Abstract
Introduction: In chest wall reconstruction, the main objectives are the restoration of the chest wall integrity, function, and aesthetic, which is often achieved with the placement of implants. We aimed to evaluate whether 3D printed models can be useful for preoperative planning and surgical treatment in chest wall reconstruction to improve the outcome of the surgery and to reduce the rate of complications. Methods: We conducted a systematic review of literature using PubMed, Scopus, Embase, and Google Scholar databases until 8 November 2021 with the following keywords: (“3D printing” or “rapid prototyping” or “three-dimensional printing” or “bioprinting”) and (“chest wall” or “rib” or “sternum” or “ribcage” or “pectus excavatum”). Results were then manually screened by two independent authors to select studies relevant to 3D printing application in chest wall reconstruction. The primary outcome was morphological correction, and secondary outcomes were changes in operating time and procedure-related complication rate. Results: Eight articles were included in our review. Four studies were related to pectus excavatum correction, two studies were related to rib fracture stabilization, and two studies were related to chest wall tumor resection and reconstruction. Seven studies reported 3D printing of a thorax model or template implants for preoperative planning and implant modeling, and one study reported 3D printing of a PEEK prosthesis for direct implantation. Four studies reported comparison with a conventionally treated control group, and three of them detected a shorter operative time in the 3D printing model-assisted group. Satisfactory morphological correction was reported in all studies, and six studies reported a good implant fitting with minimal need for intraoperative adjustments. There were no major intraoperative or postoperative complications in any of the studies. Conclusions: The use of 3D printing models in chest wall reconstruction seems to be helpful for the production of personalized implants, reducing intraoperative adjustments. Results of morphological correction and postoperative recovery after the 3D printing-assisted surgery were satisfactory in all studies with a low rate of complication. Our literature review suggests good results regarding prosthesis fitting, accuracy of surgical planning, and reduction in operative time in 3D printing-assisted procedures, although more evidence is needed to prove this observation.
1. Introduction
Chest wall reconstruction is required in different situations, such as traumatic injuries, thoracic tumors, or chest wall malformations. The main objectives in reconstructing the thoracic wall are restoring the chest wall integrity and rigidity, preserving the physiological function of the thorax, and protecting internal organs, while pursuing a good aesthetic result [1].
Conventional methods for chest wall reconstruction are represented by prosthetic replacements using biologic (allografts and homografts), synthetic (methyl methacrylate, PTFE, polypropylene), or metallic (titanium, stainless steel) meshes, bars, or plates, depending on the pathology of interest [2].
The prosthesis fitting is crucial to achieve all of the mentioned objectives, and 3D reconstruction based on CT images offers the possibility to accurately study the pathological anatomy of the chest wall, thus allowing the procedure to be meticulously planned and the prosthetic materials to be provided in advance [3]. The application of 3D reconstruction is implemented in preexisting imaging techniques that are commonly used in chest wall visualization. These are mostly CT, but also MRI and US, when appropriate in preoperative study or postoperative evaluation of complications.
In the surgical field, 3D printing has been embraced as a tool for preoperative planning, surgical simulation and training [4], patient education [5], and to produce templates or molds for surgical implants [6,7] for 3D printing prosthesis and implants (especially in orthopedic and maxillofacial surgery) [8] and in tissue engineering (3D bioprinting) [9].
Due to its recent development in the medical field, reports regarding the use of 3D printing in thoracic surgery, and specifically in chest wall reconstruction, are still limited. Nonetheless, many aspects of 3D printing-assisted procedures are of interest, including the possibility to better understand the anatomy of complex situations, such as large thoracic tumors, which often invade adjacent structures, thus making ita challenge for the surgeon to perform a successful resection while needing to achieve R0 resection margins. Another interesting application is the possibility to project custom-made implants, specifically built on the anatomy of the patient, and to rehearse the procedure on a customized model.
Analyzing current evidence on this matter, we aimed to evaluate whether 3D printed models could be useful for preoperative planning and surgical treatment in chest wall reconstruction to improve the outcome of the surgery and to reduce the rate of complications.
2. Materials and Methods
2.1. Search Strategy
We conducted a systematic review according to the PRISMA protocol [10] to assess the current use of 3D printing in chest wall reconstruction. Four medical databases (PubMed, Scopus, Embase, and Google Scholar, updated to 8 November 2021) were searched to collect studies regarding the subject of interest using the following keywords: (“3D printing” or “rapid prototyping” or “three-dimensional printing” or “bioprinting”) and (“chest wall” or “rib” or “sternum” or “ribcage” or “pectus excavatum”). We conducted an additional manual search of the bibliographies of the selected articles. The details of the database search are reported in Table 1.

Table 1.
Details of the database search.
2.2. Selection Process
We included in our review: (I) papers published in English; (II) studies that reported surgical application of 3D printing models for preoperative planning or surgical reconstruction of the chest wall; (III) original research articles. The exclusion criteria were as follows: (I) papers not published in English; (II) case reports, case series including fewer than 5 patients, reviews, abstracts, meta-analyses, brief communications, editorials, and letters; (III) papers based on the same study population, in which case we selected the most recent article to avoid duplication.
Two independent reviewers (BL, AN) analyzed the search results, and proceeded to inspect the articles’ titles and abstracts to exclude papers that were not related to the topic of interest. Full text of the remaining articles was then evaluated to determine if they met our inclusion/exclusion criteria. Disagreements were resolved through discussion with two senior reviewers (AF, MS).
The primary outcome of our review was morphological correction, secondary outcomes were changes in operating time and procedure-related complication rate.
2.3. Quality and Risk of Bias Assessment
The quality and risk of bias for each study was independently evaluated using the Downs and Black assessment checklist [11] by two authors (BL, AN). Differences between the two reviewers were solved through discussion with a third author (AF). The total score for this 27-item checklist ranges from 0 to 28 points. We considered scores as follows: Excellent (24–28 points), Good (19–23 points), Fair (14–18), Poor (<14 points). The synthetized result of the assessment is reported in Table 2, indicating an overall good methodological quality of the considered studies.

Table 2.
Quality assessment results (Downs and Black checklist).
3. Results
A total of 708 titles were identified via the search of the previously mentioned databases, whereas no additional study of interest was found via a manual search of the bibliographies of the selected studies (Figure 1). We subsequently excluded 299 papers as duplicates.
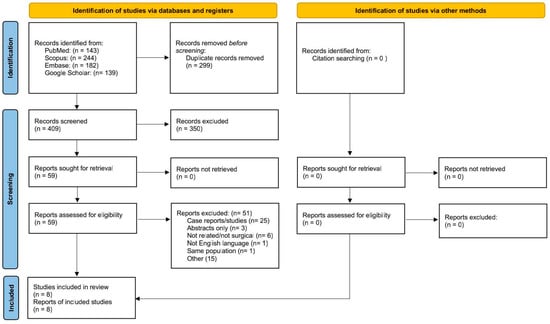
Figure 1.
Flow chart of the study according to PRISMA guidelines.
Based on the titles and abstracts, the remaining 409 papers were evaluated, and 350 of these were excluded as the object was not related to the topic of our research. The remaining 59 articles were analyzed through full-text examination by all authors, who further excluded 51 studies. Finally, a total of eight papers were selected, which constitute the subject of our systematic review.
The following data were extracted from the selected papers, as summarized in Table 3: the authors, the year of publication, the country, the study design, the number of patients that received a 3D printing assisted procedure, the application, the disease of interest, the study limitations, the structure printed, the processing software, the 3D printer, the materials.

Table 3.
Characteristics of the studies evaluated.
3.1. Study and Patient Characteristics
The eight studies included in our review were published between the year 2018 and 2021; six studies were from China, one was from Spain, and one was from Argentina. There were five prospective studies and three retrospective studies. Four studies were related to pectus excavatum correction, two to rib fracture stabilization, and two studies to chest wall tumor resection and reconstruction.
In all studies, 3D printing models were used for preoperative planning, whereas in only one study (Wang et al., 2019) it was also used for chest wall repair.
3.2. Rib Fracture Fixation
Zhou et al. [12] treated 16 patients with high complex rib fractures (identified as patients with one to three fractures in the 2nd to 4th ribs, with three fracture segments in each fractured site, the middle fracture segment ≤ 5 cm and presence of costal cartilage fracture) with framework locking-plate internal fixation combined with 3D printing. A 3D ribcage model based on 64-row spiral CT images was processed then imported into the 3D printer to obtain a true-size photosensitive resin model of the fractured ribs. The model was used to study the morphology of the fracture site, to select an appropriate incision site, and to simulate the fracture reduction. Placement, direction, length, and number of screws needed for fixation were planned and recorded based on the 3D printed rib model. During the surgery, there was no need for intraoperative adjustments to the locking plate. Follow-up at 5 to 10 months evaluated the recovery of the patients. A satisfactory chest wall reconstruction was achieved in all cases, confirmed by chest RX or CT scans after surgery.
Chen et al. [13] retrospectively evaluated the data of 48 patients who underwent surgical stabilization of rib fractures (SSRF). Patients were split into two groups based on whether or not 3D printing was used (32 patients in the conventional SSRF group vs. 16 in the 3D assisted SSRF group). Before surgery, in the 3D printing-assisted group, a 3D reconstruction was created from CT images (1 mm slice thickness), then a 3D printed model of the ribcage was produced in acrylonitrile butadiene styrene. Based on the model, the titanium plates were bent and cut to fit the patient’s ribcage profile, and the length and number of the screws was recorded. The incision site was planned according to the 3D printed model. In comparison with the conventional SSRF group, the mean operative time (175.24 vs. 125 min p = 0.003,) and the mean operative time per fixed plate (52.99 vs. 35.41 min, p < 0.001) were significantly shorter in patients in the 3D printing SSRF group. The incision length (14.19 vs. 8.71 cm, p = 0.002) and the wound length per fixed rib (4.19 vs. 2.54 cm, p< 0.001) were significantly smaller in the 3D printing SSRF group. There was no statistically significant difference in hospital stay, ICU stay, and postoperative complications (empyema, broken plates, and wound infection happened in four patients in the SSRF group) between the two groups.
3.3. Pectus Excavatum Repair
Bellia-Munzon et al. [14] prospectively collected data of 130 patients who underwent minimally invasive repair of pectus excavatum (MIRPE) with the use of 3D printing. All patients underwent a CT scan with 3D reconstruction (1 mm slice thickness). A flexed 3D printed template of the implant bars was elaborated based on the CT images and clinical information of the patient through specifically developed semiautomatic software that considered the entry sites to the thorax, the curvature, and the anteroposterior pressure to which the implant would be subjected. The 3D printed template was checked over the patient’s chest surface in a fitting ambulatory session to make modifications to the template if necessary. The number of implants was determined based on the processing software recommendations and the anatomy of the sternum (particularly the percentage of sternum that lay behind the anterior costal line) and the prevision was accurate in all patients. The final metallic prebent implants were custom made in according to the 3D printed template and manufactured in surgical steel or titanium. During the surgery the implants were placed on the patient’s chest to determine the final location and direction, then the implant tied to a tape was pulled back through a retrosternal tunnel. The removal of implants was planned two–three years later. When the tip of the sternum ended up between two bars resulting in undercorrection, the bars were placed in the crossed position instead of parallel (13.8% of patients). The number of implants introduced was 337, ranging from 1 to 4 per patient (2 or 3 bars in the majority of cases). Of the 130 patients, 120 (92.3%) had an optimal “implant-deformity” match not requiring any modifications; in seven patients (5.4%) the implant needed minimal rebending(achieved without flipping the bars); in two patients (1.5%) the implants were too short; and in one patient (0.8%) with an extremely asymmetric chest the implant was removed and rebent intraoperatively. Comparing the operative time of patients with pectus excavatum treated in the same hospital before the 3D printing model implementation (group A,) with the cohort described in the study (group B), a significant reduction was observed (group A 125.4 ± 30.7, vs. group B 87.6 ± 49.9 min; p < 0.0001), also considering the operative time adjusted to the number of bars per operation (group A 78.1 ± 31.7, vs. group B 41.8 ± 14.7 min per implant; p < 0.0001). The number of bars introduced was increased in the 3D printing group (group A 1.7 ± 0.6, vs. group B 2.6 ± 0.5 implants per patient; p < 0.0001). There were no intraoperative complications including bleeding. Postoperative complications included two patients with pleural effusion prior to discharge.
Wang et al. [15] (2020) treated six patients with pectus excavatum with the non-thoracoscopic extrapleural Nuss procedure. All patients received a 3D CT scan (1.25 mm slice thickness), and the images were exported as DICOM files to the imaging software to elaborate a 3D thoracic model. A flexible ribcage model was printed in polylactic acid. The flexibility of the materials allowed simulation of the pectus excavatum correction, thus predicting the repair efficacy. The thoracic model was used to study the dimensions of the chest deformity, to plan the trajectory of the steel bars, and to find the optimal substernal force point through simulation of the actual procedure. The repair efficacy was found to be uniform between the prediction on the 3D printed model and the surgical procedure on the patients.
Huang et al. [16] retrospectively analyzed data of 419 patients that underwent the Nuss procedure and selected 357 patients between the traditional Nuss procedure (TN, 342 patients) and the 3D printing model-assisted Nuss procedure (3DPMAN, 15 patients). In 3DPMAN, a standard spiral CT of the thorax was used to generate a 3D-reconstructed thorax model through a processing software. Custom-made template pectus bars and a predicted postoperative 3D thorax model were produced through a computer-aided design process. The template bars were 3D printed with polylactic acid, then metallic pectus bars were manufactured according to a 3D template in the 3DPMAN group and according to a metallic measuring tape in the TN group.
In the TN group, six patients (1.7%) experienced flipping of the metallic bars, two patients experienced migration (0.56%), and two patients (0.56%) experienced dislocation of the metallic bars. No patient experienced surgery-related complications or dislocation of the bars in the 3DPMAN group. A shorter operative time was observed in the 3DPMAN group compared with the TN group (60.36 vs. 74.34 min, p < 0.001), fewer pectus bar insertion (1.000 versus 1.360, p < 0.001), and better morphological correction (HI: 20.34% versus 10.06%, p < 0.001).
Gaspar Pérez et al. [17] prospectively collected data of six patients treated for pectus excavatum with the 3D printed model-assisted Nuss procedure. A diagnostic CT scan was performed in all patients (0.625 mm thickness). The number of bars needed for correction and the appropriate intercostal space for insertion were established preoperatively based on CT scan images and physical characteristics of the patients, then the CT images were uploaded to the segmentation and design software to simulate the morphological repair and establish the Nuss bar size and length. The simulated bar model and chest anatomical model were 3D printed in polylactic acid, then the customized Nuss bar was reproduced in titanium according to the 3D printed bar model. A single Nuss bar was introduced in all cases, and the median operating time was 82 min. There was no need for intraoperative bar replacement or removal, while a single patient experienced intraoperative complications (4 mm distal transfixation perforation of the lingula, with self-limited bleeding and absence of air leaks). No postoperative complications were observed in any patients. Correction was defined as highly satisfactory in all patients.
3.4. Chest Wall Tumor Resection and Reconstruction
Wu et al. [18] reviewed data of six patients with thoracic wall tumors that underwent a 3D printed assisted resection and reconstruction and 10 patients treated with the conventional surgery. The patients in the 3D assisted group underwent thin-slice CT scan, then the DICOM images were imported into the processing software to elaborate a 3D model of the chest wall tumor and adjacent structures. The 3D model, printed using a liquid photosensitive resin, was used to plan the surgery, focusing on the 3D morphology, location, and spatial relationships of the tumor. The titanium implant plate for reconstruction was designed according to the surgical resection line drawn on the 3D printed model, to best fit the thoracic wall defect. Follow-up was conducted at 15 and 90 days after the surgery. The 3D conformal titanium plates were in all cases completely consistent with chest wall defect after tumor resection, even though the tumors of the six patients were different in size and the volume was relatively large. In the 3D printing group, less intraoperative bleeding was reported in relation to the conventional surgery group. No postoperative complications, including plate displacement, were observed in the 3D = assisted group, and there were no significant changes in respiratory function after the surgery (p < 0.05). In the conventional surgery group, four patients had complications that consisted of infection and puncture of the artery or skin by the titanium plate. Postoperative pain score was lower in the 3D group. Recovery time was reported to be shortened in 3D group (1 week vs. 2 weeks).
Wang et al. [19] (2019) treated 18 patients that underwent wide resection of a chest wall tumor using 3D printed polyetheretherketone (PEEK) implants. In eight cases the tumor affected the sternum and in 10 cases the ribs. For all patients, a CT scan with slice thickness at 0.90 mm was performed using a 64-detector CT scanner. The images were imported into the processing software for surgical planning and to design 3D PEEK protheses of the ribs or the sternum. The wide excision of the tumor resulted in a defect in the anterior chest wall of at least 8 cm × 8 cm; the reconstruction followed the “sandwich technique”. The mean duration of the surgery was 174 ± 54 min, the mean blood loss was 297 ± 235 mL. No complications were observed in the 6 to 12 months after the operation. The respiratory function was tested 1 week before the operation and 3 months after the operation, showing a mean reduction in FVC of 0.39 ± 0.28 L (14.0% of the preoperative FVC value). The postoperative FVC was reduced significantly in both ribs and sternum patients (p < 0.001), whereas no significant difference was observed in postoperative MVV and FEV1/FVC.
4. Discussion
In preoperative planning, visualization of the anatomy and pathology of the patient is essential for the surgeon. Compared with standard CT images, 3D reconstruction offers the surgeon the possibility to examine and study the pathology in a more realistic way. Three-dimensional printed models place the 3D reconstruction directly into the surgeon’s hands, opening a new phase in surgical planning and training.
The results of our review highlighted some potential benefits of the 3D printing application in chest wall reconstruction. The realization of 3D thorax models contributes to a better understanding of the anatomy of the chest wall defect, and therefore enables the surgeon to plan the surgical steps in advance. An accurate selection of number, location, and direction of implants was made before the operation in all the studies of our review, with the aid of 3D reconstruction and with a 3D printed model of the thorax. Regarding selection of the incision site, Chen et al. [13] and Zhou et al. [12] recorded in their studies a smaller incision site in 3D printing-assisted surgery compared to conventional SSRF. Surgical planning and rehearsal of the procedure can contribute to reducing operative time, as stated by three studies [13,14,16] in our review that compared the 3D-assisted group operating time with that of a conventionally treated control group.
Another interesting aspect is the possibility of manufacturing a custom-made well-fitting prosthesis with minimal need for intraoperative adjustments [12,14,15,16,17,18], often needed in conventional procedures of bar implant or plate insertion [20,21]. Intraoperative adjustments of bars and plates can prolong the operative time and can cause scratching of the implants’ surface, which may damage the surrounding tissues and favor adherences. A 3D printed template fitting directly on the patient and simulation of the procedure on the thorax model allowed anticipation of the modifications needed to the implant.
In all studies, no major intraoperative or postoperative complications were found in the 3D printed-assisted surgeries. Wu et al. [18] observed less intraoperative bleeding in the 3D-assisted group compared to the conventional group. The difference between preoperative and postoperative respiratory function was assessed by two studies [18,19] with contrasting results. Wu et al. [18] found no significant difference, whereas Wang et al. [19] observed a reduced FVC and no difference in postoperative MVV and FEV1/FVC.
Huang et al. [16] reported some cases of bars’ dislocation and flipping in the traditional surgery group, compared to no cases in the 3D printing-assisted group. Referring to morphological correction, only Huang et al. [16] used a quantitative method to evaluate this outcome, detecting a better correction in patients treated with a 3D printing-assisted technique in comparison to the conventional method, whereas all the other studies recorded satisfactory morphological correction and good implant fitting but did not support this data with a statistical comparison to conventional techniques. Recovery time was directly addressed by Wu et al. [18],who noted a shorter recovery time in the 3D-assisted group and a lower postoperative pain score; and by Zhou et al. [12], who reported a NRS score of 4 or less, and consequently stopped analgesic drugs at 7 days post-procedure in the majority of patients.
For the matter of direct prosthetic printing, Wang et al. [19] interestingly reported the use of sternum and ribs prostheses that were 3D printed in PEEK as an alternative to conventional titanium prostheses due to the physical properties of PEEK, which has a low elastic modulus and similar flexural and tensile strength to the sternum and ribs, and shorter manufacturing time [22,23]. It should be noted that there is not much evidence in the literature regarding the direct implantation of 3D printed prostheses for thoracic wall reconstruction, and the majority of the studies reporting such an application at present are case reports, and were therefore not included in our review.
Production time is a known limitation in 3D printed model applications in surgery, and this is also true of thoracic wall reconstruction. Particularly in emergency situations, such as ribcage fractures associated with damage to the internal organs, time of intervention is critical and the application of 3D printing, which requires at least 5 h for the production of a thoracic model [13], may not be convenient. Conversely, in elective surgery it is possible to schedule the surgical steps in advance and, even if the production time requires several hours, the procedure can be performed without delay. Bellia-Munzon et al. [14] reported 1 working day to print the template and 3 working days for the manufacture of the final metallic bars. Chen et al. [13] reported a minimum of 5–6 h to print the ribcage model. Wang et al. [19] reported approximately 30 h to completely manufacture a PEEK implant.
Another limitation of the application of 3D printing to surgery is the cost, which comprises the printer cost, the processing software cost, and the printing materials cost. The 3D printing utilization cost may be counterbalanced by the shorter operative time [24] and by the benefits that derive from the personalization of the implants, although there is an open debate on this topic. Bellia-Munzon et al. [13] stated that the cost of the implants projected with the assistance of 3D printing is not superior to that of conventional implants. Huang et al. [15] observed that manufacturing a customized prebent bar based on CT images would be more expensive than modeling the bar based on the 3D printing template, and that the costs were further reduced by the fewer insertion of bars in the 3D printing-assisted group.
The main limitation of our review is the reduced number of experimental studies on the topic and the lack of prospective randomized studies. The recent development of 3D printing applications in the surgery field and the fact that not all hospitals are equipped with 3D printers can partly explain the reduced evidence on the subject.
Furthermore, only four of the eight studies presented a control group, which allows us to perform a stronger evaluation of the differences between 3D printing-assisted techniques and conventional techniques. The small sample size in most of the studies is also a crucial limitation, which affects the variability of the data and leads to the risk of selection bias.
5. Conclusions
The application of 3D printing models in chest wall reconstruction can be useful for designing and manufacturing personalized implants, specifically designed on the anatomy of the patient, and therefore reducing intraoperative adjustments. The results of morphological correction and postoperative recovery after 3D printing-assisted surgery were satisfactory in all studies, with a low rate of complications. Although our data point towards a better outcome regarding prosthesis fitting, accuracy of surgical planning, and reduction in operative time in 3D printing-assisted procedures, due to the limitations of our study, more evidence is needed to prove the presented findings.
Author Contributions
Conceptualization, B.L. and A.F.; methodology, A.C. and M.B.; software, A.N.; validation, G.N., G.O. and R.M.; formal analysis, D.P.; investigation, F.C.; resources, V.D.F.; data curation, G.M., F.F., A.C.I. and G.V.; writing—original draft preparation, B.L.; writing—review and editing, M.S. and A.F.; visualization, G.V.; supervision, A.F. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data were available from the articles cited in the review and reported in the References.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pascal, A.T.; Laurent, B. Prosthetic reconstruction of the chest wall. Thorac. Surg. Clin. 2010, 20, 551–558. [Google Scholar]
- Sanna, S.; Brandolini, J.; Pardolesi, A.; Argnani, D.; Mengozzi, M.; Dell’Amore, A.; Solli, P. Materials and techniques in chest wall reconstruction: A review. J. Vis. Surg. 2017, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, I.; Evans, P.L.; Goodrum, H. Chest wall reconstruction with an anatomically designed 3-D printed titanium ribs and hemi-sternum implant. 3D Print. Med. 2020, 6, 26. [Google Scholar] [CrossRef]
- Ganguli, A.; Pagan-Diaz, G.J.; Grant, L.; Cvetkovic, C.; Bramlet, M.; Vozenilek, J.; Kesavadas, T.; Bashir, R. 3D printing for preoperative planning and surgical training: A review. Biomed. Microdevices 2018, 20, 65. [Google Scholar] [CrossRef]
- Zhuang, Y.-D.; Zhou, M.-C.; Liu, S.-C.; Wu, J.-F.; Wang, R.; Chen, C.-M. Effectiveness of personalized 3D printed models for patient education in degenerative lumbar disease. Patient Educ. Couns. 2019, 102, 1875–1881. [Google Scholar] [CrossRef]
- Lu, T.; Shao, Z.; Liu, B.; Wu, T. Recent advance in patient-specific 3D printing templates in mandibular reconstruction. J. Mech. Behav. Biomed. Mater. 2020, 106, 103725. [Google Scholar] [CrossRef]
- Hay, J.A.; Smayra, T.; Moussa, R. Customized Polymethylmethacrylate Cranioplasty Implants Using 3-Dimensional Printed Polylactic Acid Molds: Technical Note with 2 Illustrative Cases. World Neurosurg. 2017, 105, 971–979. [Google Scholar]
- Diment, L.E.; Thompson, M.S.; Bergmann, J.H.M. Clinical efficacy and effectiveness of 3D printing: A systematic review. BMJ Open 2017, 7, e016891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, Y.-J.; Kim, C.-H. 3D Bioprinting Technologies for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1078, 15–28. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, S.H.; Black, N. The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-Randomised Studies of Health Care Interventions. J. Epidemiol. Community Health 1979, 52, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, D.; Xie, Z.; Yang, Y.; Chen, M.; Liang, Z.; Zhang, G.; Li, S. Application of 3D printing and framework internal fixation technology for high complex rib fractures. J. Cardiothorac. Surg. 2021, 16, 5. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Lin, K.-H.; Huang, H.-K.; Chang, H.; Lee, S.-C.; Huang, T.-W. The beneficial application of preoperative 3D printing for surgical stabilization of rib fractures. PLoS ONE 2018, 13, e0204652. [Google Scholar] [CrossRef]
- Bellia-Munzon, G.; Martinez, J.; Toselli, L.; Peirano, M.N.; Sanjurjo, D.; Vallee, M.; Martinez-Ferro, M. From bench to bedside: 3D reconstruction and printing as a valuable tool for the chest wall surgeon. J. Pediatr. Surg. 2020, 55, 2703–2709. [Google Scholar] [CrossRef]
- Wang, L.; Guo, T.; Zhang, H.; Yang, S.; Liang, J.; Guo, Y.; Shao, Q.; Cao, T.; Li, X.; Huang, L. Three-dimensional printing flexible models: A novel technique for Nuss procedure planning of pectus excavatum repair. Ann. Transl. Med. 2020, 8, 110. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Lin, K.-H.; Chen, Y.-Y.; Wu, T.-H.; Huang, H.-K.; Chang, H.; Lee, S.-C.; Chen, J.-E.; Huang, T.-W. Feasibility and Clinical Effectiveness of Three-Dimensional Printed Model-Assisted Nuss Procedure. Ann. Thorac. Surg. 2019, 107, 1089–1096. [Google Scholar] [CrossRef]
- Fillat-Gomà, F.; Coderch-Navarro, S.; Monill-Raya, N.; JE, B.A.; Martínez, S.; San Vicente Vela, B.; Jiménez-Arribas, P.; Güizzo, J.R. Initial experience with 3D printing in the use of customized Nuss bars in pectus excavatum surgery. Cir. Pediatrica 2021, 34, 186–190. [Google Scholar]
- Wu, Y.; Chen, N.; Xu, Z.; Zhang, X.; Liu, L.; Wu, C.; Zhang, S.; Song, Y.; Wu, T.; Liu, H.; et al. Application of 3D printing technology to thoracic wall tumor resection and thoracic wall reconstruction. J. Thorac. Dis. 2018, 10, 6880–6890. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, L.; Li, X.; Zhong, D.; Li, D.; Cao, T.; Yang, S.; Yan, X.; Zhao, J.; He, J.; et al. Three-Dimensional Printing PEEK Implant: A Novel Choice for the Reconstruction of Chest Wall Defect. Ann. Thorac. Surg. 2019, 107, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.; Guerriero, V.; Wong, M.C.Y.; Palo, F.; Lena, F.; Mattioli, G. Complications and trends in minimally invasive repair of pectus excavatum: A large volume, single institution experience. J. Pediatric Surg. 2021, 56, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kaghazchi, A.; Sun, B.; Woodward, A.; Forrester, J.D. Systematic Review and Meta-Analysis of Hardware Failure in Surgical Stabilization of Rib Fractures: Who, What, When, Where, and Why? J. Surg. Res. 2021, 268, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, T.; Li, X.; Huang, L. Three-dimensional printing titanium ribs for complex reconstruction after extensive posterolateral chest wall resection in lung cancer. J. Thorac. Cardiovasc. Surg. 2016, 152, e5–e7. [Google Scholar] [CrossRef] [Green Version]
- Aranda, J.L.; Jiménez, M.F.; Rodríguez, M.; Varela, G. Tridimensional titanium-printed custom-made prosthesis for sternocostal reconstruction. Eur. J. Cardiothorac. Surg. 2015, 48, e92–e94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, D.H.; Mills, P.; Duszak, R. Medical 3D Printing Cost-Savings in Orthopedic and Maxillofacial Surgery: Cost Analysis of Operating Room Time Saved with 3D Printed Anatomic Models and Surgical Guides. Acad Radiol. 2020, 27, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).