Abstract
An enzyme-loaded disk-shaped cartridge was fabricated using CO2-assisted polymer compression (CAPC), which is a polymer-bonding method that does not use heat. In this process, after the enzyme was loaded onto mesoporous silica, it was placed in a container fabricated from laminated fibrous sheets using CAPC. Subsequently, the lid and container were bonded via CAPC. Verification using the reductive decomposition reaction of an azo dye (methyl red) showed that the enzyme was not deactivated and that a reaction cartridge was created successfully.
1. Introduction
Enzymatic reactions, being used in pharmaceutical manufacturing and food industries, are indispensable in our daily lives [1,2]. However, enzymatic reactions require enzyme removal after the completion of the reaction and, to avoid this, the enzyme had to be supported or immobilized on a carrier. Various methods of immobilization have been proposed, including the use of polymers [3], cellulose [4], silica [5], ceramics [6], metal–organic frameworks [7] and carbon nanotubes [8]. One way to facilitate enzyme handling is to create a cartridge that confines the enzyme. The enzyme-loaded cartridge requires a low temperature during its fabrication to reduce enzyme deactivation. Additionally, considering the future development of applications of enzyme-loaded cartridges in food and pharmaceuticals, it is desirable to create cartridges only with safe substances approved as food additives. The use of CO2 during cartridge fabrication is one of the solutions [9].
The CO2-assisted polymer compression (CAPC) method, in which fibrous sheets are bonded using CO2 at room temperature to fabricate porous materials, is a polymer processing method that satisfies both conditions [10]. In the CAPC method, enzymes are placed in the fibrous sheets and are pressed in the presence of CO2, which is expected to encapsulate the enzymes in the sheets. Because the process is performed at room temperature, it is expected to cause little damage to the enzyme. However, to properly encapsulate the enzyme, the size of the enzyme must be smaller than the mesh of the fiber after the crimping process, and many enzymes do not fulfill this condition. This can be easily deduced from the fact that the pore size using the CAPC method is ~1 μm in previous studies [11].
Therefore, this study considered encapsulating the enzyme in a fibrous sheet after loading it onto mesoporous silica. The loading of enzymes onto mesoporous silica has been reported previously [12,13]. The disk-shaped reaction cartridge was fabricated by compressing the nonwoven fabric to reduce the mesh size and then encapsulating the mesoporous silica with the fabric. This multistep CAPC encapsulation is an application of the concept of multilayer-filter fabrication [14]. In Figure 1, azo reductase (AzoR) is used as a model enzyme, and the decomposition reaction of the azo dye is demonstrated.
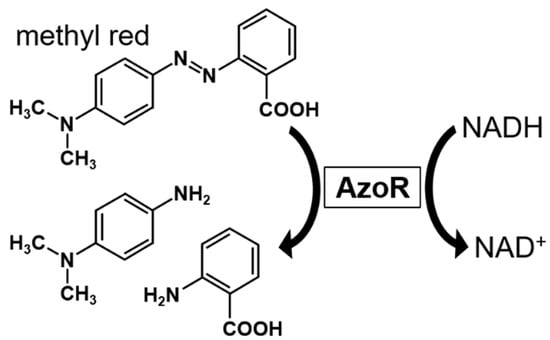
Figure 1.
Reaction scheme of methyl red degradation to 4-(N,N-dimethylamino)aniline and anthranilic acid using azo reductase (AzoR). AzoR requires reduced nicotinamide adenine dinucleotide (NADH) as a coenzyme.
2. Materials and Methods
Polyethylene terephthalate pellets (model number: TK3) were supplied by Bell Polyester Products, Inc. (Houfu, Japan). A nonwoven fabric made of polyethylene terephthalate with an average fiber diameter of 4 μm and a basis weight of 30 g/m2 was manufactured by Nippon Nozzle Co. (Kobe, Japan) using the melt blowing method. The nonwoven fabric was cut into a circle (outer diameter: 31 mm) and a donut (outer diameter: 31 mm; inner diameter: 18 mm) using a laser-processing machine. CO2 (>99.995% purity, Nippon Ekitan Co., Minato-ku, Japan) was used for CAPC treatment.
The procedure of CAPC treatment is presented in Figure 2. The stacked raw nonwoven fabrics were set in a high-pressure vessel (inner diameter: 32 mm), and the vessel was placed below a piston. The piston was lowered to the CO2 introduction position (1.2 mm for the 1st and 2nd CAPC and 2.4 mm for the 3rd CAPC). At this state, the V2 valve was closed, then the V1 valve was opened, and gaseous CO2 was introduced at a vapor pressure of 6 MPa. The CO2 was introduced while retaining the air that was initially in the high-pressure vessel. Then, the piston was lowered to the press position (0.7 mm for the 1st and 2nd CAPC and 1.8 mm for the 3rd CAPC) and stopped at the press position for a certain period. Then, the V3 valve was opened, and the CO2 was slowly evacuated over 30 s, and the V2 valve was opened to release the remaining CO2 into the atmosphere at once. The piston was then raised, and the CAPC product was taken out. The time for stopping the piston at the press position was 4 s for the 1st CAPC treatment to create the lid, 4 s for the 2nd CAPC to create the container and 20 s for the 3rd CAPC to join the lid and container. The process was performed at room temperature (22 °C) and the high-pressure vessel and piston were not heated during the process.
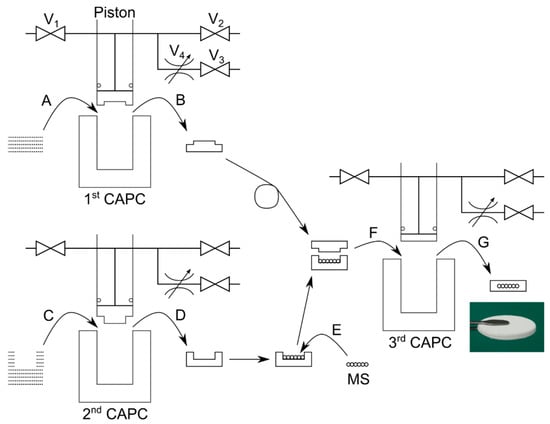
Figure 2.
Fabrication of an enzyme-loaded disk-shaped cartridge. V1 = introduction valve; V2 = exhaust valve; V3 = exhaust valve; V4 = metering valve; and MS = enzyme-loaded mesoporous silica. A, C, F = introduction into high-pressure vessel; B, D, G = removal from high-pressure vessel; and E = placement of mesoporous silica in container.
In the 1st CAPC treatment, 20 sheets of nonwoven fabric with a diameter of 31 mm were placed in a high-pressure vessel (Figure 2A) and compressed with an 18 mm recess (depth = 0.5 mm) at the tip of the piston to produce a disk with a slightly raised center (Figure 2B). This will become the lid of the container.
In the 2nd CAPC treatment, a combination of 20 sheets of nonwoven fabric with diameter 31 mm and 20 donut-shaped sheets of nonwoven fabric with diameter 31 mm and holes of diameter 18 mm were placed in a high-pressure vessel (Figure 2C) and compressed with a piston head with 18 mm protrusion (height = 1.5 mm). A disk with a concave center was formed (Figure 2D). This part will become the body of the container.
The enzyme-loaded mesoporous silica is placed in the container body using a spatula and was flattened (Figure 2E) and then the lid and container with the silica were placed in the high-pressure vessel (Figure 2F). The 3rd CAPC treatment was conducted to fabricate a disk cartridge containing the enzyme-loaded mesoporous silica (Figure 2G).
As a model reaction system, the decomposition of methyl red using AzoR was selected because the reaction progress can be verified by the color change (Figure 1). AzoR from Escherichia coli was overexpressed using BL21 Star E. coli cells (Thermo Fisher Scientific Inc., Waltham, MA, USA) and purified [15]. AzoR-loaded mesoporous silica was prepared by the following procedure. First, 100 mg of SBA-15 mesoporous silica (pore size = 4 nm, Sigma-Aldrich Japan, Meguro-ku, Japan) was measured in 5 mL tube and 4 mL of 0.25 mg/mL AzoR prepared using 25 mM Tris-HCl buffer (pH 7.5, Nippon Gene Co., Ltd., Chiyoda-ku, Japan) was added. The mixture was gently mixed using a rotator (4 °C, 17 h). Then, the mixture was centrifuged (12,000 rpm, 4 °C, 10 min) to remove the supernatant. The residue left after the removal of supernatant was washed by adding 4 mL of 25 mM Tris-HCl buffer (pH 7.5) and then the AzoR-loaded mesoporous silica was resuspended. Washing (described above) was performed again, and AzoR-loaded mesoporous silica was obtained by suction filtration using filter paper (4 μm) and drying at 30 °C for 1 h using a thermostatic bath.
Disk cartridges encapsulating AzoR-loaded mesoporous silica were prepared according to the method shown in Figure 2. The prepared enzyme-loaded cartridges were set in a holder and evaluated to see if the enzyme cartridges could be prepared with the activity to perform the reduction and decomposition reaction of azo dye (methyl red, FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) using AzoR. The enzyme-loaded cartridge was set in a holder in an incubator (MIR-154; SANYO Electric Co., Ltd., Osaka, Japan) maintained at 30 °C (Figure 3), and the enzyme reaction was evaluated by pumping the reaction substrate solution (0.05 mM methyl red, 25 mM Tris-HCl buffer (pH 7.5), 0.3 mM reduced nicotinamide adenine dinucleotide (NADH, FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) and 1 μM flavin mononucleotide (FMN, Sigma-Aldrich Japan, Meguro-ku, Japan)) using a microsyringe pump (Nexus 3000; Chemyx Inc., Stafford, TX, USA). The decolourization rate of methyl red was evaluated by measuring the absorbance of the decreased methyl red at 430 nm using the spectrophotometer (SpectraMax M2e, Molecular Devices, LLC., San Jose, CA, USA). The amount of enzyme-loaded mesoporous silica contained in the disk-shaped cartridge is 36 mg. A disk cartridge encapsulating mesoporous silica without AzoR was used for comparative evaluation. The amount of mesoporous silica contained in the disk-shaped cartridge is 23 mg. The pumped liquid flow rates are 0.1 mL/min (1 mL collected in 10 min) for 1–6 mL and 0.0333 mL/min (1 mL collected in 30 min) for 7–15 mL of feed volume.
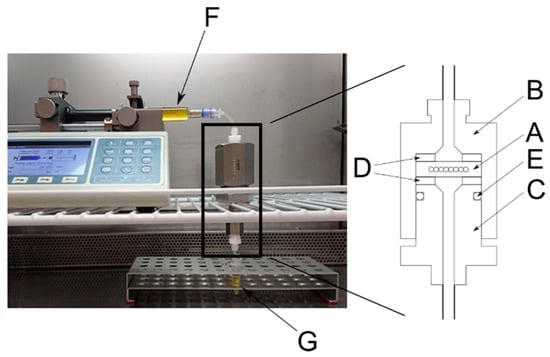
Figure 3.
Setup of the enzyme reaction evaluation system. A = disk-shaped cartridge; B = sample holder; C = sample holder; D = gaskets; E = O-ring; F = syringe; and G = sample collector.
An RH-2000 (Hirox Co., Ltd., Suginami-ku, Japan) optical microscope was used for cross-section imaging, and a TM-1000 scanning electron microscope (Hitachi High-Tech Co., Minato-ku, Japan) was used to observe the sample surfaces.
3. Results and Discussion
Figure 4 shows scanning electron microscopy images. The raw nonwoven fabric had large holes between fibers (Figure 4a). Even the enzyme-loaded mesoporous silica could not be retained in the nonwoven fabric. However, in the container fabricated using 2nd CAPC treatment, the fibers were dense, and the holes were small (Figure 4b). Fabricating the container with a sufficiently small pore size is necessary to prevent the mesoporous silica (Figure 4c) from spilling out.
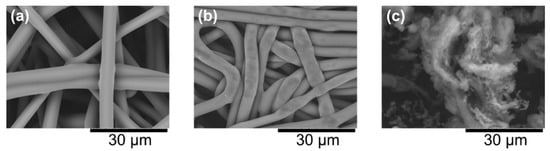
Figure 4.
Scanning electron microscope images of (a) raw nonwoven fabric, (b) container fabricated using 2nd CAPC and (c) SBA-15 mesoporous silica.
In order to confirm that the mesoporous silica is encapsulated, the results of a CAPC product using mesoporous silica colored with methylene blue are shown in Figure 5. Figure 5 shows a cross section cut with scissors and photographed with an optical microscope. The blue band is the layer of mesoporous silica encapsulated in the material. Since the mesoporous silica is encapsulated as a powder, blue dots can be seen on the upper and lower layers, which are the powder scattered during the cutting process. This observation shows that the mesoporous silica is confined in a band shape.
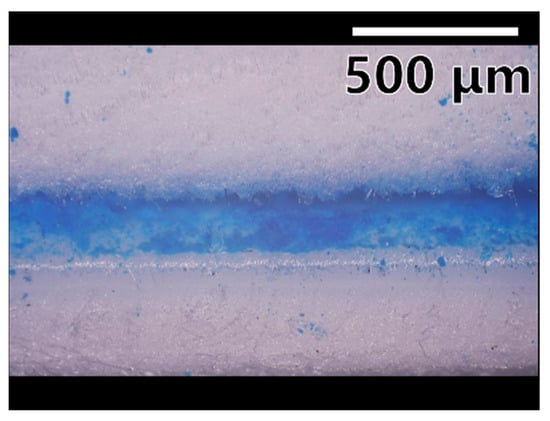
Figure 5.
Results of optical microscope observations. A sample was prepared using blue-colored SBA-15 mesoporous silica, and cross section cut with scissors was observed.
Figure 6 shows the evaluation results of the enzymatic reaction. Though the decolorization reaction did not proceed in the cartridge loaded with mesoporous silica without enzymes, the reductive degradation (decolorization) of methyl red was observed when the disk cartridge encapsulating AzoR-loaded mesoporous silica was used. The decolorization rate varied with the pumping rate (residence time). From these results, it was clear that the enzyme was trapped in the cartridge with its activity maintained. Furthermore, the enzyme was not deactivated via CAPC treatment, indicating that the enzyme-loaded disk cartridge was successfully created through CAPC. This unique flow reactor system based on the CAPC technique enables the efficient continuous decomposition reaction of azo dye and effective regulation of enzymatic activity by means of accurate control of the flow rate, leading the resolution of important issues such as continuous and scale-up operations in a batch-wise experiment [16].
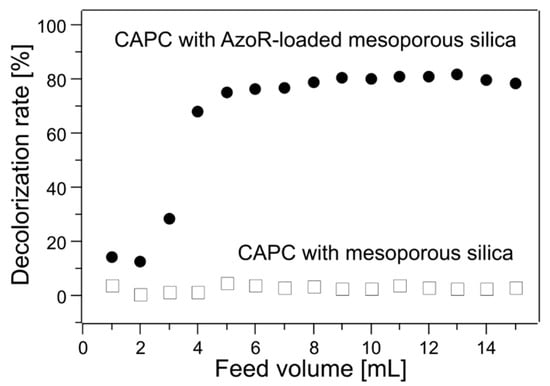
Figure 6.
Enzyme reaction evaluation results. Black circle = disk-shaped cartridge with enzyme-loaded mesoporous silica. White square = disk-shaped cartridge with only mesoporous silica.
The enzyme could be loaded without deactivation because the CAPC is performed at room temperature without applying any heat. Although high-pressure CO2 was used during fabrication, the enzymes were not destroyed in the high-pressure condition under isotropic pressure. This is evident from the fact that the enzymatic reaction occurred in supercritical CO2 at high pressures [17].
4. Conclusions
This study successfully produced a disk-shaped cartridge carrying an enzyme using the CAPC method. The cartridge was fabricated by applying the CAPC treatment three times. The nonwoven sheets were stacked and compressed to fabricate a lid with the 1st CAPC and a container with the 2nd CAPC. Then, enzyme-loaded mesoporous silica was placed in the container and covered with the lid and 3rd CAPC treatment was performed to obtain the enzyme-loaded cartridge. When AzoR was selected as the enzyme for the demonstration of activity and experiment was conducted, the decolorization reaction of methyl red proceeded, showing that the enzyme was not deactivated using high-pressure CO2 and the disk-shaped reaction cartridge could be prepared successfully.
The success of the method was verified in an experiment in which the flow rate was extremely low. However, scaling up this method for use in the food industry is a challenge. The thin and small cartridge seems suitable for pretreatment for tests such as blood diagnosis. In this research, we only fabricated a disk-shaped cartridge in which the enzyme was supported using the CAPC method and demonstrated that enzyme reactions could be performed. Optimization according to the application of the cartridge will be considered in future research.
Author Contributions
Conceptualization, T.A. and S.-i.M.; methodology, T.A. and S.-i.M.; formal analysis, T.A. and S.-i.M.; writing—original draft preparation, T.A.; writing—review and editing, S.-i.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vogel, A.; May, O. Industrial Enzyme Applications; Wiley-VCH: Weinheim, Germany, 2019; ISBN 978-3527343850. [Google Scholar]
- Sheldon, R.A.; Brady, D. Broadening the scope of biocatalysis in sustainable organic synthesis. ChemSusChem 2019, 12, 2859–2881. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, S.M.; Velasco-Lozano, S.; Orrego, A.H.; Rocha-Martín, J.; Moreno-Pérez, S.; Fraile, J.M.; López-Gallego, F.; Guisán, J.M. Functionalization of porous cellulose with glyoxyl groups as a carrier for enzyme immobilization and stabilization. Biomacromolecules 2021, 22, 927–937. [Google Scholar] [CrossRef]
- Kuo, P.C.; Lin, Z.X.; Wu, T.Y.; Hsu, C.-H.; Lin, H.-P.; Wu, T.-S. Effects of morphology and pore size of mesoporous silicas on the efficiency of an immobilized enzyme. RSC Adv. 2021, 11, 10010–10017. [Google Scholar] [CrossRef]
- Mulinari, J.; Oliveira, J.V.; Hotza, D. Lipase immobilization on ceramic supports: An overview on techniques and materials. Biotechnol. Adv. 2020, 42, 107581. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.K.; Falcaro, P.; Doonan, C.J. Metal-organic framework-based enzyme biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.W.; Barua, S.; Sardar, M.; Khare, S.K. Immobilization of Transglutaminase on multi-walled carbon nanotubes and its application as bioinspired hydrogel scaffolds. Int. J. Biol. Macromol. 2020, 163, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Balaban, M.O.; Ferrentino, G. Dense Phase Carbon Dioxide: Food and Pharmaceutical Apprications; Wiley-Blackwell: Ames, IO, USA, 2012; ISBN 978-0813806495. [Google Scholar]
- Aizawa, T. A new method for producing porous polymer materials using carbon dioxide and a piston. J. Supercrit. Fluids 2018, 133, 38–41. [Google Scholar] [CrossRef]
- Aizawa, T. Fabrication of porosity-controlled polyethylene terephthalate porous materials using a CO2-assisted polymer compression method. RSC Adv. 2018, 8, 3061–3068. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, S.; Yokoyama, T.; Ishii, R.; Itoh, T.; Tomon, E.; Hamakawa, S.; Tsunoda, T.; Mizukami, F.; Nanbu, H.; Hanaoka, T.A. An enzyme-encapsulated microreactor for efficient theanine synthesis. Chem. Commun. 2012, 48, 7058–7060. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Chiba, M.; Tsunoda, T.; Yamaguchi, A. Enzyme immobilization in mesoporous silica for enhancement of thermostability. J. Nanosci. Nanotechnol. 2018, 18, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T. Novel strategy for fabricating multi-layer porous membranes with varying porosity. ACS Omega 2020, 5, 24461–24466. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Yatome, C.; Ishida, N.; Kitade, Y. Putative ACP Phosphodiesterase Gene (acpD) Encodes an Azoreductase. J. Biol. Chem. 2001, 276, 46394–46399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Anke, M.K.; Szymanska, K.; Tischler, D. Immobilization of Rhodococcus opacus 1CP azoreductase to obtain azo dye degrading biocatalysts operative at acidic pH. Int. Biodeterior. Biodegrad. 2017, 118, 89–94. [Google Scholar] [CrossRef]
- Dias, A.L.B.; dos Santos, P.; Martínez, J. Supercritical CO2 technology applied to the production of flavor ester compounds through lipase-catalyzed reaction: A review. J. CO2 Util. 2018, 23, 159–178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).