Comparison of Clinical Parameters in Dental Implant Therapy between Implant Site Development Using Porcine- and Bovine-Derived Xenografts
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
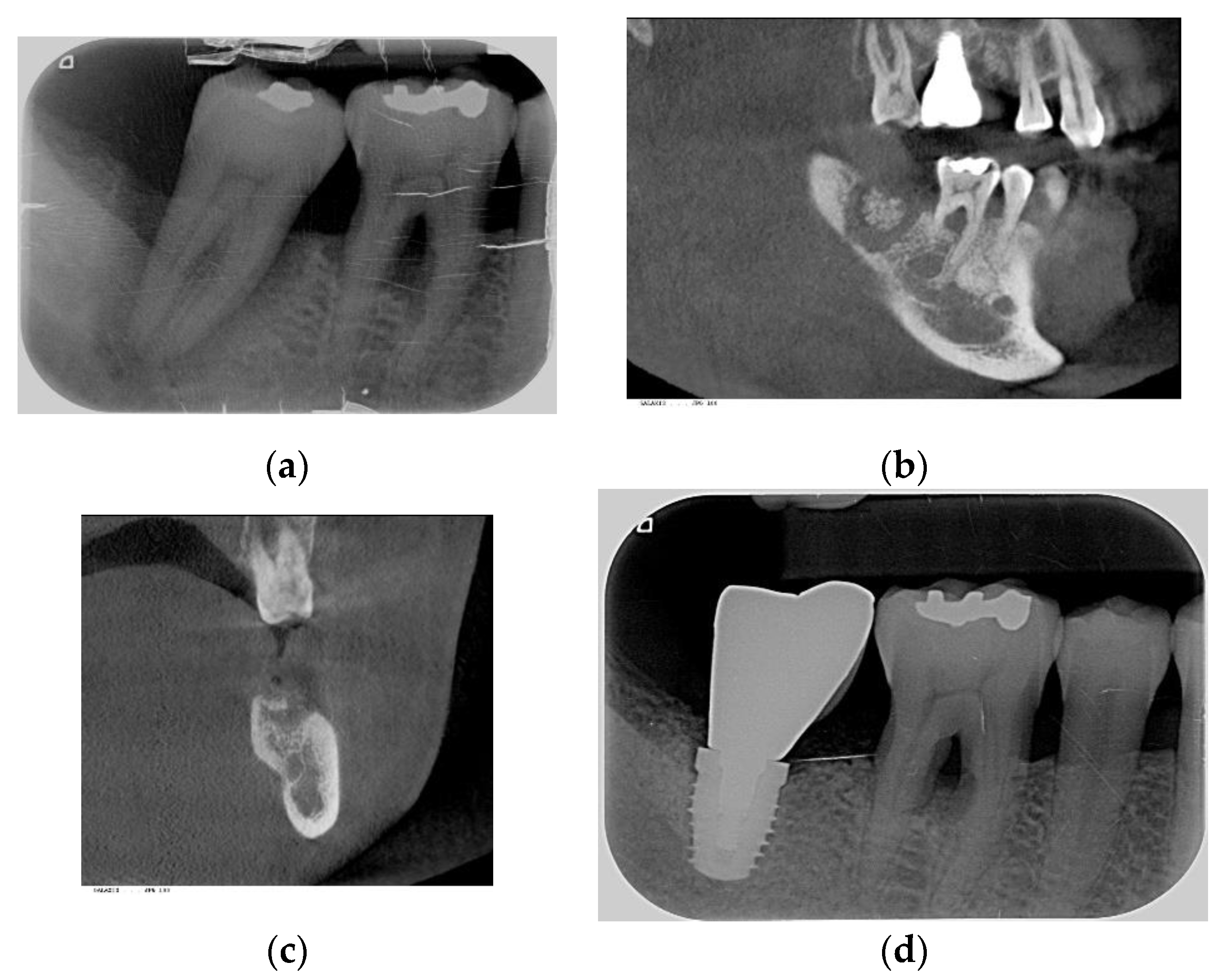
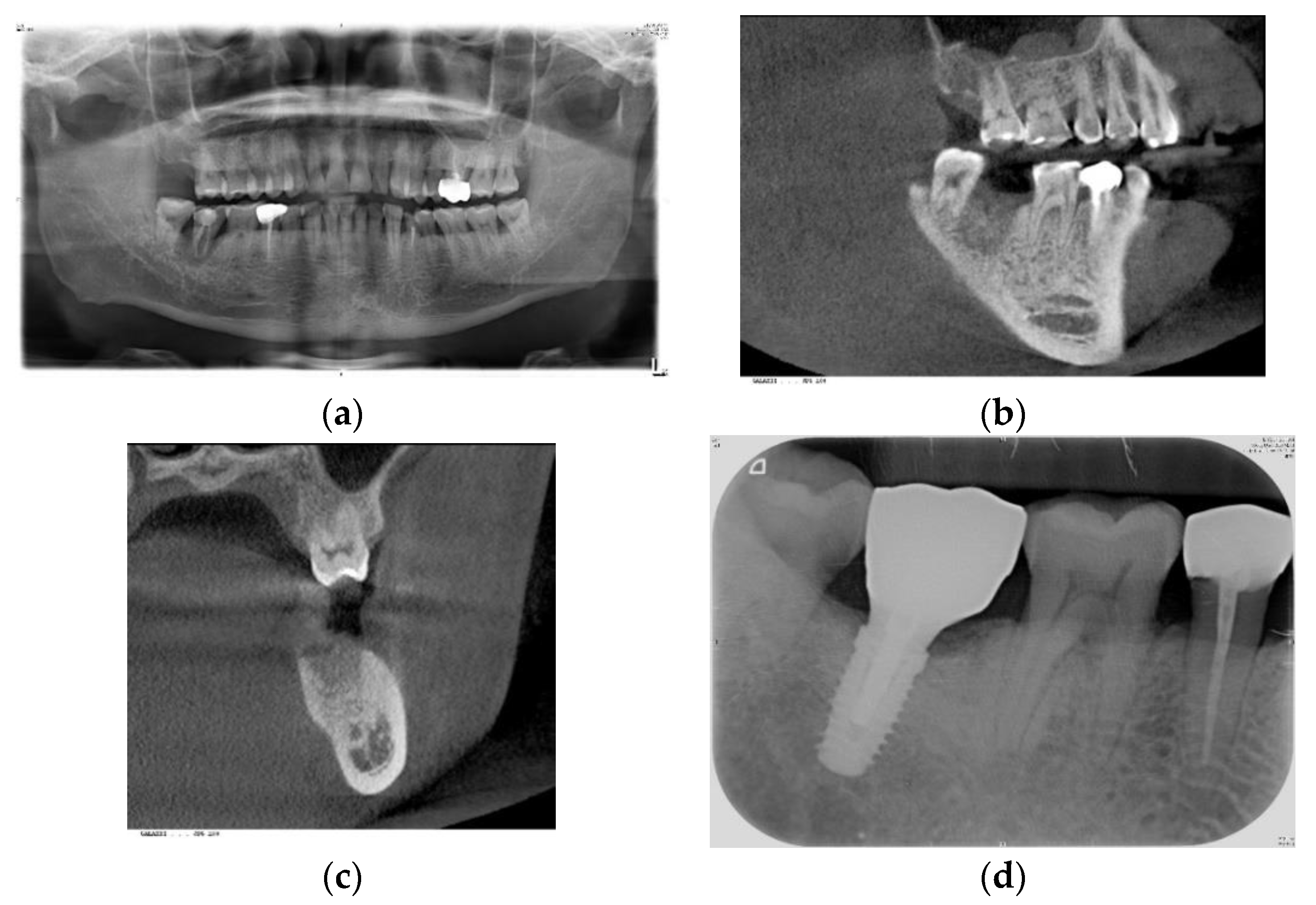
2.2. Procedures Performed
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Conditions vs. Xenograft Materials
3.1.1. Maxillary Sites
3.1.2. Mandible Sites
3.2. Implant Site Development vs. Xenograft Materials
3.2.1. Maxillary Teeth
3.2.2. Mandible Teeth
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corbella, S.; Taschieri, S.; Francetti, L.; Weinstein, R.; Del Fabbro, M. Histomorphometric Results After Postextraction Socket Healing with Different Biomaterials: A Systematic Review of the Literature and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2017, 32, 1001–1017. [Google Scholar] [CrossRef]
- Messina, A.M.; Marini, L.; Oh, D.S.; Marini, E. A Step-by-Step Procedure for Bone Regeneration Using Calcium Phosphate Scaffolds: From Site Preparation to Graft Placement. J. Craniofac. Surg. 2019, 30, 149–153. [Google Scholar] [CrossRef]
- Verket, A.; Lyngstadaas, S.P.; Tiainen, H.; Ronold, H.J.; Wohlfahrt, J.C. Impact of particulate deproteinized bovine bone mineral and porous titanium granules on early stability and osseointegration of dental implants in narrow marginal circumferential bone defects. Int. J. Oral Maxillofac. Surg. 2018, 47, 1086–1094. [Google Scholar] [CrossRef]
- Antonelli, A.; Bennardo, F.; Brancaccio, Y.; Barone, S.; Femiano, F.; Nucci, L.; Minervini, G.; Fortunato, L.; Attanasio, F.; Giudice, A. Can Bone Compaction Improve Primary Implant Stability? An In Vitro Comparative Study with Osseodensification Technique. Appl. Sci. 2020, 10, 8623. [Google Scholar] [CrossRef]
- Testori, T.; Iezzi, G.; Manzon, L.; Fratto, G.; Piattelli, A.; Weinstein, R.L. High temperature-treated bovine porous hydroxyapatite in sinus augmentation procedures: A case report. Int. J. Periodontics Restor. Dent. 2012, 32, 295–301. [Google Scholar]
- Lee, J.H.; Yi, G.S.; Lee, J.W.; Kim, D.J. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal Implant Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, H.K.; Yun, J.H.; Cho, K.S. Randomized Clinical Trial of Maxillary Sinus Grafting using Deproteinized Porcine and Bovine Bone Mineral. Clin. Implant Dent. Relat. Res. 2017, 19, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; DeVilliers, P.; Grande, M.; Stefanelli, L.V.; Carlo, S.D.; Pompa, G. Histologic evaluation of bone healing of adjacent alveolar sockets grafted with bovineand porcine-derived bone: A comparative case report in humans. Regen. Biomater. 2017, 4, 125–128. [Google Scholar]
- Iezzi, G.; Degidi, M.; Piattelli, A.; Mangano, C.; Scarano, A.; Shibli, J.A.; Perrotti, V. Comparative histological results of different biomaterials used in sinus augmentation procedures: A human study at 6 months. Clin. Oral Implant. Res. 2012, 23, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes--comparison with human bone. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Shin, J.W.; Yang, Y.I.; Kim, Y.K.; Park, K.D.; Lee, J.W.; Jo, I.H.; Kim, Y.J. In vitro study of osteogenic differentiation of bone marrow stromal cells on heat-treated porcine trabecular bone blocks. Biomaterials 2004, 25, 527–535. [Google Scholar] [CrossRef]
- Bae, E.B.; Kim, H.J.; Ahn, J.J.; Bae, H.Y.; Kim, H.J.; Huh, J.B. Comparison of Bone Regeneration between Porcine-Derived and Bovine-Derived Xenografts in Rat Calvarial Defects: A Non-Inferiority Study. Materials 2019, 12, 3412. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Chen, M.Y.; Hsieh, D.J.; Periasamy, S.; Yen, K.C.; Chuang, C.T.; Wang, H.C.; Tseng, F.W.; Kuo, J.C.; Chien, H.H. Evaluating the bone-regenerative role of the decellularized porcine bone xenograft in a canine extraction socket model. Clin. Exp. Dent. Res. 2020, 7, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cha, J.K.; Kim, C.S. Alveolar ridge regeneration of damaged extraction sockets using deproteinized porcine versus bovine bone minerals: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2018, 20, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.C.; Jung, U.W.; You, H.; Lee, J.S. Randomized clinical trial of ridge preservation using porcine bone/cross-linked collagen vs. bovine bone/non-cross-linked collagen: Cone beam computed tomographic analysis. Clin. Oral Implant. Res. 2017, 28, 1492–1500. [Google Scholar] [CrossRef]
- Koo, T.H.; Song, Y.W.; Cha, J.K.; Jung, U.W.; Kim, C.S.; Lee, J.S. Histologic analysis following grafting of damaged extraction sockets using deproteinized bovine or porcine bone mineral: A randomized clinical trial. Clin. Oral Implant. Res. 2020, 31, 93–102. [Google Scholar] [CrossRef]
- Lai, V.J.; Michalek, J.E.; Liu, Q.; Mealey, B.L. Ridge preservation following tooth extraction using bovine xenograft compared with porcine xenograft: A randomized controlled clinical trial. J. Periodontol. 2020, 91, 361–368. [Google Scholar] [CrossRef]
- Misch, C.E. Bone character: Second vital implant criterion. Dent. Today 1988, 7, 39–40. [Google Scholar]
- Mardas, N.; Dereka, X.; Donos, N.; Dard, M. Experimental model for bone regeneration in oral and cranio-maxillo-facial surgery. J. Invest. Surg. 2014, 27, 32–49. [Google Scholar] [CrossRef]
- Guarnieri, R.; Stefanelli, L.; De Angelis, F.; Mencio, F.; Pompa, G.; Di Carlo, S. Extraction Socket Preservation Using Porcine-Derived Collagen Membrane Alone or Associated with Porcine-Derived Bone. Clinical Results of Randomized Controlled Study. J. Oral Maxillofac. Res. 2017, 8, e5. [Google Scholar] [CrossRef]
- Guarnieri, R.; Testarelli, L.; Stefanelli, L.; De Angelis, F.; Mencio, F.; Pompa, G.; Di Carlo, S. Bone Healing in Extraction Sockets Covered with Collagen Membrane Alone or Associated with Porcine-Derived Bone Graft: A Comparative Histological and Histomorphometric Analysis. J. Oral Maxillofac. Res. 2017, 8, e4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, L.C.; Tsai, I.M. Comparison of Early Implant Failure Rates between Subjects with and without Orthodontic Treatment Before Dental Implantation. J. Oral Implantol. 2019, 45, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Iezzi, G.; Mazzoni, S.; Piattelli, A.; Perrotti, V.; Barone, A. Regenerative properties of collagenated porcine bone grafts in human maxilla: Demonstrative study of the kinetics by synchrotron radiation microtomography and light microscopy. Clin. Oral Investig. 2018, 22, 505–513. [Google Scholar] [CrossRef]
- Chang, L.-C.; Cheng, Y.-M. The Effect of Different Socket Types on Implant Therapy While Using Flapless Ridge Preservation. Appl. Sci. 2021, 11, 970. [Google Scholar] [CrossRef]
- Chang, L.C. Risk factors associated with early failure of maxillary versus mandibular implants: A retrospective study. Int. J. Oral Implantol. 2020, 13, 55–63. [Google Scholar]
| Bovine Bone, BioOss (n = 65) | Porcine Bone, MinerOss (n = 39) | p-Value | ||
|---|---|---|---|---|
| Age (Year) | 50.6 (11.7) | 55.8 (11.1) | 0.029 * | |
| Sex | Female | 23 (35.4%) | 17 (43.6%) | 0.414 |
| Male | 42 (64.6%) | 22 (56.4%) | ||
| Systemic disease | Healthy | 30 (46.2%) | 16 (41.0%) | 0.770 |
| Diabetes mellitus | 4 (6.2%) | 1 (2.6%) | ||
| Osteoporosis | 2 (3.1%) | 2 (5.1%) | ||
| Other disease | 29 (44.6%) | 20 (51.3%) | ||
| Smoking status | No | 49 (75.4%) | 33 (84.6%) | 0.400 |
| Former | 15 (23.1%) | 5 (12.8%) | ||
| Current | 1 (1.5%) | 1 (2.6%) | ||
| Tooth site | Anterior | 8 (12.3%) | 7 (17.9%) | 0.727 |
| Premolar | 23 (35.4%) | 12 (30.8%) | ||
| Molar | 34 (52.3%) | 20 (51.3%) | ||
| Extraction etiology | Non-periodontitis | 25 (38.5%) | 24 (61.5%) | 0.027 * |
| Periodontitis | 40 (61.5%) | 15 (38.5%) | ||
| Coronal seal | None | 6 (9.2%) | 2 (5.1%) | <0.001 * |
| Membrane | 19 (29.2%) | 35 (89.7%) | ||
| Collagen plug | 40 (61.5%) | 2 (5.1%) | ||
| Time of ridge recontouring | RP | 41 (63.1%) | 17 (43.6%) | 0.067 |
| RA | 24 (36.9%) | 22 (56.4%) | ||
| Bovine Bone, BioOss (n = 45) | Porcine Bone, MinerOss (n = 19) | p-Value | ||
|---|---|---|---|---|
| Age (Year) | 49.2 (9.0) | 54.0 (9.6) | 0.061 | |
| Sex | Female | 19 (42.2%) | 7 (36.8%) | 0.784 |
| Male | 26 (57.8%) | 12 (63.2%) | ||
| Systemic disease | Healthy | 19 (42.2%) | 12 (63.2%) | 0.284 |
| Diabetes mellitus | 1 (2.2%) | 1 (5.3%) | ||
| Osteoporosis | 2 (4.4%) | 0 (0.0%) | ||
| Other disease | 23 (51.1%) | 6 (31.6%) | ||
| Smoking status | No | 34 (75.6%) | 13 (68.4%) | 0.658 |
| Former | 10 (22.2%) | 5 (26.3%) | ||
| Current | 1 (2.2%) | 1 (5.3%) | ||
| Tooth site | Anterior | 2 (4.4%) | 1 (5.3%) | 1.000 |
| Premolar | 12 (26.7%) | 5 (26.3%) | ||
| Molar | 31 (68.9%) | 13 (68.4%) | ||
| Extraction etiology | Non-periodontitis | 26 (57.8%) | 12 (63.2%) | 0.784 |
| Periodontitis | 19 (42.2%) | 7 (36.8%) | ||
| Coronal Seal | None | 5 (11.1%) | 2 (10.5%) | <0.001 * |
| Membrane | 8 (17.8%) | 17 (89.5%) | ||
| Collagen plug | 32 (71.1%) | 0 (0.0%) | ||
| Time of ridge recontouring | RP | 31 (68.9%) | 7 (36.8%) | 0.026 * |
| RA | 14 (31.1%) | 12 (63.2%) | ||
| Bovine Bone, BioOss (n = 65) | Porcine Bone, MinerOss (n = 39) | p-Value | ||
|---|---|---|---|---|
| Primary stability ‡ | <20 N | 13 (20.6%) | 14 (35.9%) | 0.231 |
| 20–35 N | 22 (34.9%) | 10 (25.6%) | ||
| ≥35 N | 28 (44.4%) | 15 (38.5%) | ||
| Bone density | D1–D3 | 9 (13.8%) | 14 (35.9%) | 0.014 * |
| D4 | 56 (86.2%) | 25 (64.1%) | ||
| BG | Yes | 36 (55.4%) | 23 (59.0%) | 0.838 |
| No | 29 (44.6%) | 16 (41.0%) | ||
| SL | Yes | 35 (53.8%) | 18 (46.2%) | 0.544 |
| No | 30 (46.2%) | 21 (53.8%) | ||
| Post-operative infection | Yes | 4 (6.2%) | 6 (15.4%) | 0.170 |
| No | 61 (93.8%) | 33 (84.6%) | ||
| Implant survival | Early failure | 3 (4.6%) | 0 (0.0%) | 0.290 |
| Survival | 62 (95.4%) | 39 (100.0%) | ||
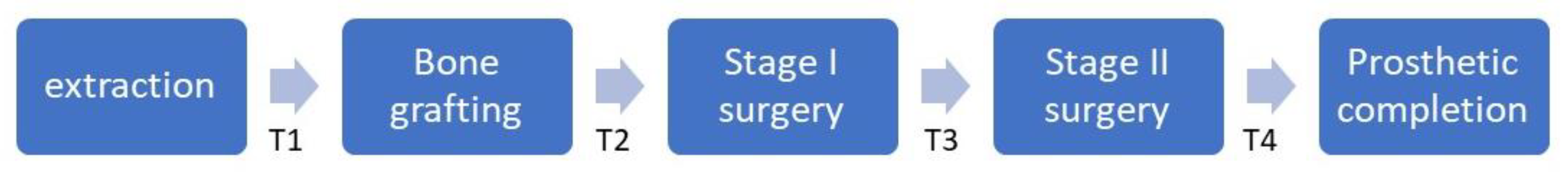
| Extraction–bone grafting (T1, day) | RP | 0 | 0 | NA |
| RA | 84.0 (36.0, 158.5) | 40.5 (35.0, 50.0) | 0.030 * | |
| Bone grafting–Stage I (T2, day) † | 110.0 (95.0, 135.0) | 123.0 (107.0, 141.0) | 0.090 | |
| Stage I–Stage II (T3, day) † | 261.0 (231.0, 343.0) | 238.0 (208.0, 288.0) | 0.054 | |
| Stage II–prosthetic completion (T4, day) † | 99.0 (67.0, 127.0) | 103.0 (68.0, 112.0) | 0.587 | |
| Bone grafting–Stage II (T2 + T3, day) † | 393.0 (346.0, 463.0) | 373.0 (336.0, 419.0) | 0.302 | |
| Bone grafting–completion (T2 + T3 + T4, day) † | 477.0 (431.0, 584.0) | 469.0 (420.0, 530.0) | 0.283 | |
| Extraction–completion (T1 + T2 + T3 + T4; day) | RP | 481.0 (442.0, 581.0) | 463.0 (435.0, 530.0) | 0.287 |
| RA | 593.0 (462.5, 746.5) | 517.0 (468.0, 600.0) | 0.248 | |
| Follow-up (day) † | 2095.0 (1526.0, 2566.0) | 743.0 (567.0, 807.0) | <0.001 * | |
| Bovine Bone, BioOss (n = 45) | Porcine Bone, MinerOss (n = 19) | p-Value | ||
|---|---|---|---|---|
| Primary stability | <20 N | 2 (4.4%) | 4 (21.1%) | 0.036 * |
| 20–35 N | 9 (20.0%) | 6 (31.6%) | ||
| ≥35 N | 34 (75.6%) | 9 (47.4%) | ||
| Bone density | D1–D3 | 23 (51.1%) | 4 (21.1%) | 0.030 * |
| D4 | 22 (48.9%) | 15 (78.9%) | ||
| BG | Yes | 27 (60.0%) | 11 (57.9%) | >0.999 |
| No | 18 (40.0%) | 8 (42.1%) | ||
| Post-operative infection | Yes | 4 (8.9%) | 1 (5.3%) | >0.999 |
| No | 41 (91.1%) | 18 (94.7%) | ||
| Implant survival | Early failure | 1 (2.2%) | 0 (0.0%) | >0.999 |
| Survival | 44 (97.8%) | 19 (100.0%) | ||
| Extraction–bone grafting (T1, day) † | RP | 0 | 0 | >0.999 |
| RA | 41.0 (20.0, 117.0) | 42.0 (37.0, 55.0) | 0.771 | |
| Bone grafting–Stage I (T2, day) † | 110.0 (89.0, 133.0) | 140.0 (121.0, 151.0) | 0.003 * | |
| Stage I–Stage II (T3, day) † | 135.0 (113.0, 176.0) | 145.0 (119.0, 166.0) | 0.853 | |
| Stage II–prosthetic completion (T4, day) † | 112.0 (96.0, 132.0) | 98.0 (72.0, 127.0) | 0.174 | |
| Bone grafting–Stage II (T2 + T3, day) † | 266.0 (208.0, 314.0) | 287.0 (263.0, 306.0) | 0.383 | |
| Bone grafting–completion (T2 + T3 + T4, day) † | 390.0 (336.0, 417.0) | 378.0 (326.0, 415.0) | 0.356 | |
| Extraction–completion (T1 + T2 + T3 + T4, day) † | RP | 385.0 (322.0, 417.0) | 407.0 (354.0, 415.0) | 0.524 |
| RA | 466.5 (399.0, 500.0) | 407.5 (345.5, 440.0) | 0.054 | |
| Follow-up (day)† | 1970.0 (1565.0, 2241.0) | 555.0 (352.0, 773.0) | <0.001 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.-C. Comparison of Clinical Parameters in Dental Implant Therapy between Implant Site Development Using Porcine- and Bovine-Derived Xenografts. Technologies 2021, 9, 72. https://doi.org/10.3390/technologies9040072
Chang L-C. Comparison of Clinical Parameters in Dental Implant Therapy between Implant Site Development Using Porcine- and Bovine-Derived Xenografts. Technologies. 2021; 9(4):72. https://doi.org/10.3390/technologies9040072
Chicago/Turabian StyleChang, Li-Ching. 2021. "Comparison of Clinical Parameters in Dental Implant Therapy between Implant Site Development Using Porcine- and Bovine-Derived Xenografts" Technologies 9, no. 4: 72. https://doi.org/10.3390/technologies9040072
APA StyleChang, L.-C. (2021). Comparison of Clinical Parameters in Dental Implant Therapy between Implant Site Development Using Porcine- and Bovine-Derived Xenografts. Technologies, 9(4), 72. https://doi.org/10.3390/technologies9040072





