1. Introduction
Hospital-acquired pneumonia (HAP) refers to any pneumonia contracted by a patient in a hospital at least 48–72 h after being admitted [
1]. It is an infection that was not present before the patient came to the hospital and therefore is distinguished from community-acquired pneumonia. HAP is the second most common hospital-acquired infection. It is the most common cause of death among hospital-acquired infections [
2] and is usually caused by a bacterial infection. Barber et al., in their review study, recognized HAP as a major cause of deaths, notably in patients with severe underlying conditions, and suggested that the development of new diagnostic tools and therapeutic methods was urgent to face the epidemic of antibiotic-resistant pathogens [
3].
Factors that increase the risk of HAP include weakened cough, mechanical ventilation, tracheostomy tubes, suctioning and weakened immune system from disease or medications [
2]. To diagnose HAP, there must be evidence on the chest x-ray as well as fever, elevated white blood cell count, or the presence of secretions from the lungs. Hospitalized patients with HAP receive antibiotic therapy, while other lung medications may be given to help loosen and remove thick secretions from the lungs. Oxygen may be provided to maintain good oxygen levels. Some of the components for HAP prevention include clean hands, use antibacterial hand gel before and after each patient interaction, and the use of gloves during direct patient contact.
For some patients, HAP often progresses to septicemia, then severe sepsis or even septic shock. Early recognition of these patients might help in reducing morbidity and mortality. According to de Lange et al., elevated systemic levels of pro-inflammatory cytokines at the time of diagnosis of hospital-acquired pneumonia appear to be indicative of subsequent progression to septic shock [
4]. HAP is different from community pneumonia since it is very often caused by methicillin-resistant staphylococcus aureus (MRSA).
The progression of a disease can be described as a temporal sequence of diagnosis events. In the case of pneumonia, the disease may, unfortunately, oftentimes progress to septicemia. Septicemia is a bacterial infection that spreads into the bloodstream [
5]. Severe sepsis, that could be the next phase of a worsening septicemia, is the body’s response to that infection, during which the immune system will trigger extreme and potentially dangerous, whole-body inflammation [
6]. Sepsis, in turn, when it causes dangerously low blood pressure (shock), is called septic shock.
Almirante et al. studied the predictors of mortality among patients with Candida bloodstream infection is Spain and observed a very high (44%) mortality rate for patients with candidemia [
7]. There is a scarcity of studies that examine differences in the mortality rate between hospital and community-onset bloodstream infections. One of these studies was conducted by Diekama et al., who examined the epidemiology and outcome of nosocomial and community-onset bloodstream infections, and found that bloodstream infections cause substantial morbidity and mortality. According to this study, the crude mortality for community-onset infections was 14%, but was much higher, at 34%, for nosocomial infections [
8]. Other researchers have shown that a bloodstream infection has a severe impact on critically ill patients. To determine the excess mortality attributable to hospital-acquired bloodstream infections, Smith et al. found that among critically ill patients with bloodstream infections, observed mortality significantly exceeded the predicted value. Critically ill patients who develop nosocomial bloodstream infections are at greater risk of death than patients with comparable severity of illness without this complication [
9]. These results are similar to those of a previous study that examined hospital mortality among patients in a Surgical intensive care unit (SICU). According to that study, nosocomial bloodstream infection complicated 2.67 per 100 admissions to the SICU and the crude mortality rates for a patient who developed a bloodstream infection was 50% and only 15% for patients who did not [
10]. There are a few studies that study HAP-specific bloodstream infection and its association with crude mortality. Sopena et al. studied, in a prospective design, non-ICU HAP and found that the mean incidence of HAP was 3 ± 1.4 cases per 1000 hospital admissions, and mortality was 26%, with 13.9% being attributed to pneumonia [
11].
While the aforementioned studies examined the impact of bloodstream infections on hospital outcomes of care, including inpatient mortality, none of them, to the authors’ knowledge has examined the progression of bloodstream infections and how each phase of a pneumonia-induced bloodstream infection is associated with an increase to the likelihood of hospital death. Existing studies follow a traditional statistical design approach and were not designed to study, in a data science manner, the bloodstream infection as a progressive clinical event of multiple steps.
Since the progression of a bloodstream infection can be described as a temporal sequence of diagnoses (septicemia → severe sepsis → septic shock), this sequence makes up a clinical event. Event recognition is the detection of events that are considered relevant for processing [
12]. Examples of event recognition consist of recognizing human activities on video content [
13] and extracting interesting stories from social media. In healthcare, there are diverse event recognition paradigms, such as the identification of cardiac arrhythmia events [
14], and the spread of an epidemic [
15]. At the hospital, the association of those attributes that may form sequential clinical events have been explained in previous studies [
16]. The knowledge of interesting clinical events can benefit medical education, quality assurance hospital committees who study high-risk inpatient scenarios, and clinicians who are informed about scenarios that are likely to unfold during a patient encounter.
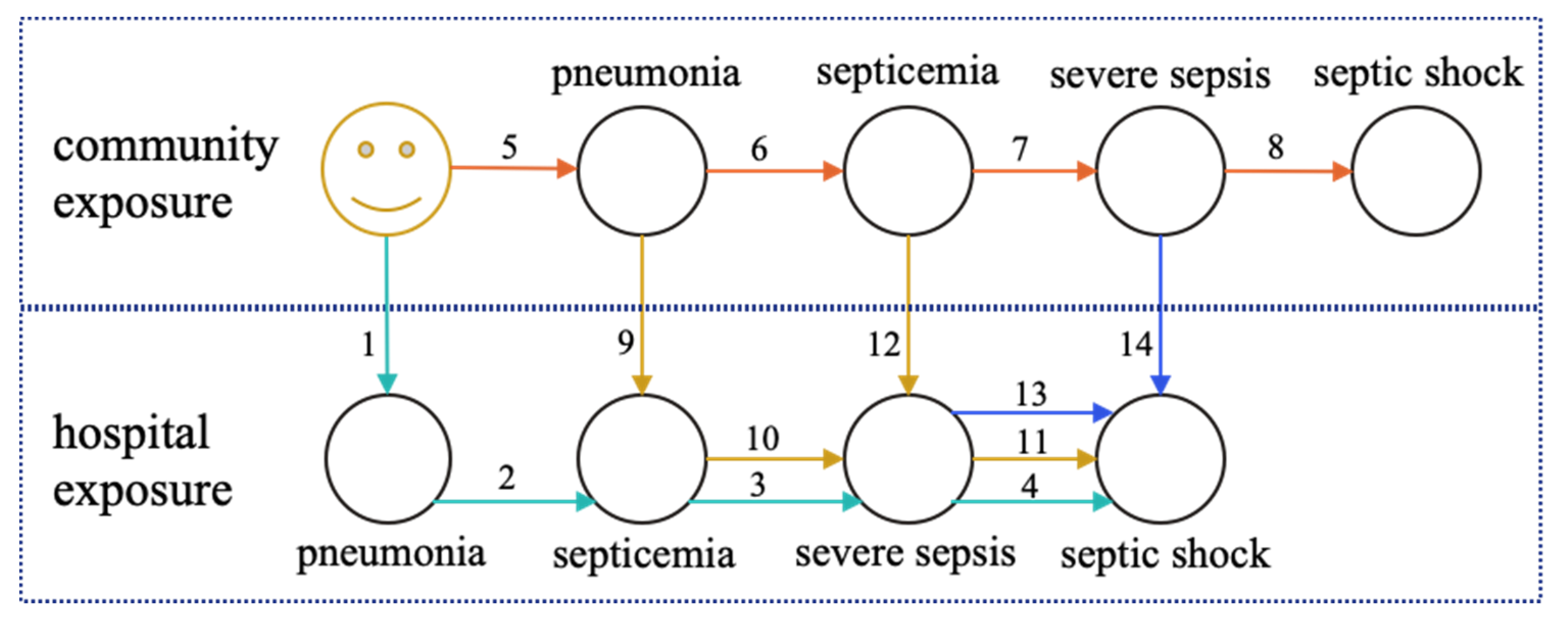
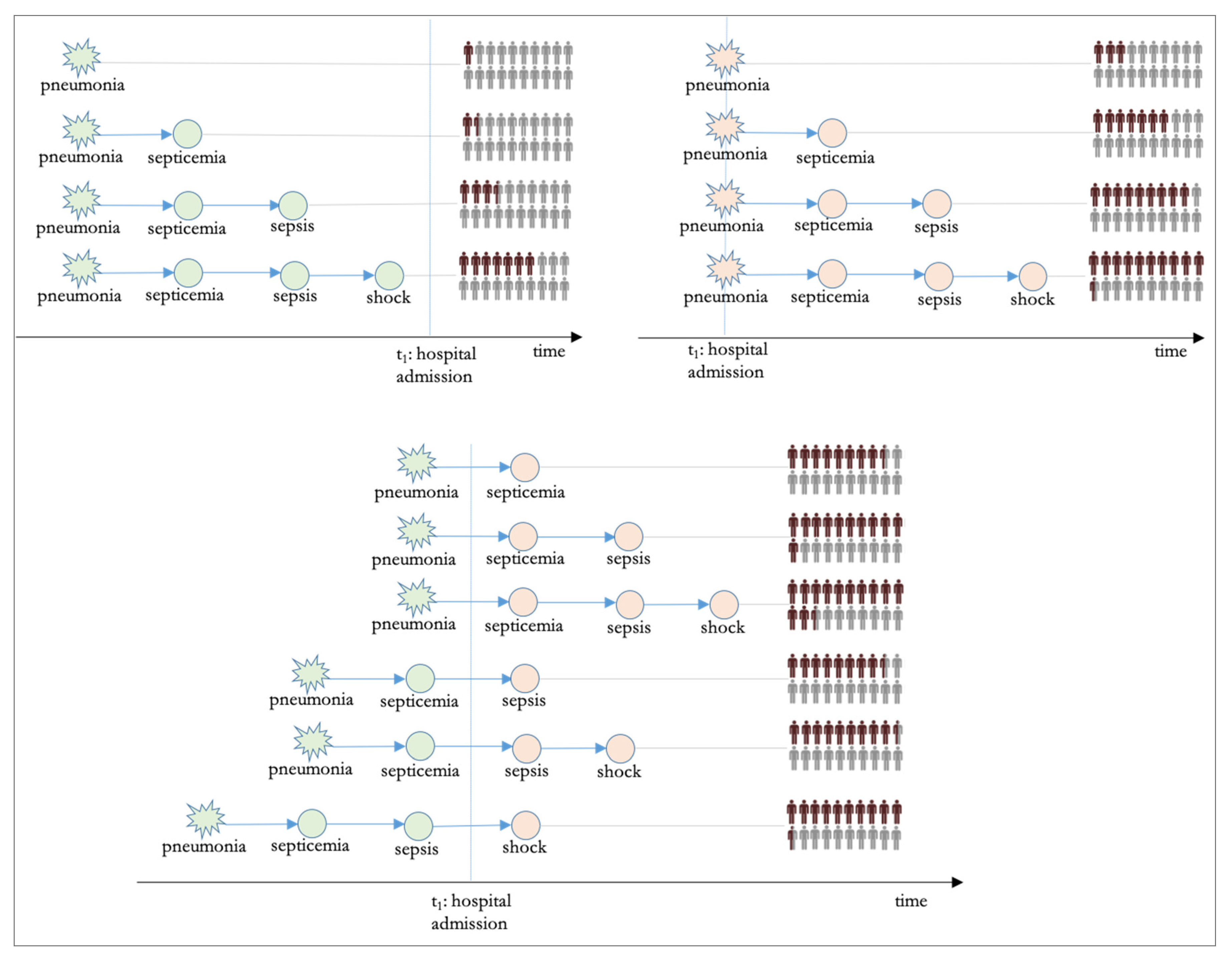
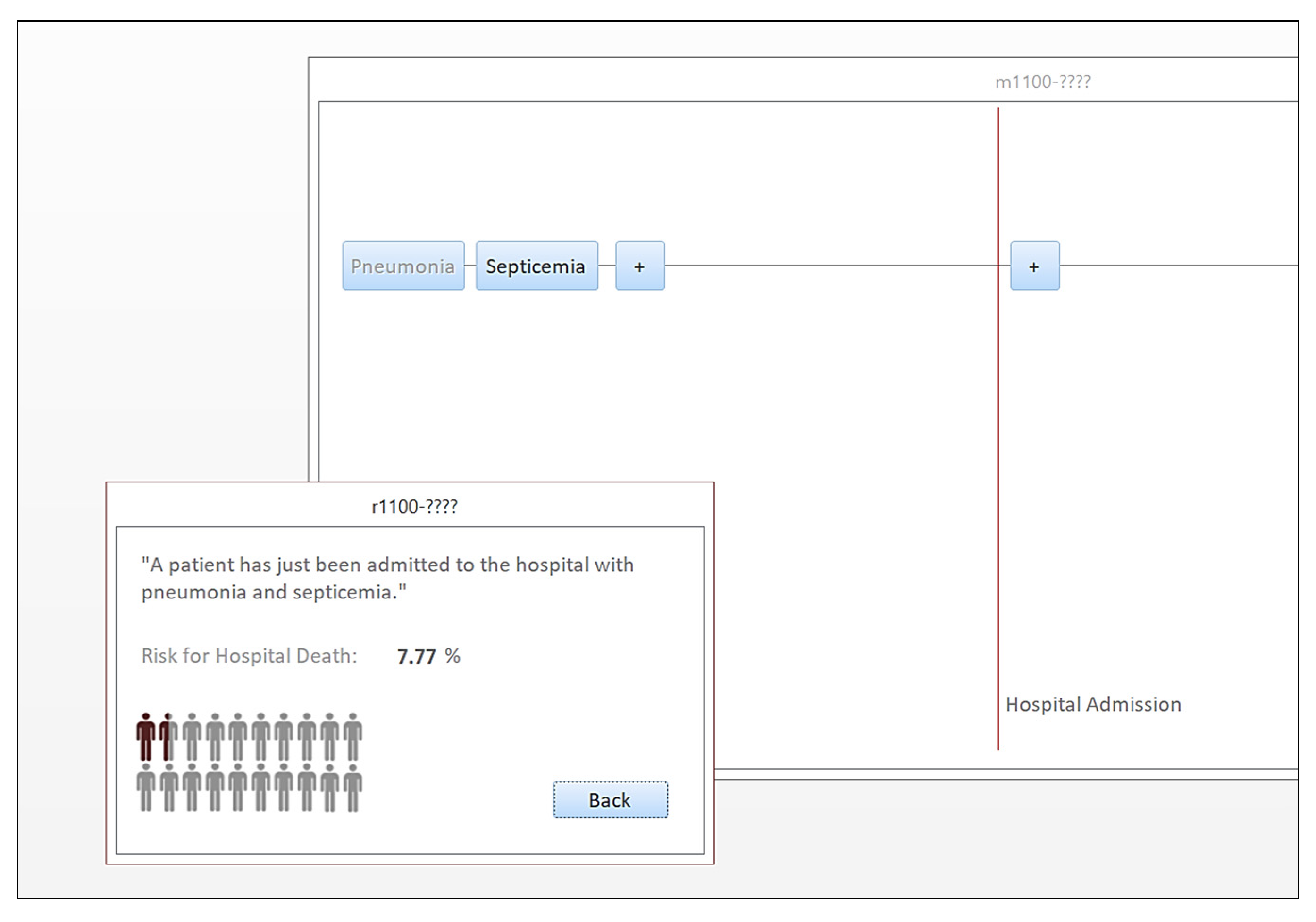
In acknowledgment of this opportunity, we designed a retrospective cross-sectional study to represent the progression of pneumonia-induced bloodstream infections using graph theory principles, where each path of the graph is a different scenario of bloodstream-infection progression, and we furthermore estimated the likelihood for inpatient death for each path. To disseminate results more effectively, we also developed a prototype applet to navigate various pneumonia-induced bloodstream infection paths of the graph, interactively. The study contribution is summarized below: (a) A new approach is introduced for the study of the effect of pneumonia-induced bloodstream infections on hospital mortality. In this approach, the representation of the progression is based on directed graphs. (b) The attributable risk for inpatient death is quantified for each step of the progression by examining each path as a predictor using multivariate analysis. (c) A prototype applet is made available, allowing the user to navigate various paths of the graph and learn about the risk for inpatient mortality. This applet can be used as a data-driven tool in health curricula.
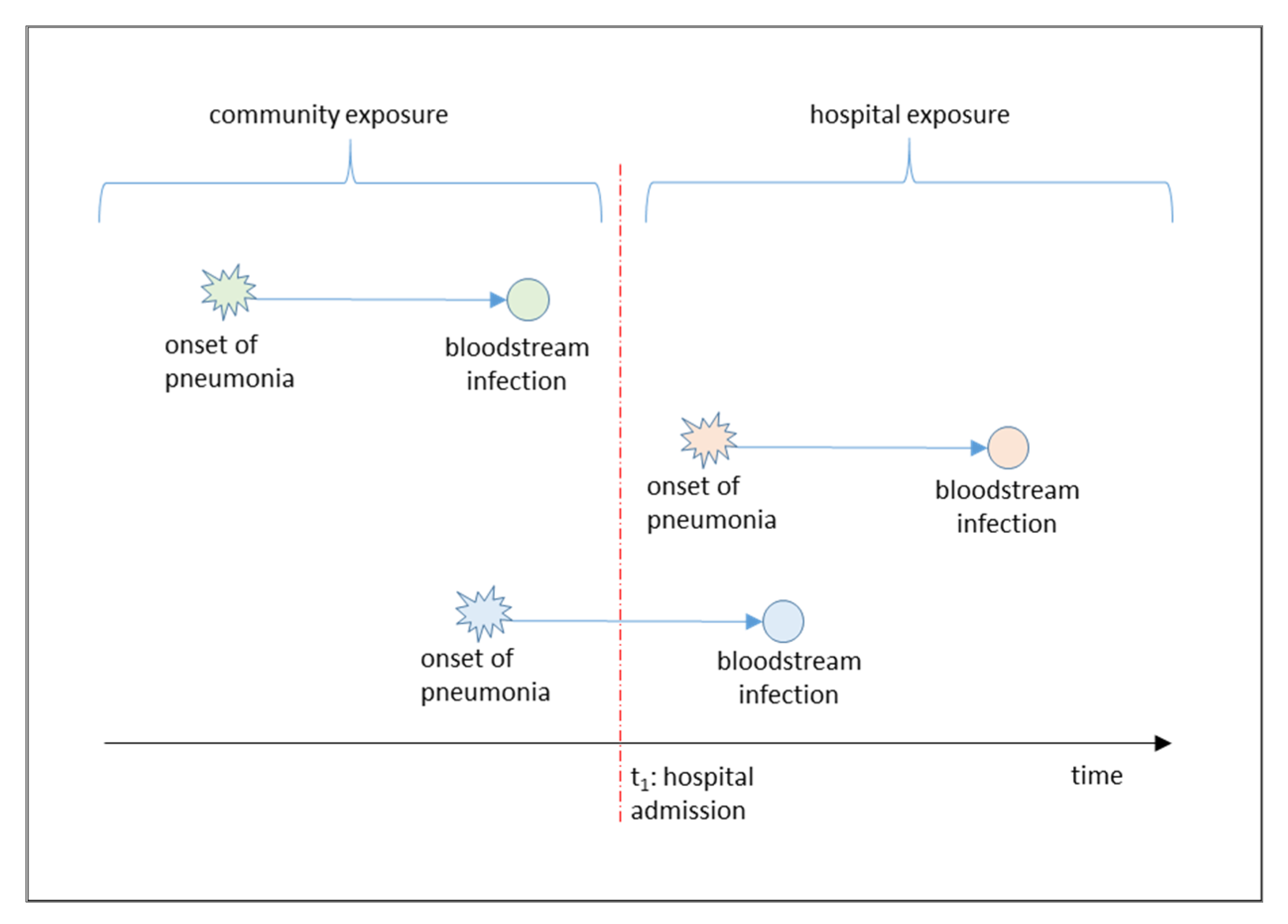
Figure 1 shows the contextual framework of the study, with three different types of pneumonia-induced bloodstream infection events: Those with community onset and progression, those with hospital onset and progression, and mixed events with community onset and hospital progression.
4. Discussion
This study used a novel representation of pneumonia-induced bloodstream infections, based on graph theory. The study used a dataset with real medical claims data from the Centers for Medicare and Medicaid Services. The scenarios that the research examined were based on the established clinical knowledge that bloodstream infection progression is sequential and ordered, with septicemia always preceding sepsis, which always precedes septic shock, with many articles examining this progression [
20]. Each stage of the infection was represented as a graph node, and the graph paths were therefore different scenarios of bloodstream infections. The probability of death was found to be lower when a patient was admitted to the hospital with community pneumonia that had not progressed to a bloodstream infection. At that time point, and considering that it is yet unknown whether the patient will develop a nosocomial infection or not, the mortality is only 4.99%. Patients with community pneumonia typically respond well to antibiotics, which they usually receive early on, soon after admission to the hospital. Apparently, this is a prophylaxis from further progression to a life-threatening bloodstream infection. On the other hand, when pneumonia is HAP, hospital mortality is much higher, at 13.71%. The main reason for this finding is that these cases of pneumonia are most likely to be methicillin resistant. Additionally, when a HAP-induced bloodstream infection develops in the hospital, the crude mortality is much higher compared to pneumonia-induced bloodstream infections imported from the community (45.75% vs. 17.04%, for the progression up the stage of severe sepsis). Septicemia and sepsis have been extensively studied in the literature as risk factors for hospital death. The Centers for Disease Control and Prevention (CDC) make available US State-level statistics of septicemia mortality [
21], and it is evident that septicemia is a major risk factor. In the present study, we have furthermore shown that the likelihood for death is alarmingly high when septicemia is induced by pneumonia and further progresses to severe sepsis.
The mixed scenarios are of particular interest. These are scenarios where pneumonia began in the community, but the bloodstream infection progressed in-hospital. Some of these scenarios were found to have the highest rates of crude hospital mortality, at up to 63%. Those very high mortality rates can be explained by considering that a patient whose—already known—pneumonia cannot be treated and progresses to a life-threatening bloodstream infection is a critical patient who has a high risk of dying because of the underlying case mix of his/her conditions. Another explanation is that the pneumonia brought from the community may not be easy to treat because of its being caused by antibiotic-resistant microorganisms, despite those being atypical in the community.
The results of our study confirm the knowledge that HAPs are more lethal than community cases of pneumonia [
8,
22]. Additionally, the study confirms the knowledge that a pneumonia-induced bloodstream infection is associated with very high crude mortality rates [
11]. What this research adds to existing knowledge is the breakdown of the risk for each phase of a bloodstream infection. The progression in the various scenarios was represented in the form of clinical events with temporal sequences that can be displayed on computer graphs. The authors believe that approaches similar to the proposed 14-path directed graph can be further used in future research to study bloodstream infections as hospital events and examine the attributable risk for each progression phase.
The primary intended audience for the prototype navigator applet consists of university students and their instructors in health science programs, such as nursing and health administration. In such programs, students discuss the significance of preventive measures in lowering the risk for hospital infections and its implications in hospital quality. Therefore, navigating interactive graphs to create scenarios of bloodstream infections and discussing the risk in class can enhance data-driven aspects of college education.
The authors would finally like to discuss two limitations of this work and strategies for future improvements. First of all, the dataset that the research used includes Medicare admissions in US hospitals. Medicare patients are, in their majority, >65 years old, and therefore the results should be interpreted cautiously for younger inpatient cases. Secondly, the research only examined pneumonia-induced bloodstream infections, and therefore the end-user has limited events to explore via the graph representation of events, a total of fourteen. Future work can extend the scope by examining other sources of bloodstream infections, such as UTIs and abscesses, to create a universal bloodstream infection navigator which covers more scenarios than the pneumonia-induced ones. Additionally, with more data records in hand, future work could make it be possible to explore bloodstream infection progression scenarios for specific patient demographics and age groups. This was not possible in the present work, because it would lead to over filtering the dataset and having an insufficient amount of records per path. Finally, the authors want to discuss that there is a non-perfect link between the node of pneumonia and that of septicemia. This is because a small portion of patients with pneumonia could have developed septicemia triggered by other acute conditions that coexist with pneumonia, and this inconsistency has not been quantified.