Investigation of AgI-Based Solid Solutions with Ag2CO3
Abstract
:1. Introduction
2. Materials and Methods
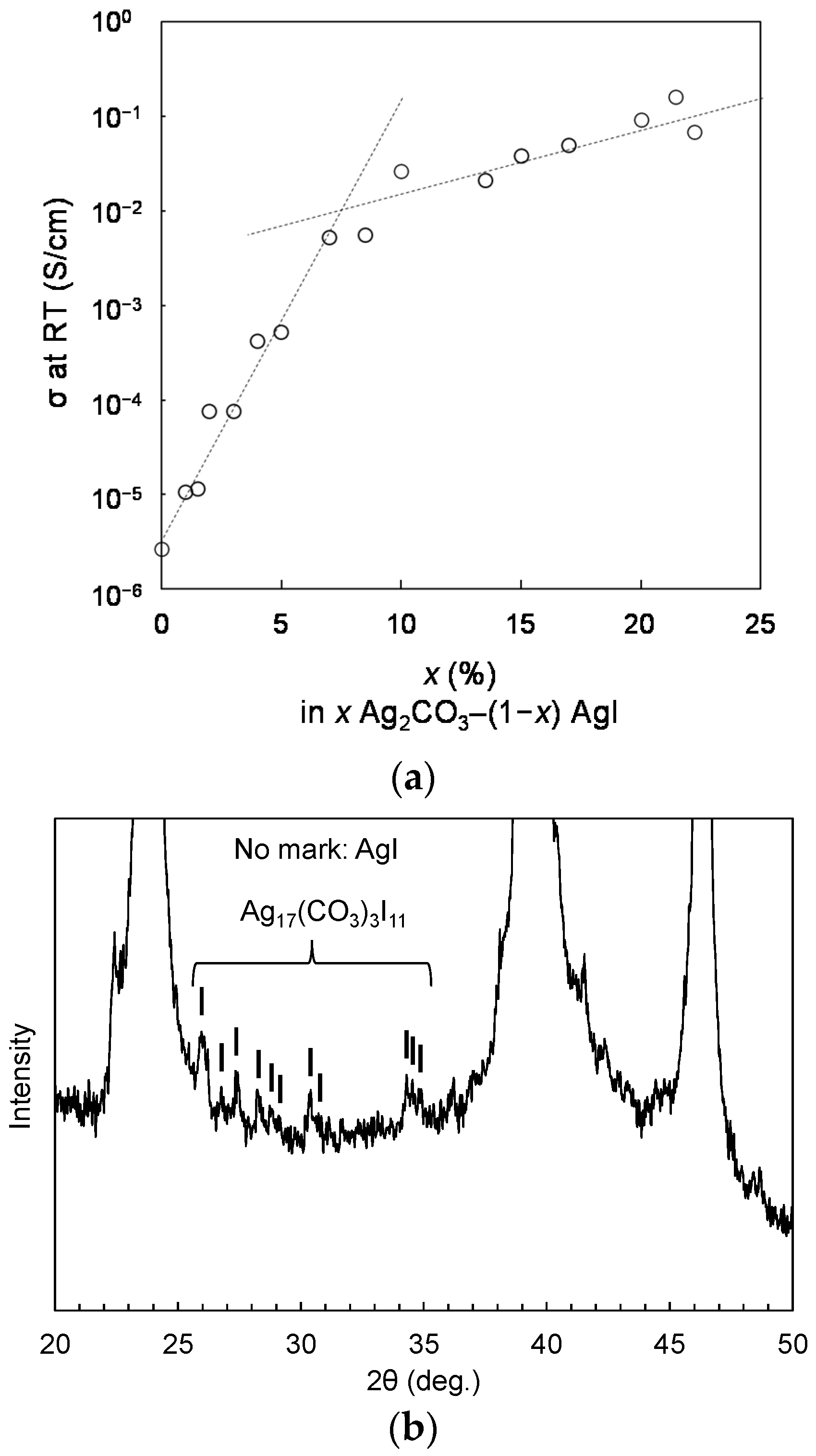
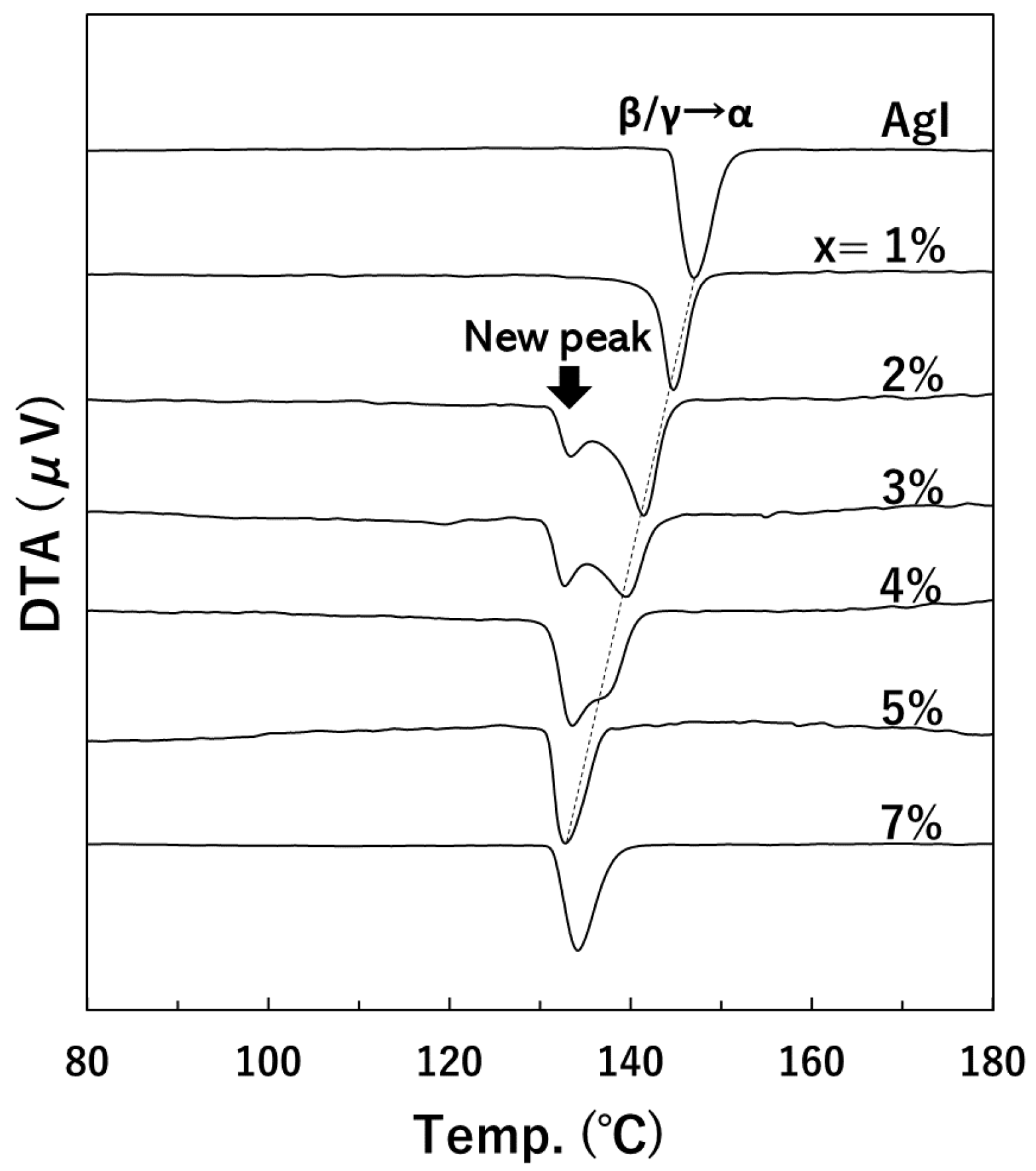
3. Results
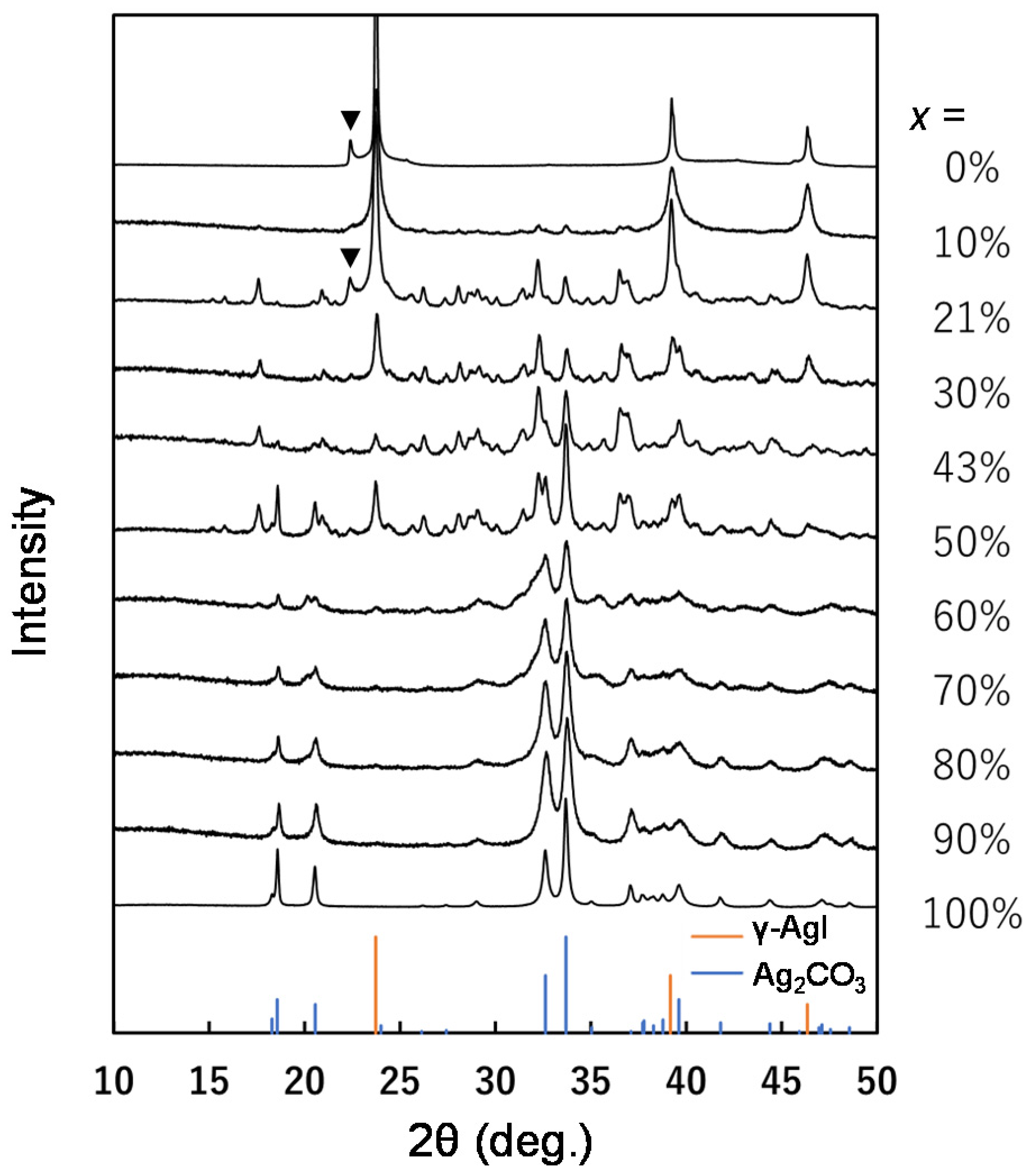
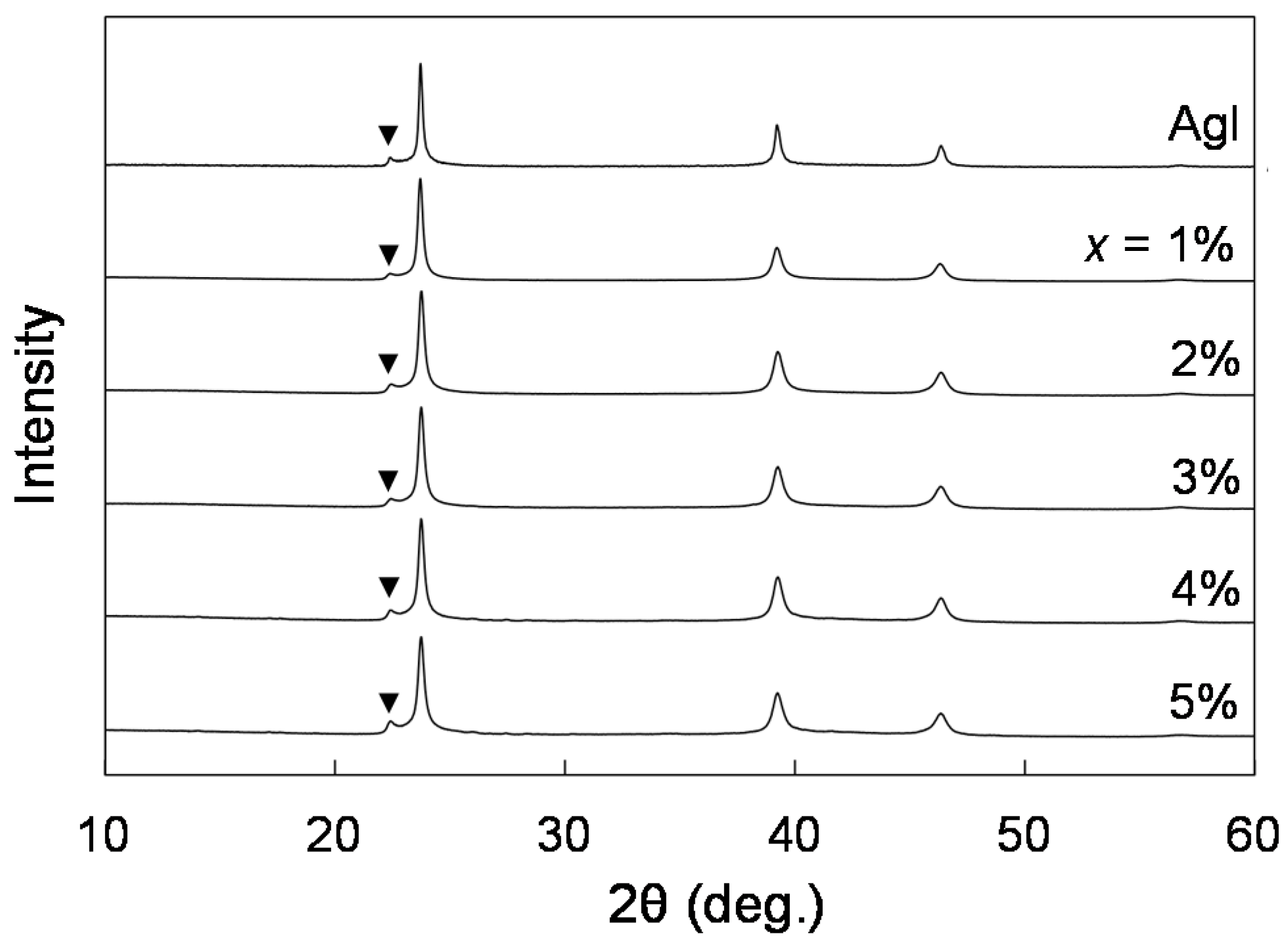
Phase Identification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakuda, A. Favorable composite electrodes for all-solid-state batteries. J. Ceram. Soc. Jpn. 2018, 126, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-K. Promising All-Solid-State Batteries for Future Electric Vehicles. ACS Energy Lett. 2020, 5, 3221–3223. [Google Scholar] [CrossRef]
- Tubandt, C.; Lorenz, E. Molekularzustand und elektrisches Leitvermögen kristallisierter Salze. Z. Phys. Chem. 1914, 87, 513–542. [Google Scholar] [CrossRef]
- Strock, L.W. Kristallstruktur des Hochtemperatur—Jodsilbers α-AgJ. Z. Phys. Chem. 1934, B25, 441–459. [Google Scholar] [CrossRef]
- Strock, L.W. Ergänzung und Berichtigung zu: “Kristallstruktur des Hochtemperatur-Jodsilbers α-AgJ”. Z. Phys. Chem. 1936, B31, 132–136. [Google Scholar] [CrossRef]
- Cava, R.J.; Reidinger, F.; Wuensch, B.J. Single-crystal neutron-diffraction study of AgI between 23° and 300 °C. Solid State Commun. 1977, 24, 411–416. [Google Scholar] [CrossRef]
- Suzuki, R.; Watanabe, Y.; Yamane, H.; Kitaura, M.; Uchida, K.; Matsushima, Y. Crystal structure of silver carbonate iodide Ag10(CO3)3I4. Acta Crystallogr. 2021, E77, 734–738. [Google Scholar] [CrossRef]
- Watanabe, Y.; Suzuki, R.; Kato, K.; Yamane, H.; Kitaura, M.; Ina, T.; Uchida, K.; Matsushima, Y. Superionic Ag+ conductor Ag17(CO3)3I11. Inorg. Chem. 2021, 60, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.N.; Greene, P.D. Potassium iodide + silver iodide phase diagram. High ionic conductivity of KAg4I5. Trans. Faraday Soc. 1966, 62, 2069–2075. [Google Scholar] [CrossRef]
- Owens, B.B.; Argue, G.R. High-Conductivity Solid Electrolytes: MAg4I5. Science 1967, 157, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.N.; Greene, P.D. Solids with high ionic conductivity in group 1 halide systems. Trans. Faraday Soc. 1967, 63, 424–430. [Google Scholar] [CrossRef]
- Bradley, J.N.; Greene, P.D. Relationship of structure and ionic mobility in solid MAg4I5. Trans. Faraday Soc. 1967, 63, 2516–2521. [Google Scholar] [CrossRef]
- Owens, B.B.; Argue, G.R. High conductivity solid electrolyte system Rbl-AgI. J. Electrochem. Soc. 1970, 117, 898–900. [Google Scholar] [CrossRef]
- Takahashi, T.; Ikeda, S.; Yamamoto, O. Solid-state ionics—Solids with high ionic conductivity in the systems silver iodide–silver oxyacid salts. J. Electrochem. Soc. 1972, 119, 477–482. [Google Scholar] [CrossRef]
- Takahashi, T.; Ikeda, S.; Yamamoto, O. Solid-state ionics: A new high ionic conductivity solid electrolyte Ag6I4WO4 and use of this compound in a solid-electrolyte cell. J. Electrochem. Soc. 1973, 120, 647–651. [Google Scholar] [CrossRef]
- Chan, L.Y.Y.; Geller, S. Crystal structure and conductivity of 26-silver 18-iodide tetratungstate, Ag26I18W4O16. J. Solid State Chem. 1977, 21, 331–347. [Google Scholar] [CrossRef]
- PDXL: Integrated X-ray Powder Diffraction Software; Rigaku Co.: Tokyo, Japan, 2018.
- Hull, S.; Keen, D.A. Pressure-induced phase transitions in AgCl, AgBr, and AgI. Phys. Rev. B 1999, 59, 750–761. [Google Scholar] [CrossRef]
- Quaranta, N.E.; Bazan, J.C. Thermodynamic data for solid AgI and its phase transitions from ENF measurements. Solid State Ionics 1983, 11, 71–75. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, K.; Matsushima, Y. Investigation of AgI-Based Solid Solutions with Ag2CO3. Technologies 2021, 9, 54. https://doi.org/10.3390/technologies9030054
Uchida K, Matsushima Y. Investigation of AgI-Based Solid Solutions with Ag2CO3. Technologies. 2021; 9(3):54. https://doi.org/10.3390/technologies9030054
Chicago/Turabian StyleUchida, Kento, and Yuta Matsushima. 2021. "Investigation of AgI-Based Solid Solutions with Ag2CO3" Technologies 9, no. 3: 54. https://doi.org/10.3390/technologies9030054
APA StyleUchida, K., & Matsushima, Y. (2021). Investigation of AgI-Based Solid Solutions with Ag2CO3. Technologies, 9(3), 54. https://doi.org/10.3390/technologies9030054





