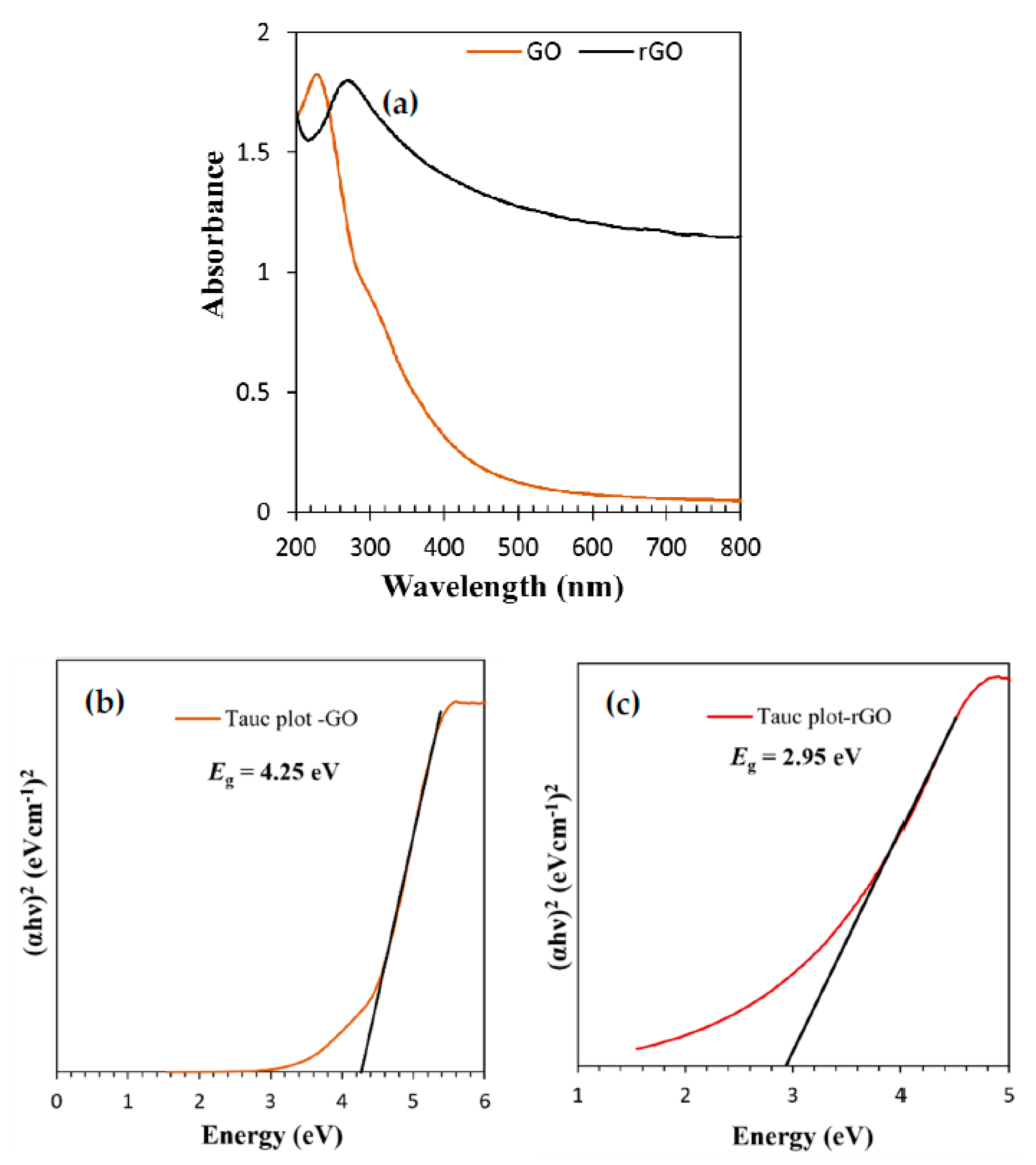
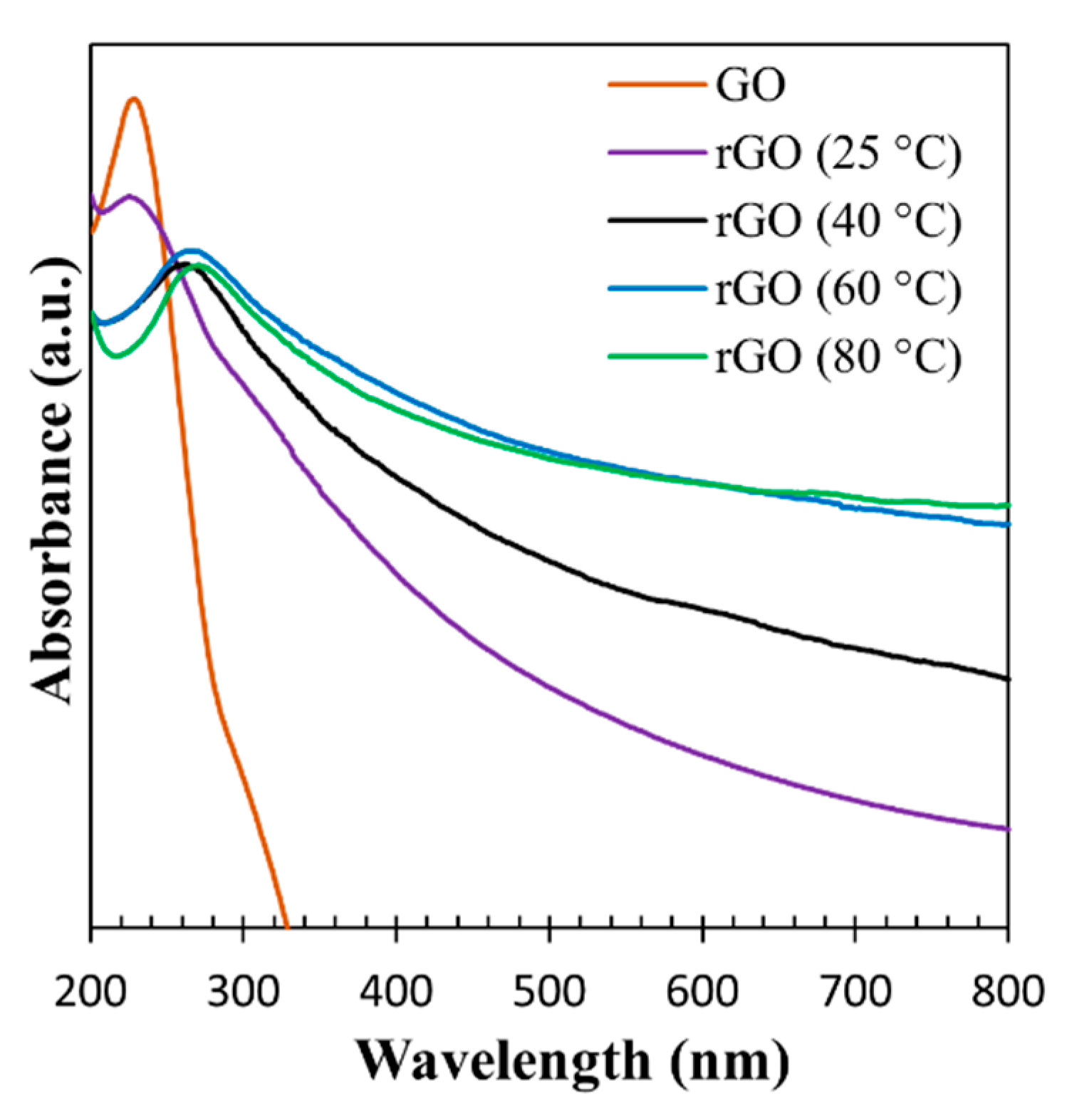
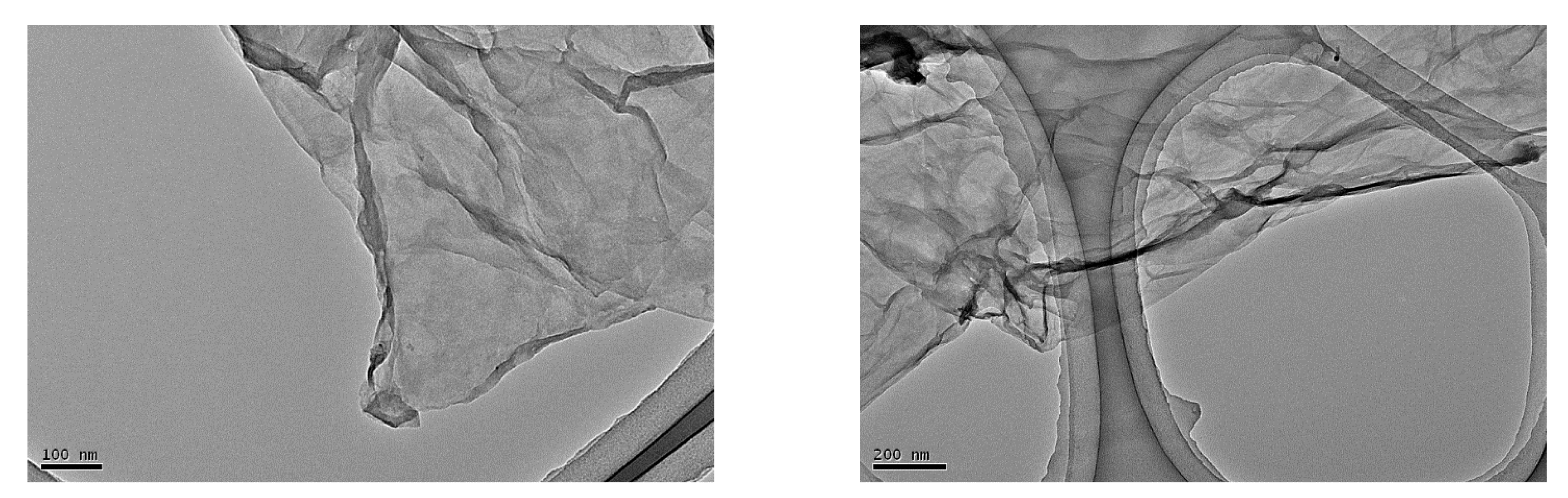
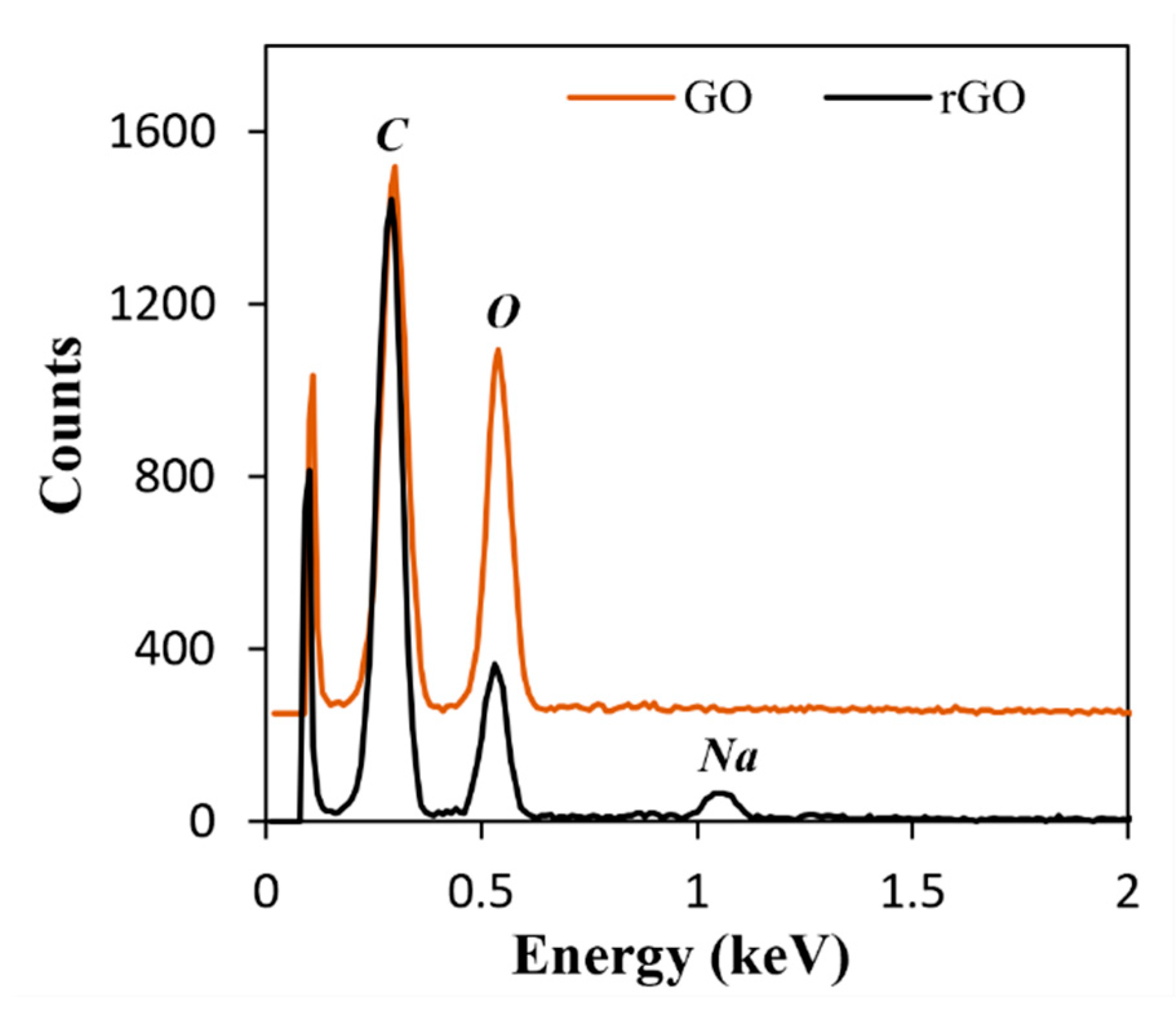
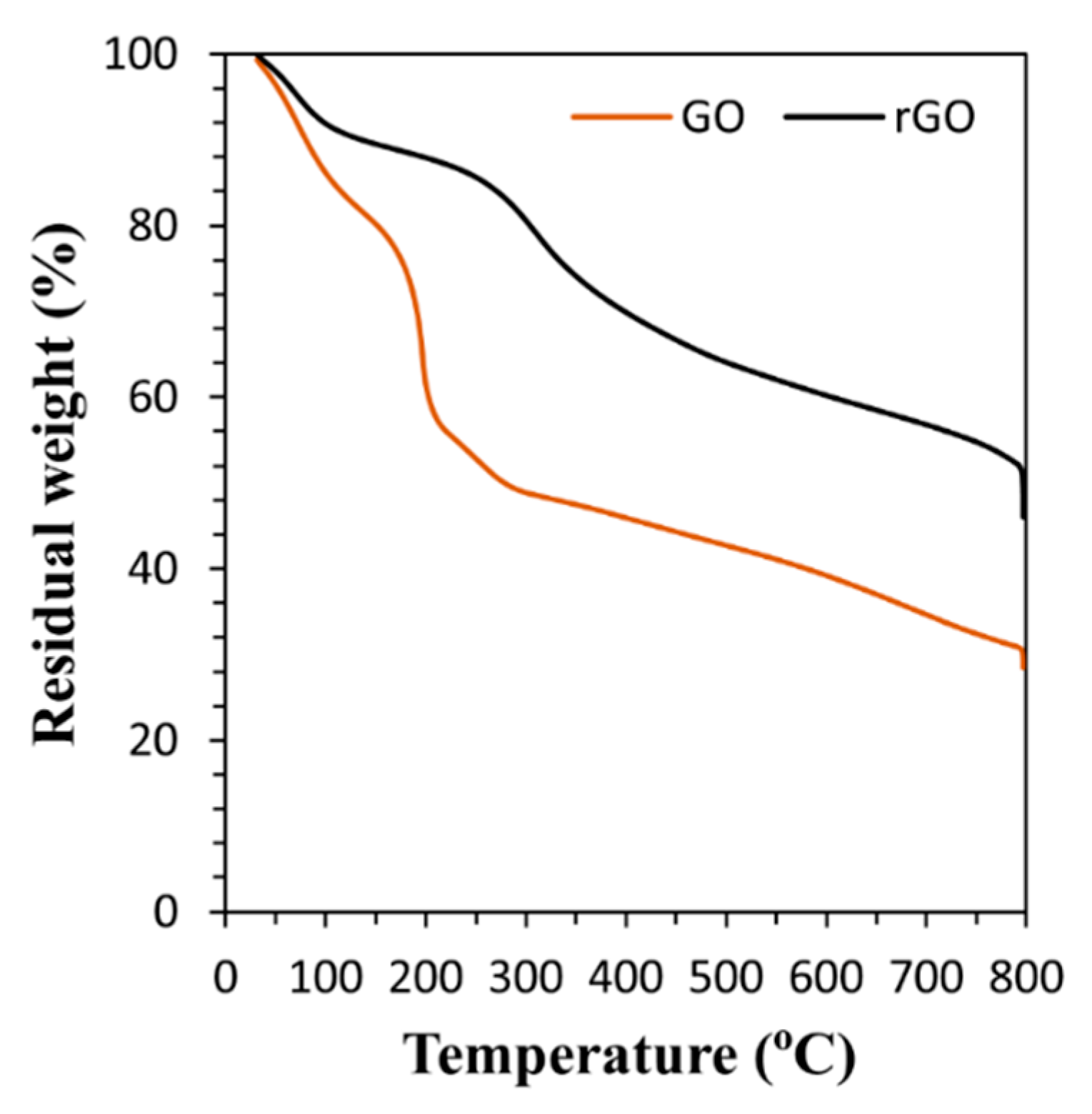
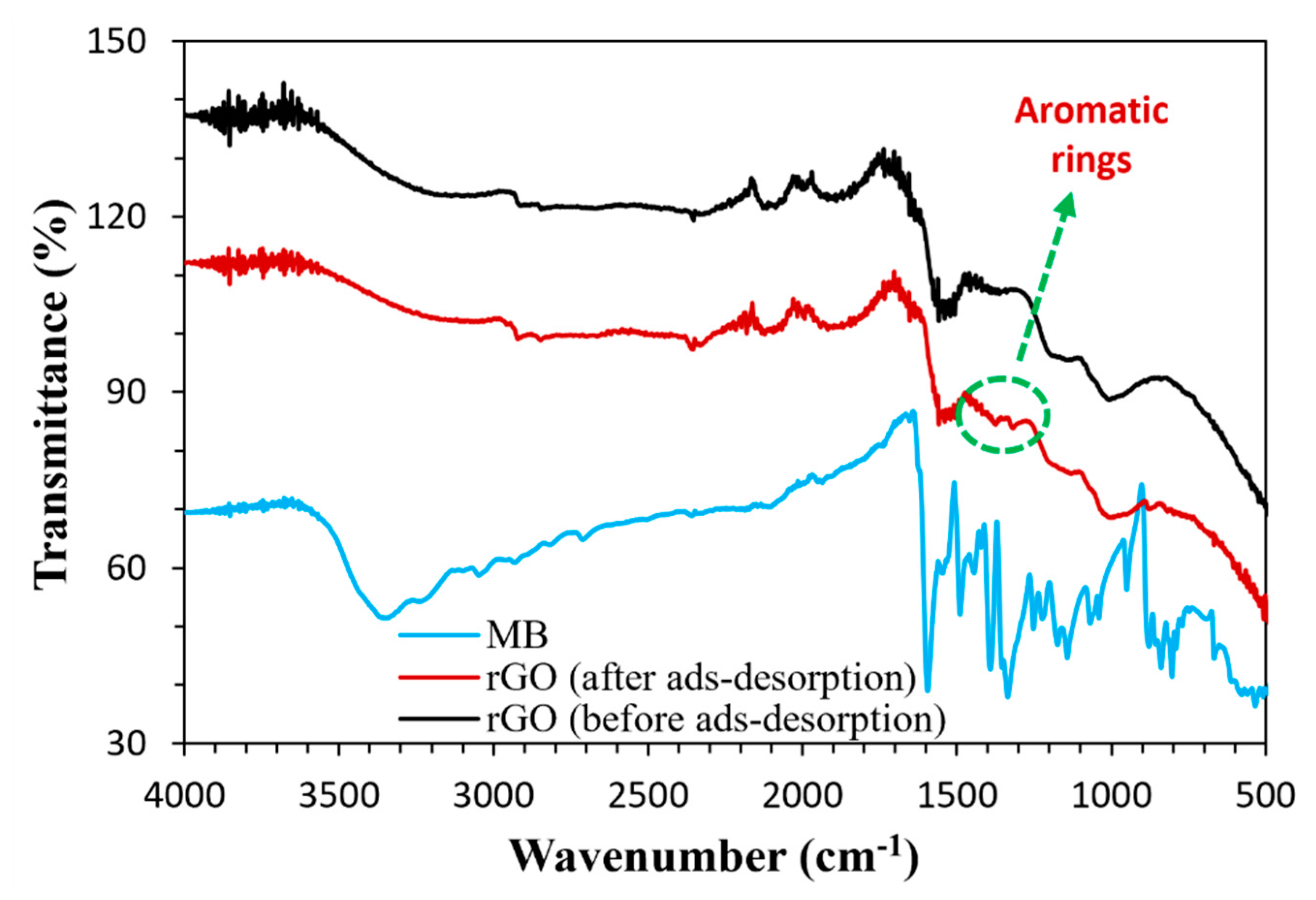
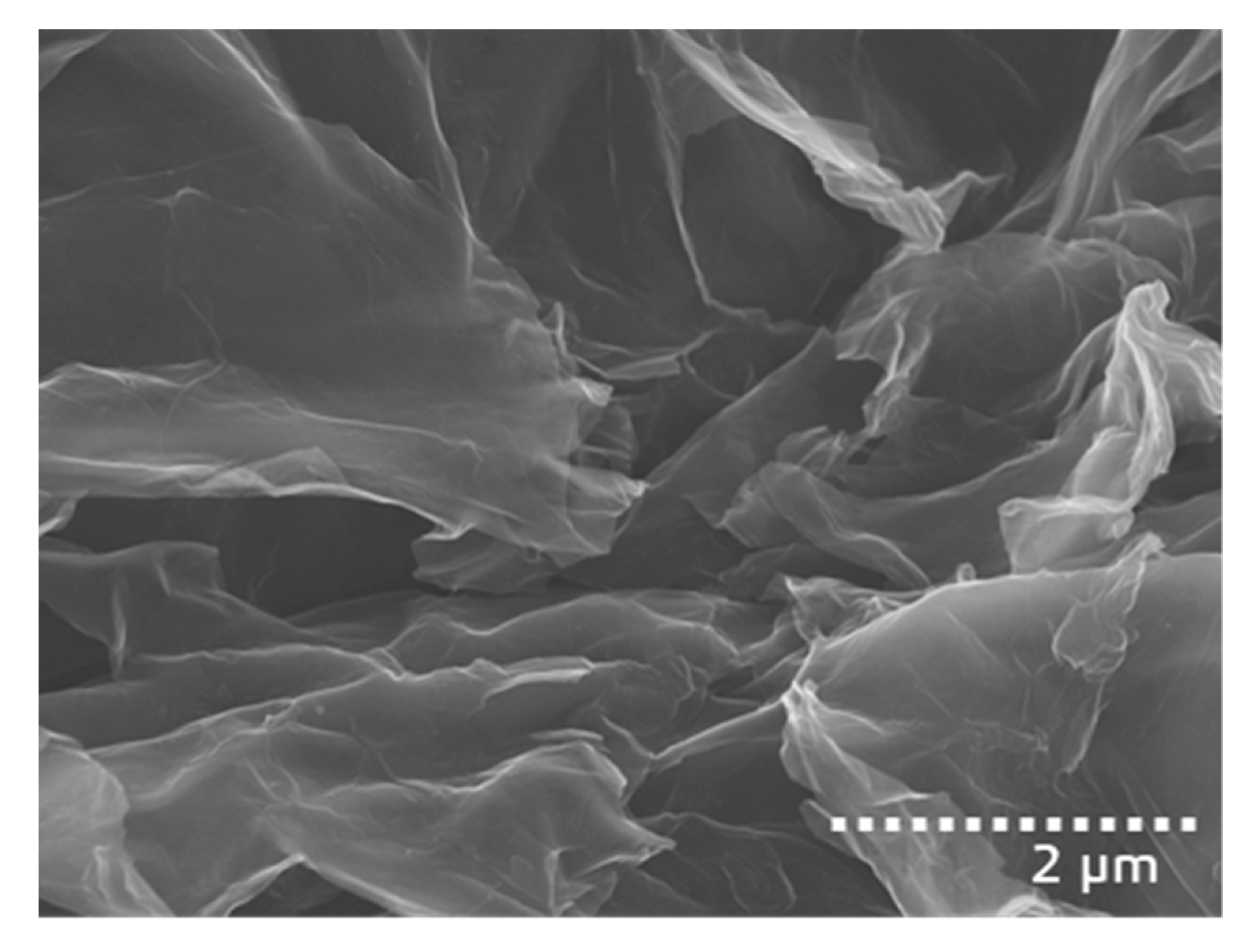
3.1. Characterization
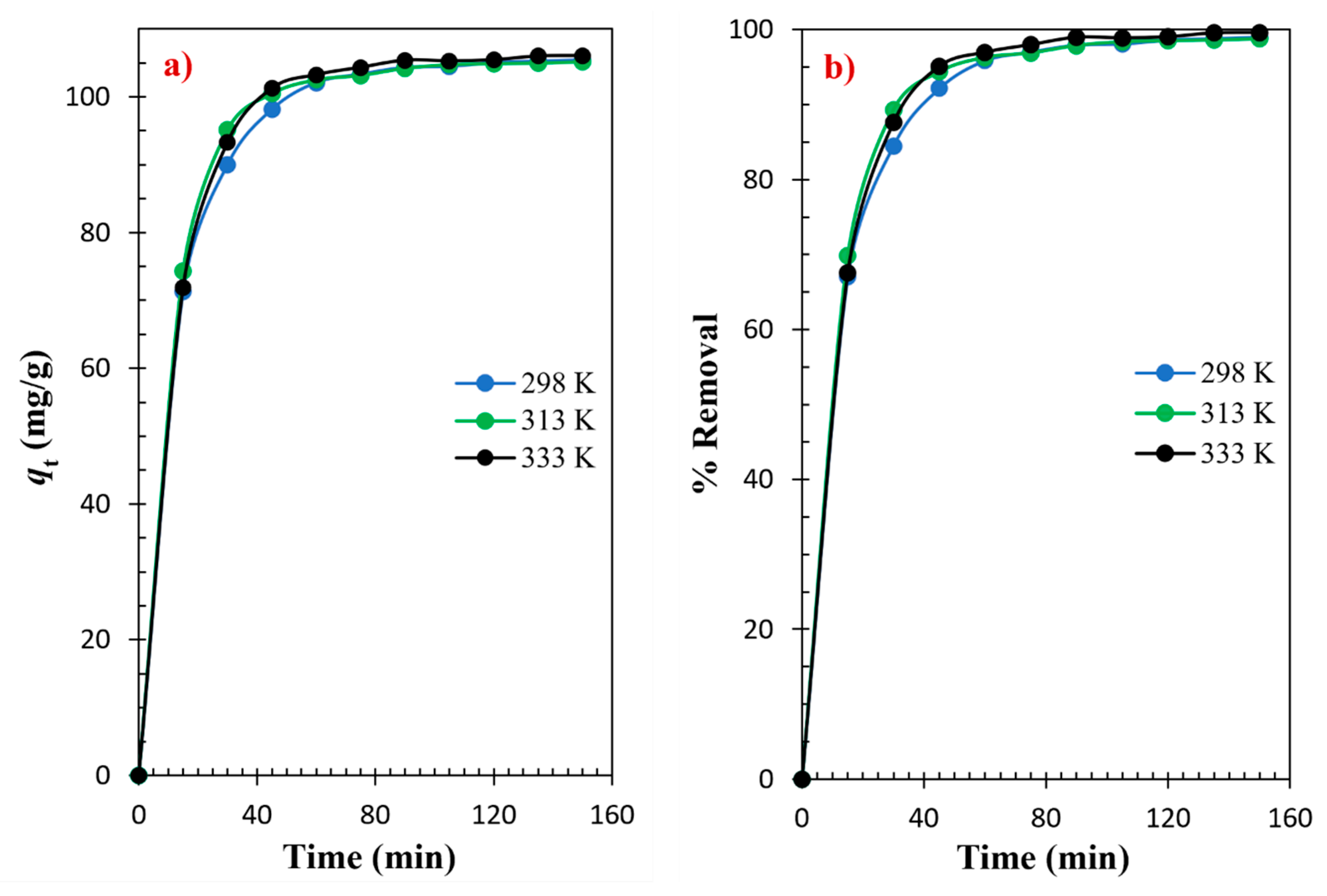
3.2. Adsorptions Kinetics
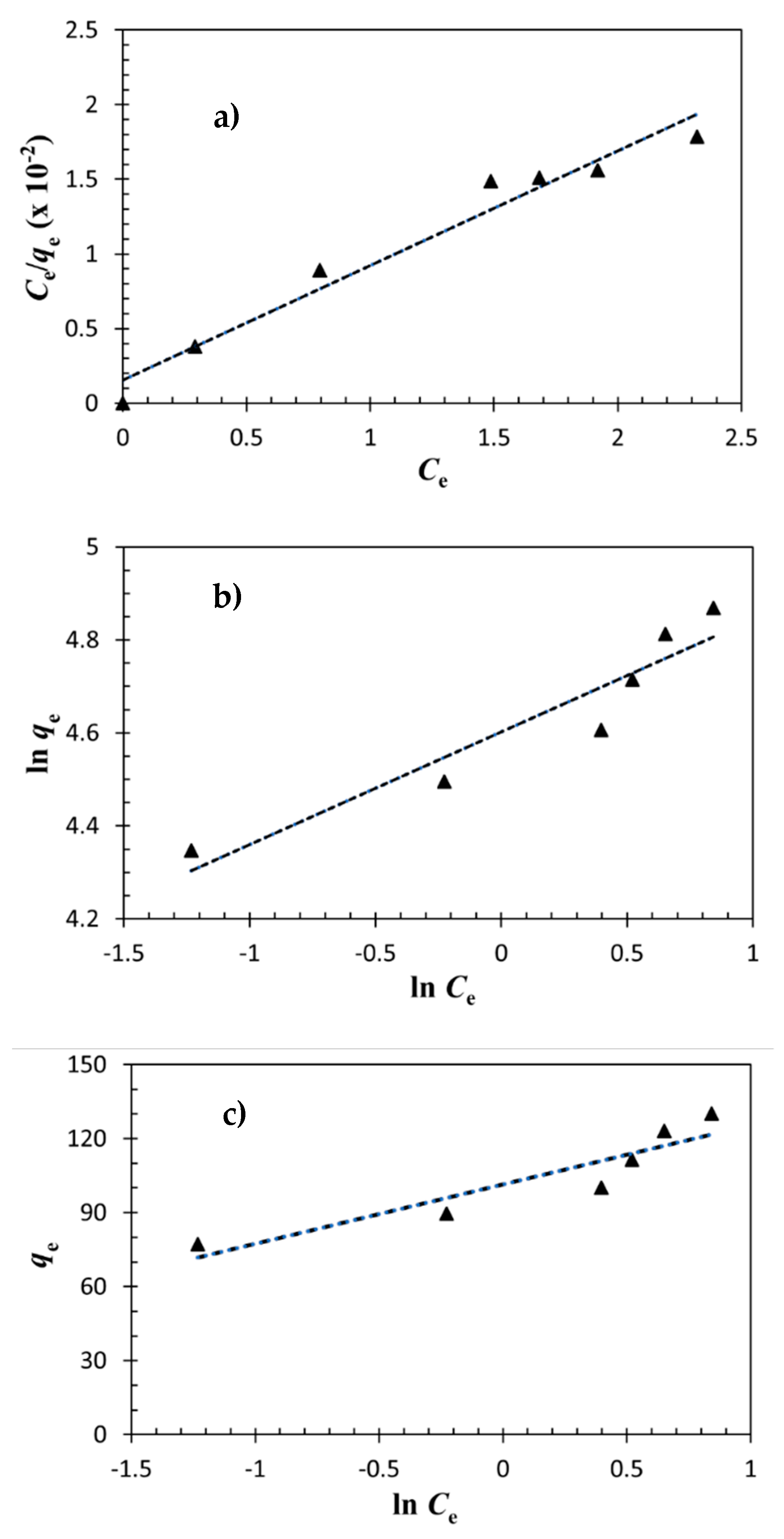
3.3. Adsorption Isotherm
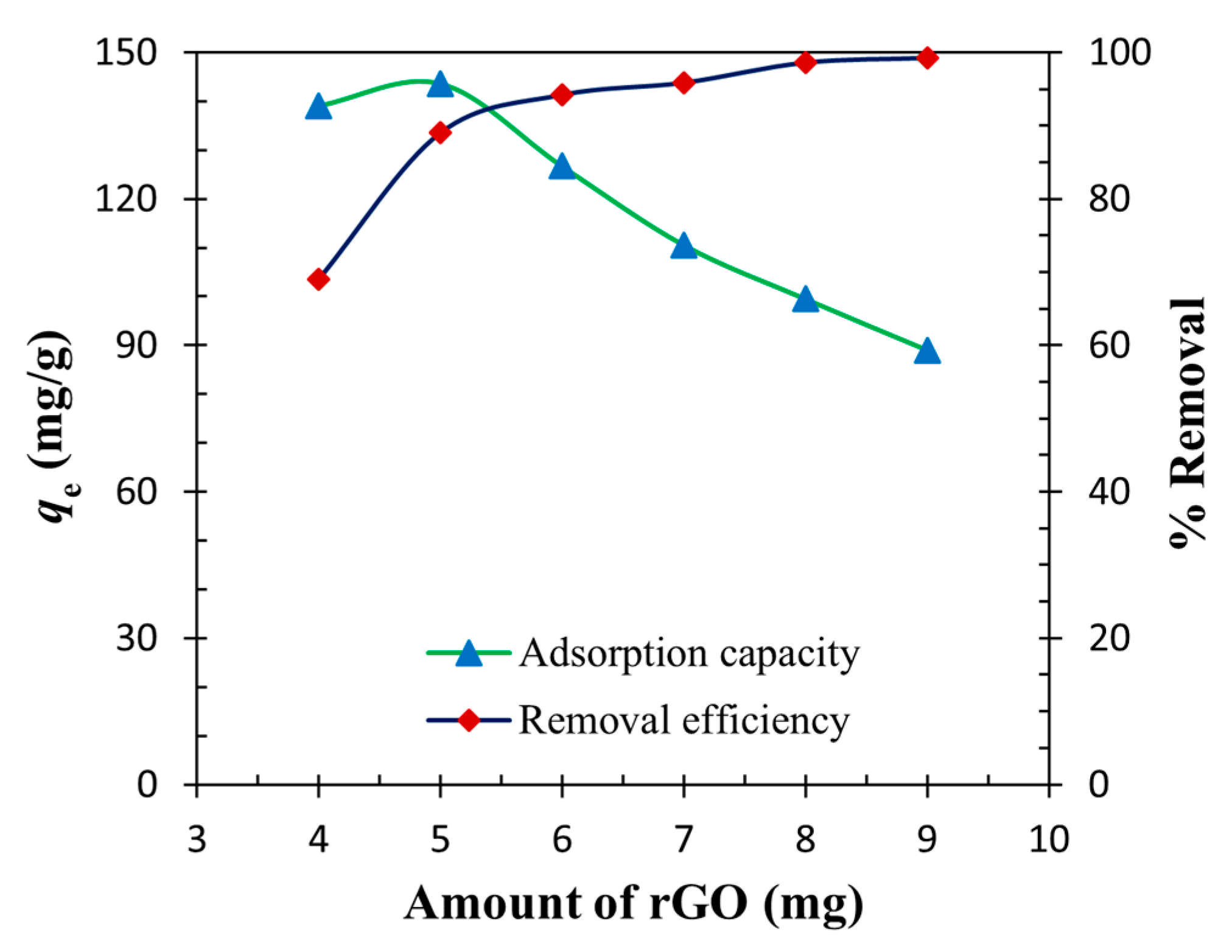
3.4. Effect of Mass of rGO
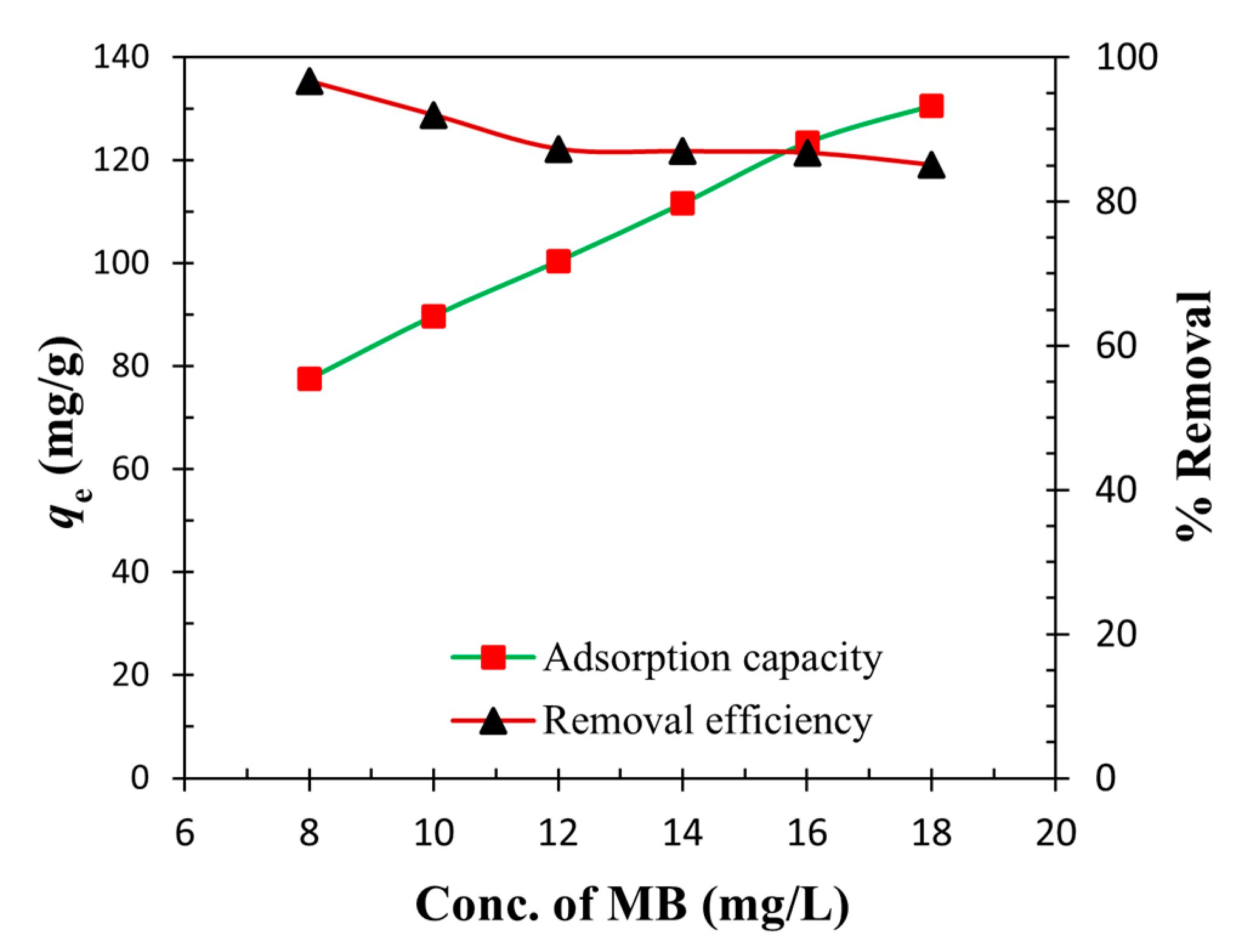
3.5. Effect of the Initial Concentration of MB
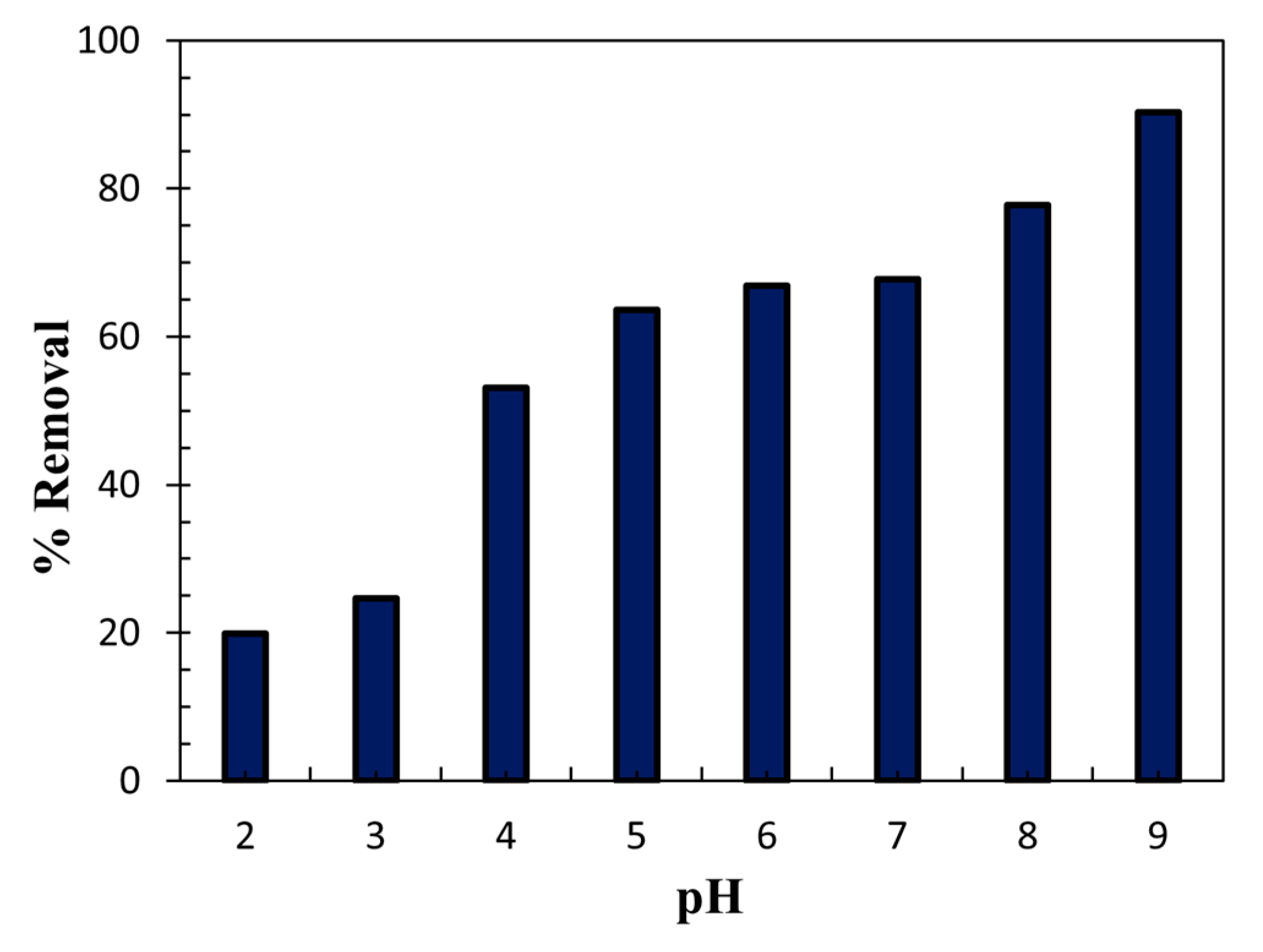
3.6. Effect of pH
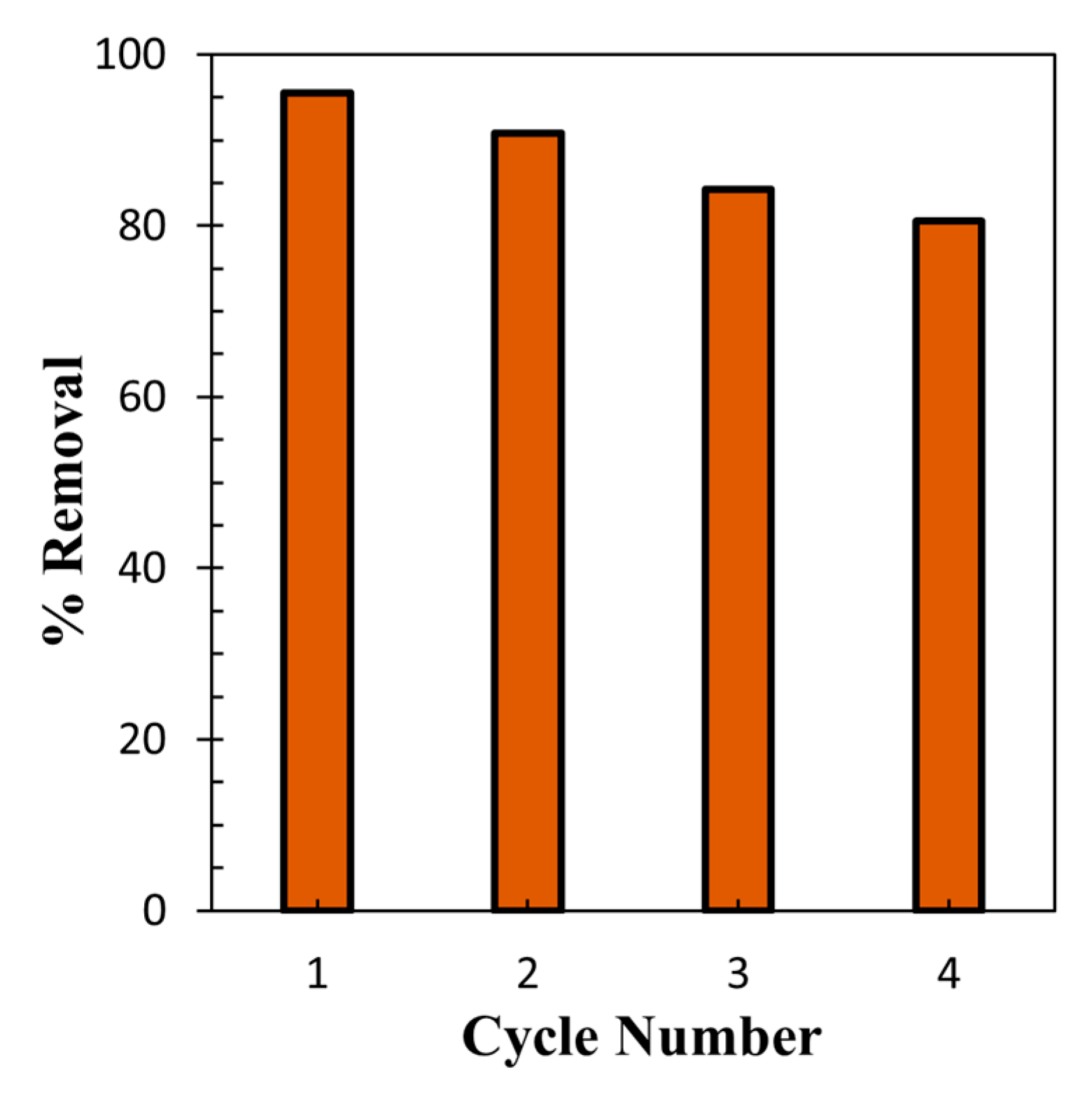
3.7. Recycling and Reuse Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Fu, S.; Yuan, B.; Li, Y.; Deng, Z. Toward a Universal “Adhesive Nanosheet” for the Assembly of Multiple Nanoparticles Based on a Protein-Induced Reduction/Decoration of Graphene Oxide. J. Am. Chem. Soc. 2010, 132, 7279–7281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing Sugar: New Functional Molecules for the Green Synthesis of Graphene Nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Samorì, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, J.M.; León, V.; Lucío, M.I.; Prato, M.; Vázquez, E. Production of ready-to-use few-layer graphene in aqueous suspensions. Nat. Protoc. 2018, 13, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Yin, J. Facile Synthesis of Soluble Graphene via a Green Reduction of Graphene Oxide in Tea Solution and Its Biocomposites. ACS Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; Qi, D.; Lin, J. Green Synthesis of Porous Spherical Reduced Graphene Oxide and Its Application in Immobilized Pectinase. ACS Omega 2020, 5, 32706–32714. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Siddiqui, R.; Sharma, P.; Kumar, R.; Verma, G.; Saini, A. Green synthesis of peptide functionalized reduced graphene oxide (rGO) nano bioconjugate with enhanced antibacterial activity. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel insight into the adsorption of Cr(VI) and Pb(II) ions by MOF derived Co-Al layered double hydroxide @hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Shen, Z.; Lin, J. Graphene and graphene-based composites as Li-ion battery electrode materials and their ap-plication in full cells. J. Mater. Chem. A 2017, 5, 15423–15446. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Mahesh, K.; Le, N.; Kemp, K.; Timilsina, R.; Tiwari, R.N.; Kim, K.S. Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions. Carbon 2013, 56, 173–182. [Google Scholar] [CrossRef]
- Chia, J.S.Y.; Tan, M.; Khiew, P.; Chin, J.; Lee, H.W.; Bien, D.; Siong, C.W. A novel one step synthesis of graphene via sonochemical-assisted solvent exfoliation approach for electrochemical sensing application. Chem. Eng. J. 2014, 249, 270–278. [Google Scholar] [CrossRef]
- Goud, K.Y.; Hayat, A.; Catanante, G.; Satyanarayana, M.; Gobi, K.V.; Marty, J.L. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochimica Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
- Jiang, C.; An, D.; Wang, Z.; Zhang, S.; An, X.; Bo, J.; Yan, G.; Moon, K.-S.; Wong, C. A sustainable reduction route of graphene oxide by industrial waste lignin for versatile applications in energy and environment. J. Clean. Prod. 2020, 268, 122019. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.C.; Bonaccorso, F.S.; Fal’Ko, V.; Novoselov, K.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2014, 7, 4598–4810. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application. Macromol. Symp. 2017, 376. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Synthesis of high-performance nickel hydroxide nanosheets/gadolinium doped-α-MnO2 composite nanorods as cathode and Fe3O4/GO nanospheres as anode for an all-solid-state asymmetric supercapacitor. J. Energy Chem. 2022, 64, 475–484. [Google Scholar] [CrossRef]
- Feng, H.; Cheng, R.; Zhao, X.; Duan, X.; Li, J. A low-temperature method to produce highly reduced graphene oxide. Nat. Commun. 2013, 4, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, K.; Huang, H.-H.; Joshi, R.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, W.; Zhang, W.; Zheng, X.; Wu, M.-B.; Wei, W.; Tao, Y.; Li, Z.; Yang, Q.-H. Reduction of Graphene Oxide by Hydrogen Sulfide: A Promising Strategy for Pollutant Control and as an Electrode for Li-S Batteries. Adv. Energy Mater. 2013, 4, 1301565. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Liu, C.F.; Zhan, L.; Li, Y.F.; Huang, C.Z. Green and easy synthesis of biocompatible graphene for use as an anticoagulant. RSC Adv. 2012, 2, 2322–2328. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Ghosh, T.K.; Rana, D.; Roy, I.; Bhattacharyya, A.; Sarkar, G.; Chakraborty, M.; Chattopadhyay, D. Studies on synthesis of reduced graphene oxide (RGO) via green route and its electrical property. Mater. Res. Bull. 2016, 79, 41–51. [Google Scholar] [CrossRef]
- Bhattacharya, G.; Sas, S.; Wadhwa, S.; Mathur, A.; McLaughlin, J.; Roy, S.S. Aloe vera assisted facile green synthesis of reduced graphene oxide for electrochemical and dye removal applications. RSC Adv. 2017, 7, 26680–26688. [Google Scholar] [CrossRef] [Green Version]
- Manchala, S.; Tandava, V.S.R.K.; Jampaiah, D.; Bhargava, S.K.; Shanker, V. Novel and Highly Efficient Strategy for the Green Synthesis of Soluble Graphene by Aqueous Polyphenol Extracts of Eucalyptus Bark and Its Applications in High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11612–11620. [Google Scholar] [CrossRef]
- Gan, L.; Li, B.; Chen, Y.; Yu, B.; Chen, Z. Green synthesis of reduced graphene oxide using bagasse and its application in dye removal: A waste-to-resource supply chain. Chemosphere 2018, 219, 148–154. [Google Scholar] [CrossRef]
- Wijaya, R.; Andersan, G.; Santoso, S.P.; Irawaty, W. Green Reduction of Graphene Oxide using Kaffir Lime Peel Extract (Citrus hystrix) and Its Application as Adsorbent for Methylene Blue. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maddinedi, S.B.; Mandal, B.K.; Vankayala, R.; Kalluru, P.; Pamanji, S.R. Bioinspired reduced graphene oxide nanosheets using Terminalia chebula seeds extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, B.; Tabrizi, M.A. Green-synthesis of reduced graphene oxide nanosheets using rose water and a survey on their characteristics and applications. RSC Adv. 2013, 3, 13365–13371. [Google Scholar] [CrossRef]
- Arias, F.A.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Gomez, C.V.; Arias, M.; Caputi, L.S. The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide. Nanomaterials 2020, 10, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.-Y.; Hsieh, S.-L.; Ciou, J.-Y.; Huang, Y.-W.; Leang, J.-Y.; Chen, M.-H.; Hou, C.-Y. Lemon juice bioactivity in vitro increased with lactic acid fermentation. Int. J. Food Prop. 2020, 24, 28–40. [Google Scholar] [CrossRef]
- ‘World’s Top Lemon Producing Countries. Available online: www.atlasbig.com (accessed on 8 October 2021).
- Cicero, N.; Corsaro, C.; Salvo, A.; Vasi, S.; Giofrè, S.V.; Ferrantelli, V.; Di Stefano, V.; Mallamace, D.; Dugo, G. The metabolic profile of lemon juice by proton HR-MAS NMR: The case of the PGI Interdonato Lemon of Messina. Nat. Prod. Res. 2015, 29, 1–9. [Google Scholar] [CrossRef]
- Alfadul, S.M.; Hassan, B.H. Chemical Composition of Natural Juices Combining Lemon and Dates. ETP Int. J. Food Eng. 2016. [Google Scholar] [CrossRef]
- Mucci, A.; Parenti, F.; Righi, V.; Schenetti, L. Citron and lemon under the lens of HR-MAS NMR spectroscopy. Food Chem. 2013, 141, 3167–3176. [Google Scholar] [CrossRef]
- Yu, L.; Liao, Z.; Zhao, Y.; Zeng, X.; Yang, B.; Bai, W. Metabolomic analyses of dry lemon slice during storage by NMR. Food Front. 2020, 1, 180–191. [Google Scholar] [CrossRef]
- Mosae Selvakumar, P.; Antonyraj, C.A.; Babu, R.; Dakhsinamurthy, A.; Manikandan, N.; Palanivel, A. Green synthesis and antimicrobial activity of monodispersed silver nanoparticles synthesized using lemon extract. Synth. React. Inorg. Met. Nano-Metal. Chem. 2016, 46, 291–294. [Google Scholar] [CrossRef]
- Sujitha, M.V.; Kannan, S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mahiuddin, M.; Ochiai, B. Green synthesis of crystalline bismuth nanoparticles using lemon juice. RSC Adv. 2021, 11, 26683–26686. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (lemon) phenomenon—A review of the chemistry, pharmacological prop-erties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Gurbani, N.; Han, C.-P.; Marumoto, K.; Liu, R.-S.; Choudhary, R.J.; Chouhan, N. Biogenic Reduction of Graphene Oxide: An Efficient Superparamagnetic Material for Photocatalytic Hydrogen Production. ACS Appl. Energy Mater. 2018, 1, 5907–5918. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Poudel, M.B.; Yu, C.; Kim, H.J. Synthesis of conducting bifunctional polyaniline@Mn-TiO2 nanocomposites for supercapacitor electrode and visible light driven photocatalysis. Catalysts 2020, 10, 546. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, Y.; Podkolzin, S.G.; Lee, W. Band gap of reduced graphene oxide tuned by controlling functional groups. J. Mater. Chem. C 2020, 8, 4885–4894. [Google Scholar] [CrossRef]
- Mathkar, A.; Tozier, D.; Cox, P.; Ong, P.; Galande, C.; Balakrishnan, K.; Reddy, A.L.M.; Ajayan, P.M. Controlled, Stepwise Reduction and Band Gap Manipulation of Graphene Oxide. J. Phys. Chem. Lett. 2012, 3, 986–991. [Google Scholar] [CrossRef]
- Velasco-Soto, M.A.; Pérez-García, S.A.; Alvarez-Quintana, J.; Cao, Y.; Nyborg, L.; Licea-Jiménez, L. Selective band gap ma-nipulation of graphene oxide by its reduction with mild reagents. Carbon 2015, 93, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Kim, H.S. Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 10160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Ahmad, A.; Khan, S.; Ahmad, S.; Khan, I.; Zada, S.; Fu, P. Algal extracts based biogenic synthesis of reduced graphene oxides (rGO) with enhanced heavy metals adsorption capability. J. Ind. Eng. Chem. 2018, 72, 117–124. [Google Scholar] [CrossRef]
- Siong, V.L.E.; Lee, K.M.; Juan, J.C.; Lai, C.W.; Tai, X.H.; Khe, C.S. Removal of methylene blue dye by solvothermally reduced graphene oxide: A metal-free adsorption and photodegradation method. RSC Adv. 2019, 9, 37686–37695. [Google Scholar] [CrossRef]
- Penki, T.R.; Valurouthu, G.; Shivakumara, S.; Sethuraman, V.A.; Munichandraiah, N. In situ synthesis of bismuth (Bi)/reduced graphene oxide (rGO) nanocomposites as high-capacity anode materials for a Mg-ion battery. N. J. Chem. 2018, 42, 5996–6004. [Google Scholar] [CrossRef] [Green Version]
- Qiao, S.-J.; Xu, X.-N.; Qiu, Y.; Xiao, H.-C.; Zhu, Y.-F. Simultaneous Reduction and Functionalization of Graphene Oxide by 4-Hydrazinobenzenesulfonic Acid for Polymer Nanocomposites. Nanomaterials 2016, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Ramesha, G.; Kumara, A.V.; Muralidhara, H.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Sampath, S. Electrochemical reduction of oriented graphene oxide films: An in-situ Raman spectro electro-chemical study. J. Phys. Chem. C 2009, 113, 7985–7989. [Google Scholar] [CrossRef]
- Kolya, H.; Kuila, T.; Kim, N.H.; Lee, J.H. Bioinspired silver nanoparticles/reduced graphene oxide nanocomposites for catalytic reduction of 4-nitrophenol, organic dyes and act as energy storage electrode material. Compos. Part B Eng. 2019, 173, 106924. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Chu, H.-J.; Lee, C.-Y.; Tai, N.-H. Green reduction of graphene oxide by Hibiscus sabdariffa L. to fabricate flexible graphene electrode. Carbon 2014, 80, 725–733. [Google Scholar] [CrossRef]
- Hou, D.; Liu, Q.; Cheng, H.; Zhang, H.; Wang, S. Green reduction of graphene oxide via Lycium barbarum extract. J. Solid State Chem. 2017, 246, 351–356. [Google Scholar] [CrossRef]
- Wang, C.; Ma, G.; Zhou, J.; Zhang, M.; Ma, X.; Duo, F.; Chu, L.; Huang, J.; Su, X. Glycine-functionalized reduced graphene oxide for methylene blue removal. Appl. Organomet. Chem. 2019, 33, e5077. [Google Scholar] [CrossRef]
- Shukla, S.P.; Kisku, G.C. Linear and non-linear kinetic modeling for adsorption of disperse dye in batch process. Res. J. Environ. Toxicol. 2015, 9, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, B.; Niu, W.; Hong, S.; Saif, B.; Wang, S.; Dong, C.; Shuang, S. β-Cyclodextrin modified graphene oxide–magnetic nanocomposite for targeted delivery and pH-sensitive release of stereoisomeric anti-cancer drugs. RSC Adv. 2015, 5, 89299–89308. [Google Scholar] [CrossRef]
- Mao, B.; Sidhureddy, B.; Thiruppathi, A.R.; Wood, P.C.; Chen, A. Efficient dye removal and separation based on graphene oxide nanomaterials. New J. Chem. 2020, 44, 4519–4528. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ochiai, B. Detailed study on rapid removal of cationic dyes using TiO2 -poly(3-chloro-2-hydroxypropyl meth-acrylate) nanocomposite. J. Electrochem. Soc. 2019, 166, B3240–B3245. [Google Scholar] [CrossRef]
- Huang, T.; Yan, M.; He, K.; Huang, Z.; Zeng, G.; Chen, A.; Peng, M.; Li, H.; Yuan, L.; Chen, G. Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. J. Colloid Interface Sci. 2019, 543, 43–51. [Google Scholar] [CrossRef]
- Zarrabi, M.; Haghighi, M.; Alizadeh, R.; Mahboob, S. Solar-light-driven photodegradation of organic dyes on sono-dispersed ZnO nanoparticles over graphene oxide: Sono vs. conventional catalyst design. Sep. Purif. Technol. 2018, 211, 738–752. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; Gemeay, A.H.; El-Sharkawy, R.G. Effective disposal of methylene blue using green immobilized silver na-noparticles on graphene oxide and reduced graphene oxide sheets through one-pot synthesis. Environ. Monit. Assess. 2020, 192, 355. [Google Scholar] [CrossRef] [PubMed]







| Models and Equations | Temperature | Parameters | |||
|---|---|---|---|---|---|
| K1 (×10−2/min) | qcal (mg/g) | qexp (mg/g) | R2 | ||
| pseudo–first order | 298 K 313 K 333 K | 6.1 6.2 6.2 | 93.4 77.1 86.6 | 104.3 104.2 105.4 | 0.998 0.977 0.989 |
| K2 (×10−3 g/(mg·min) | qcal (mg/g) | qexp (mg/g) | R2 | ||
| pseudo–second order | 298 K 313 K 333 K | 1.4 1.9 1.6 | 111.1 108.7 111.1 | 104.3 104.2 105.4 | 0.999 0.999 0.999 |
| kdif (mg/(g·min1/2)) | Ri | C (mg/g) | R2 | ||
| Intraparticle diffusion | 298 K | 3.4 | 0.34 | 69.6 | 0.733 |
| 313 K | 2.8 | 0.28 | 75.5 | 0.659 | |
| 333 K | 3.2 | 0.32 | 72.6 | 0.667 | |
| R2 | |||||
| Boyd kinetic | 298 K | 0.998 | |||
| 313 K | 0.977 | ||||
| 333 K | 0.989 | ||||
| Models and Equations | Constants | |
|---|---|---|
| Langmuir | qm (mg/g) | 130.2 |
| RL (C0 = 8 mg/L C0 = 10 mg/L C0 = 12 mg/L C0 = 14 mg/L C0 = 16 mg/L C0 = 18 mg/L) | 0.0245 0.0197 0.0164 0.0141 0.0124 0.011 | |
| b (L/mg) | 4.98 | |
| R2 | 0.988 | |
| Freundlich | n | 4.11 |
| KF (mg/g(L/mg)1/n) | 99.7 | |
| R2 | 0.898 | |
| Temkin | bT | 102.76 |
| ; considering | AT (L/g) | 67.33 |
| B (J/mol) | 24.1 | |
| R2 | 0.852 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahiuddin, M.; Ochiai, B. Lemon Juice Assisted Green Synthesis of Reduced Graphene Oxide and Its Application for Adsorption of Methylene Blue. Technologies 2021, 9, 96. https://doi.org/10.3390/technologies9040096
Mahiuddin M, Ochiai B. Lemon Juice Assisted Green Synthesis of Reduced Graphene Oxide and Its Application for Adsorption of Methylene Blue. Technologies. 2021; 9(4):96. https://doi.org/10.3390/technologies9040096
Chicago/Turabian StyleMahiuddin, Md., and Bungo Ochiai. 2021. "Lemon Juice Assisted Green Synthesis of Reduced Graphene Oxide and Its Application for Adsorption of Methylene Blue" Technologies 9, no. 4: 96. https://doi.org/10.3390/technologies9040096
APA StyleMahiuddin, M., & Ochiai, B. (2021). Lemon Juice Assisted Green Synthesis of Reduced Graphene Oxide and Its Application for Adsorption of Methylene Blue. Technologies, 9(4), 96. https://doi.org/10.3390/technologies9040096







