A Bayesian Study of the Dynamic Effect of Comorbidities on Hospital Outcomes of Care for Congestive Heart Failure Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
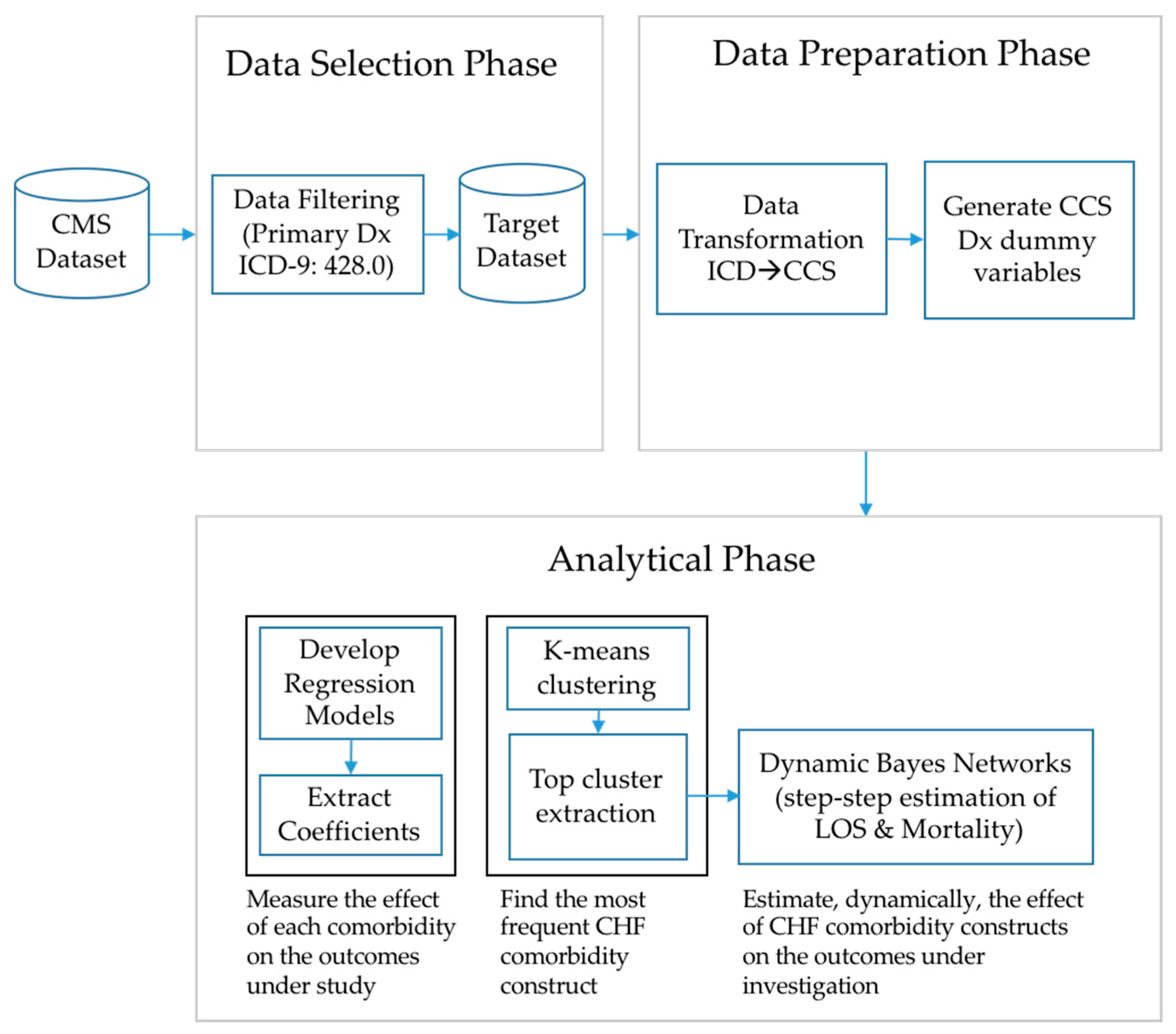
2.2. Data Selection and Preparation
2.3. Analytical Phase
3. Results
3.1. Data Description
3.2. Descriptive Analysis: CHF Comorbidities with the Longest LOS and Highest Hospital Mortality
3.3. Analytical Phase-Task 1: Coefficient Analysis
3.3.1. Length of Stay
3.3.2. Hospital Mortality Rate
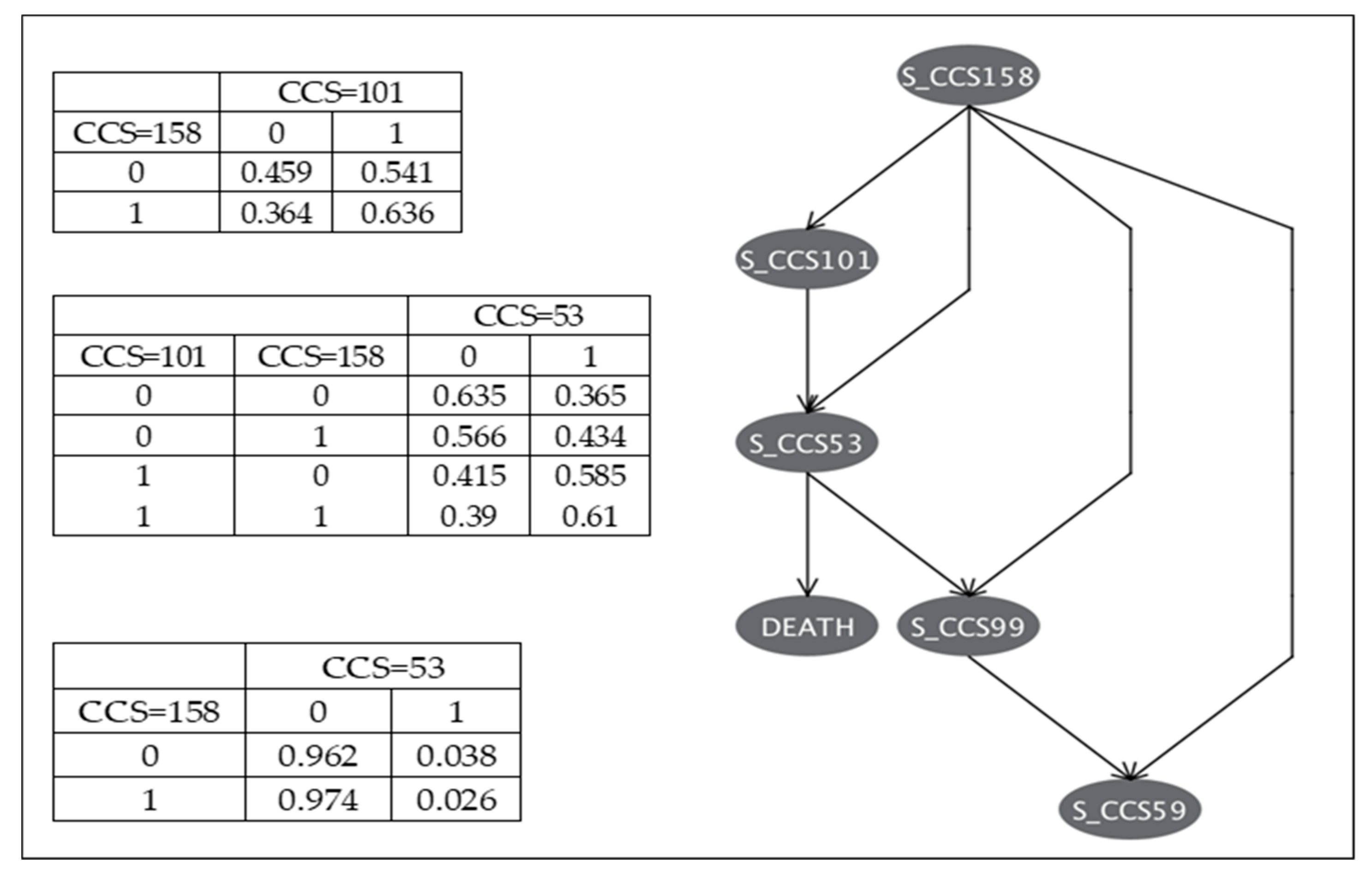
3.4. Analytical Phase-Task 2: Dynamic Navigation of CHF Comorbidity Scenarios and Their Effect on Outcomes
3.4.1. Directed Acyclic Graphs for Comorbidity Construct Scenarios
3.4.2. Bayesian Networks of CHF Comorbidities
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Prevention and Control. Chronic Diseases in America. 2019. Available online: https://www.cdc.gov/chronicdisease/resources/infographic/chronic-diseases.htm (accessed on 12 September 2019).
- U.S. Department of Health and Human Services. Multiple Chronic Conditions—A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions, Washington, DC, USA, December 2010. Available online: http://www.hhs.gov/ash/initiatives/mcc/mcc_framework.pdf (accessed on 12 September 2019).
- Feinstein, A.R. The pre-therapeutic classification of co-morbidity in chronic disease. J. Chronic Dis. 1970, 23, 455–468. [Google Scholar] [CrossRef]
- Piccirillo, J.F.; Costas, I. The impact of comorbidity on outcomes. ORL 2004, 66, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Jones, R. Chronic disease and comorbidity. Br. J. Gen. Pract. 2010, 60, 394. [Google Scholar] [CrossRef][Green Version]
- Comlossy, M. Chronic Disease Prevention and Management; National Conference of State Legislatures: Denver, CO, USA, 2013; Available online: http://www.ncsl.org/documents/health/ChronicDTK13.pdf (accessed on 12 September 2019).
- Centers for Disease Control. The Power of Prevention: Chronic Disease the Public Health Challenge of the 21st Century. 2009. Available online: www.cdc.gov/chronicdisease/pdf/2009-Power-of-Prevention.pdf (accessed on 12 September 2019).
- Gijsen, R.; Hoeymans, N.; Schellevis, F.G.; Ruwaard, D.; Satariano, W.A.; van den Bos, G.A.M. Causes and consequences of comorbidity: A review. J. Clin. Epidemiol. 2001, 54, 661–674. [Google Scholar] [CrossRef]
- Tinker, A. How to Improve Patient Outcomes for Chronic Diseases and Comorbidities. 2014. Available online: http://www.healthcatalyst.com/wp-content/uploads/2014/04/How-to-Improve-Patient-Outcomes.pdf (accessed on 12 September 2019).
- Buja, A.; Claus, M.; Perin, L.; Rivera, M.; Corti, M.C.; Avossa, F.; Schievano, E.; Rigon, S.; Toffanin, R.; Baldo, V.; et al. Multimorbidity patterns in high-need, high-cost elderly patients. PLoS ONE 2018, 13, e0208875. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.A.; Briss, P.A.; Ling, S.M.; Parrish, R.G.; Salive, M.E.; Finke, B.S. Multimorbidity patterns in the United States: Implications for research and clinical practice. J. Gerontol. Ser. A 2015, 71, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, I.; von Leitner, E.-C.; Schön, G.; Koller, D.; Hansen, H.; Kolonko, T.; Kaduszkiewicz, H.; Wegscheider, K.; Glaeske, G.; van den Bussche, H. Multimorbidity patterns in the elderly: A new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS ONE 2010, 5, e15941. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Valderas, J.M.; Yen, L.; Dawda, P.; Jowsey, T.; McRae, I.S. Multimorbidity and comorbidity of chronic diseases among the senior Australians: Prevalence and patterns. PLoS ONE 2014, 9, e83783. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.L.; Starfield, B.; Anderson, G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch. Intern. Med. 2002, 162, 2269–2276. [Google Scholar] [CrossRef]
- Wen, T.; He, S.; Attenello, F.; Cen, S.Y.; Kim-Tenser, M.; Adamczyk, P.; Amar, A.P.; Sanossian, N.; Mack, W.J. The impact of patient age and comorbidities on the occurrence of “never events” in cerebrovascular surgery: An analysis of the Nationwide Inpatient Sample. J. Neurosurg. 2014, 121, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Nobili, A.; Licata, G.; Salerno, F.; Pasina, L.; Tettamanti, M.; Franchi, C.; Vittorio, L.D.; Marengoni, A.; Corrao, S.; Iorio, A.; et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur. J. Clin. Pharmacol. 2011, 67, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Parappil, A.; Depczynski, B.; Collett, P.; Marks, G.B. Effect of comorbid diabetes on length of stay and risk of death in patients admitted with acute exacerbations of COPD. Respirology 2010, 15, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Wiler, J.L.; Handel, D.A.; Ginde, A.A.; Aronsky, D.; Genes, N.G.; Hackman, J.L.; Hilton, J.A.; Hwang, U.; Kamali, M.; Pines, J.M.; et al. Predictors of patient length of stay in 9 emergency departments. Am. J. Emerg. Med. 2012, 30, 1860–1864. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.A.; Humblet, O.; Hiatt, R.A.; Adler, N.E. Big data and disease prevention: From quantified self to quantified communities. Big Data 2013, 1, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Vaduganathan, M.; Fonarow, G.C.; Bonow, R.O. Rehospitalization for heart failure: Problems and perspectives. J. Am. Coll. Cardiol. 2013, 61, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, J.B.; Anderson, G.F.; Gerstenblith, G.; Weller, W.; Niefeld, M.; Herbert, R.; Wu, A.W. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J. Am. Coll. Cardiol. 2003, 42, 1226–1233. [Google Scholar] [CrossRef]
- Marti, C.N.; Fonarow, G.C.; Gheorghiade, M.; Butler, J. Timing and duration of interventions in clinical trials for patients with hospitalized heart failure. Circ. Heart Fail. 2013, 6, 1095–1101. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adams, K.F.; Fonarow, G.C.; Costanzo, M.R.; Berkowitz, R.L.; LeJemtel, T.H.; Cheng, M.L.; Wynne, J. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J. Am. Coll. Cardiol. 2005, 46, 57–64. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Giamouzis, G.; Parissis, J.; Starling, R.C.; Boudoulas, H.; Skoularigis, J.; Butler, J.; Filippatos, G. Reframing the association and significance of co-morbidities in heart failure. Eur. J. Heart Fail. 2016, 18, 744–758. [Google Scholar] [CrossRef]
- Szwejkowski, B.R.; Elder, D.H.J.; Shearer, F.; Jack, D.; Choy, A.M.J.; Pringle, S.D.; Struthers, A.D.; George, J.; Lang, C.C. Pulmonary hypertension predicts all-cause mortality in patients with heart failure: A retrospective cohort study. Eur. J. Heart Fail. 2012, 14, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Scrutinio, D.; Passantino, A.; Guida, P.; Ammirati, E.; Oliva, F.; Braga, S.S.; Rovere, M.T.L.; Lagioia, R.; Frigerio, M. Prognostic impact of comorbidities in hospitalized patients with acute exacerbation of chronic heart failure. Eur. J. Intern. Med. 2016, 34, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kucharska-Newton, A.M.; Heiss, G.; Ni, H.; Stearns, S.C.; Puccinelli-Ortega, N.; Wruck, L.M.; Chambless, L. Identification of heart failure events in medicare claims: The atherosclerosis risk in communities (ARIC) study. J. Card. Fail. 2016, 22, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Menis, M.; Forshee, R.A.; Kumar, S.; McKean, S.; Warnock, R.; Izurieta, H.S.; Gondalia, R.; Johnson, C.; Mintz, P.D.; Walderhaug, M.O.; et al. Babesiosis occurrence among the elderly in the United States, as recorded in large Medicare databases during 2006–2013. PLoS ONE 2015, 10, e0140332. [Google Scholar] [CrossRef] [PubMed]
- Healthcare Cost and Utilization Project. Clinical Classifications Software (CCS) for ICD-9-CM. Available online: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp (accessed on 12 September 2019).
- Lawson, C.A.; Solis-Trapala, I.; Dahlstrom, U.; Mamas, M.; Jaarsma, T.; Kadam, U.T.; Stromberg, A. Comorbidity health pathways in heart failure patients: A sequences-of-regressions analysis using cross-sectional data from 10,575 patients in the Swedish Heart Failure Registry. PLoS Med. 2018, 15, e1002540. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.C.; Ho, H.C.; Yu, M.; Chapital, A.D.; Koss, W.; Takanishi, D.M. Pre-existing cardiac disease, troponin I elevation and mortality in patients with severe sepsis and septic shock. Anaesth. Intensive Care 2008, 36, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Young, J.B.; Abraham, W.T.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; She, L.; Sun, J.L.; Yancy, C.W.; et al. Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE-HF registry). Am. J. Cardiol. 2008, 101, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Olier, I.; Rashid, M.; Mamas, M.A.; Gale, C.P.; Doherty, P.; Sperrin, M.; Kontopantelis, E.; Peat, G. Impact of co-morbid burden on mortality in patients with coronary heart disease, heart failure, and cerebrovascular accident: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2016, 3, 20–36. [Google Scholar]
- Mebazaa, A.; Tolppanen, H.; Mueller, C.; Lassus, J.; DiSomma, S.; Baksyte, G.; Januzzi, J. Acute heart failure and cardiogenic shock: A multidisciplinary practical guidance. Intensive Care Med. 2016, 42, 147–163. [Google Scholar] [CrossRef]
- Lee, D.S.; Austin, P.C.; Rouleau, J.L.; Liu, P.P.; Naimark, D.; Tu, J.V. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. Jama 2003, 290, 2581–2587. [Google Scholar] [CrossRef]
- Krumholz, H.M.; Chen, Y.-T.; Vaccarino, V.; Wang, Y.; Radford, M.J.; Bradford, W.D.; Horwitz, R.I. Correlates and impact on outcomes of worsening renal function in patients ≥65 years of age with heart failure. Am. J. Cardiol. 2000, 85, 1110–1113. [Google Scholar] [CrossRef]
- Forman, D.E.; Butler, J.; Wang, Y.; Abraham, W.T.; O’Connor, C.M.; Gottlieb, S.S.; Loh, E.; Massie, B.M.; Rich, M.W.; Stevenson, L.W.; et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J. Am. Coll. Cardiol. 2004, 43, 61–67. [Google Scholar] [CrossRef] [PubMed]
| Entire Dataset | Primary CCS = CHF Only | |||||
|---|---|---|---|---|---|---|
| Variable | N (%) | Mean (SD) | Range | N (%) | Mean (SD) | Range |
| Length of Stay (days) | 5.92 (±11.42) | 0–3086 | 5.19 (±5.13) | 0–161 | ||
| Hospital Mortality (%) | 3.16 (±17.4) | 0–1 | 3.17 (±17.5) | 0–1 | ||
| Primary CCS = 108 * | 25,647 (4.5%) | 25,647 (100%) | ||||
| Secondary CCS = 158 * | 136,391 (24.1%) | 12,385 (48.3%) | ||||
| Secondary CCS = 53 * | 241,228 (42.7%) | 13,186 (51.4%) | ||||
| Secondary CCS = 59 * | 148,528 (26.3%) | 9260 (36.1%) | ||||
| Secondary CCS = 99 * | 127,175 (22.5%) | 11,613 (45.3%) | ||||
| Secondary CCS = 101 * | 183,386 (32.5%) | 15,053 (58.7%) | ||||
| CHF Comorbidity | LOS (days) | N |
| Gangrene (CCS = 248) | 15.70 | 64 |
| Shock (CCS = 249) | 14.76 | 450 |
| Intestinal obstruction w/o hernia (CCS = 145) | 13.21 | 127 |
| Septicemia (CCS = 2) | 13.00 | 456 |
| Acute post-hemorrhagic anemia (CCS = 60) | 12.58 | 356 |
| Aspiration pneumonitis (CCS = 129) | 12.15 | 300 |
| Acute cerebrovascular disease (CCS = 109) | 11.20 | 135 |
| Cardiac arrest and ventricular fibrillation (CCS = 107) | 10.84 | 238 |
| MHSA: Adjustment disorders (CCS = 650) | 10.77 | 53 |
| Complication of surgical /medical procedure (CCS = 238) | 10.49 | 533 |
| CHF Comorbidity | Mortality (%) | N |
| Cardiac arrest and ventricular fibrillation (CCS = 107) | 51.7 | 238 |
| Shock (CCS = 249) | 32.9 | 450 |
| Peritonitis and intestinal abscess (CCS = 148) | 26.9 | 26 |
| Septicemia (CCS = 2) | 21.9 | 456 |
| Aspiration pneumonitis (CCS = 129) | 19.3 | 300 |
| Prolapse of female genital organs (CCS = 170) | 18.2 | 11 |
| Intestinal obstruction w/o hernia (CCS = 145) | 18.1 | 127 |
| Liver Ca and intrahepatic bile duct (CCS = 16) | 17.2 | 29 |
| Cancer of the esophagus (CCS = 12) | 15.8 | 38 |
| Gangrene (CCS = 248) | 15.6 | 64 |
| CHF Comorbidity | b | S.E. | p-value |
|---|---|---|---|
| Gangrene (CCS = 248) | 6.89 | 0.55 | <0.001 |
| Shock (CCS = 249) | 4.96 | 0.21 | <0.001 |
| Adjustment disorders (CCS = 650) | 4.73 | 0.59 | <0.001 |
| Intestinal obstruction w/o hernia (CCS = 145) | 4.32 | 0.38 | <0.001 |
| Aspiration pneumonitis (CCS = 129) | 3.63 | 0.25 | <0.001 |
| Acute cerebrovascular disease (CCS = 109) | 3.53 | 0.38 | <0.001 |
| Acute hemorrhage anemia (CCS = 60) | 3.46 | 0.24 | <0.001 |
| Diseases of the mouth (CCS = 137) | 2.89 | 0.61 | <0.001 |
| Complications (surg./med) (CCS = 238) | 2.89 | 0.19 | <0.001 |
| Septicemia (CCS = 2) | 2.71 | 0.21 | <0.001 |
| CHF Comorbidity | O.R. | S.E. | p-value |
|---|---|---|---|
| Cardiac arrest and ventric. fibril. (CCS = 107) | 30.50 | 0.17 | <0.001 |
| Peritonitis and intestinal abscess (CCS = 148) | 14.42 | 0.63 | <0.001 |
| Prolapse female gen. organs (CCS = 170) | 12.92 | 0.87 | <0.01 |
| Cancer of the esophagus (CCS = 12) | 10.03 | 0.54 | <0.001 |
| Cancer of the liver (CCS = 16) | 8.07 | 0.63 | <0.001 |
| Shock (CCS = 249) | 6.72 | 0.15 | <0.001 |
| Gangrene (CCS = 248) | 4.04 | 0.50 | <0.01 |
| Acute cerebrovascular disease (CCS = 109) | 3.55 | 0.32 | <0.001 |
| Intestinal obstruction w/o hernia (CCS = 145) | 3.15 | 0.32 | <0.001 |
| Respiratory failure; arrest (CCS = 131) | 2.76 | 0.08 | <0.001 |
| CHF Comorbidities | Clustered Instances |
|---|---|
| Cluster 1: ‘Disorders of lipid metabolism’ (CCS = 53), ‘deficiency and other anemia’ (CCS = 59), ‘hypertension with complications and secondary hypertension’ (CCS = 99), ‘coronary atherosclerosis and other heart disease’ (CCS = 101), ‘chronic kidney disease’ (CCS = 158) | 7565 (29%) |
| Cluster 2: ‘Fluid and electrolyte disorders’ (CCS = 55), ‘nutritional endocrine; and metabolic disorders’ (CCS = 58), ‘COPD and bronchiectasis’ (CCS = 127), ‘respiratory failure’ (CCS = 131) | 2181 (9%) |
| Cluster 3: ‘Essential hypertension’ (CCS = 98) | 4562 (18%) |
| Cluster 4: ‘Essential hypertension’ (CCS = 98), ‘disorders of lipid metabolism’ (CCS = 53), ‘coronary atherosclerosis and other heart disease’ (CCS = 101), ‘cardiac dysrhythmias’ (CCS = 106) | 5759 (22%) |
| Cluster 5: ‘Cardiac dysrhythmias’ (CCS = 106), ‘fluid and electrolyte disorders’ (CCS = 55), ‘deficiency and other anemia’ (CCS = 59), ‘hypertension with complications/secondary hypertension’ (CCS = 99), ‘chronic kidney disease’ (CCS = 158), ‘heart valve disorders’ (CCS = 96), ‘pulmonary heart disease’ (CCS = 103) | 2098 (8%) |
| Cluster 6: ‘Deficiency and other anemia’ (CCS = 59), ‘hypertension with complications and secondary hypertension’ (CCS = 99), ‘chronic kidney disease’ (CCS = 158), ‘coronary atherosclerosis and other heart disease’ (CCS = 101), ‘COPD and bronchiectasis’ (CCS = 127), ‘respiratory failure; insufficiency; arrest (adult)’ (CCS = 131), ‘diabetes mellitus without complications’ (CCS = 49), ‘acute and unspecified renal failure’ (CCS = 157) | 2284 (9%) |
| Cluster 7: ‘Respiratory failure; arrest’ (CCS = 131), ‘cardiac dysrhythmias’ (CCS = 106), ‘fluid and electrolyte disorders’ (CCS = 55), ‘essential hypertension’ (CCS = 98), ‘screening and history of mental health and substance abuse’ (CCS = 663) | 1198 (5%) |
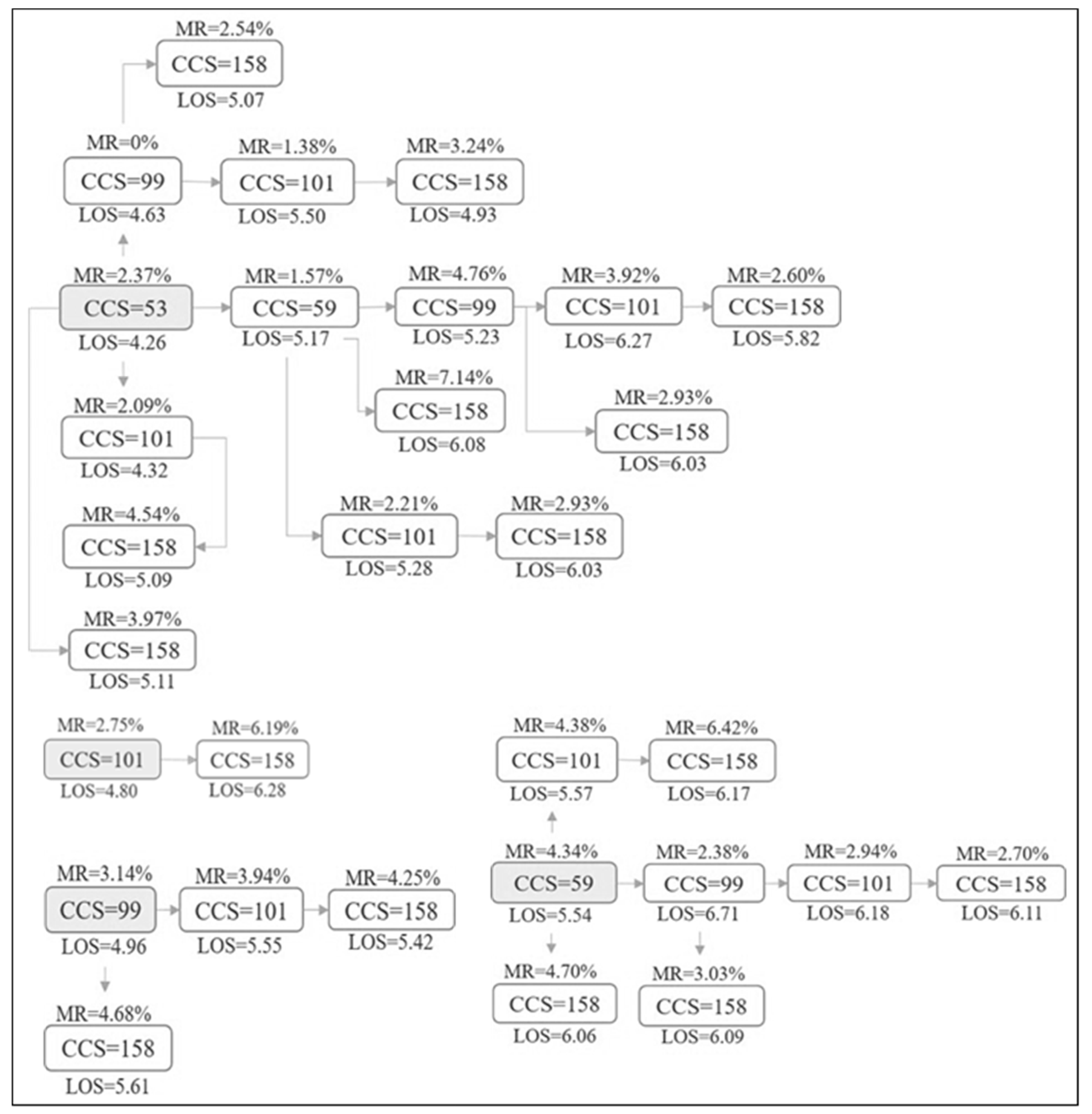
| Different Paths of CHF Comorbidities | N | Mortality rate (%) (95% C.I) | Mean LOS (days) (95% C.I) |
|---|---|---|---|
| No comorbidity from cluster | 2800 | 3.68 (2.98–4.37) | 4.76 (4.55–4.97) |
| 53 | 1598 | 2.37 (1.63–3.12) | 4.26 (4.10–4.43) |
| 53+59 | 508 | 1.57 (0.49–2.65) | 5.17 (4.83–5.51) |
| 53+99 | 95 | 0.00 (0.00–0.00) | 4.63 (3.76–5.49) |
| 53+101 | 3008 | 2.09 (1.58–2.60) | 4.32 (4.20–4.44) |
| 53+158 | 79 | 3.79 (0.00–8.01) | 5.11 (4.25–5.98) |
| 53+59+99 | 21 | 4.76 (0.00–14.09) | 5.23 (3.61–6.86) |
| 53+59+101 | 995 | 2.21 (1.34–3.08) | 5.29 (4.98–5.60) |
| 53+59+158 | 56 | 7.14 (1.24–13.04) | 6.80 (5.56–8.04) |
| 53+99+101 | 144 | 1.38 (0.00–3.31) | 5.50 (4.41–6.59) |
| 53+99+158 | 902 | 2.54 (1.56–3.53) | 5.08 (4.79–5.36) |
| 53+101+158 | 220 | 4.55 (1.79–7.30) | 5.09 (4.57–5.62) |
| 53+59+99+101 | 51 | 3.92 (0.00–9.30) | 6.27 (4.53–8.01) |
| 53+59+99+158 | 920 | 2.93 (1.88–3.98) | 6.04 (5.72–6.35) |
| 53+59+101+158 | 133 | 6.01 (1.98–10.05) | 6.16 (5.19–7.13) |
| 53+99+101+158 | 2308 | 3.25 (2.52–3.97) | 4.93 (4.74–5.12) |
| 53+59+99+101+158 | 2148 | 2.61 (1.93–3.28) | 5.82 (5.60–6.04) |
| 59 | 898 | 4.34 (0.68–3.01) | 5.54 (5.25–5.82) |
| 59+99 | 42 | 2.38 (0.00–7.04) | 6.71 (4.94–8.48) |
| 59+101 | 684 | 4.38 (2.88–5.89) | 5.57 (5.22–5.93) |
| 59+158 | 170 | 4.70 (1.52–7.89) | 6.06 (5.36–6.77) |
| 59+99+101 | 34 | 2.94 (0.00–8.79) | 6.18 (4.26–8.01) |
| 59+99+158 | 1088 | 3.03 (2.04–4.02) | 6.10 (5.74–6.45) |
| 59+101+158 | 218 | 6.42 (3.17–9.67) | 6.18 (5.57–6.78) |
| 59+99+101+158 | 1294 | 2.71 (1.82–3.58) | 6.11 (5.77–6.45) |
| 99 | 127 | 3.15 (0.11–6.19) | 4.96 (4.07–5.85) |
| 99+101 | 76 | 3.94 (0.00–8.32) | 5.55 (4.28–6.82) |
| 99+158 | 1046 | 4.68 (3.43–5.94) | 5.62 (5.16–6.08) |
| 99+101+158 | 1317 | 4.25 (3.17–5.33) | 5.43 (5.17–5.69) |
| 101 | 2181 | 2.75 (2.06–3.44) | 4.80 (4.59–5.01) |
| 101+158 | 242 | 6.20 (3.16–9.23) | 6.28 (5.48–7.08) |
| 158 | 244 | 7.79 (4.42–11.15) | 5.88 (4.84–6.93) |
| Total Comorbidities Present | N | Mortality Rate (%) (95% C.I) | Mean LOS (days) (95% C.I) |
|---|---|---|---|
| No comorbidity from cluster | 2800 | 3.68 (2.98–4.37) | 4.76 (4.55–4.97) |
| 1 out of 5 comorbidities | 5048 | 3.17 (2.69–3.65) | 4.82 (4.68–4.95) |
| 2 out of 5 comorbidities | 5950 | 3.03 (2.59–3.46) | 4.94 (4.82–5.07) |
| 3 out of 5 comorbidities | 4995 | 3.32 (2.84–3.81) | 5.52 (5.38–5.66) |
| 4 out of 5 comorbidities | 4706 | 3.12 (2.63–3.61) | 5.52 (5.37–5.67) |
| All five comorbidities | 2148 | 2.61 (1.93–3.28) | 5.82 (5.60–6.04) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zikos, D.; Zimeras, S.; Ragina, N. A Bayesian Study of the Dynamic Effect of Comorbidities on Hospital Outcomes of Care for Congestive Heart Failure Patients. Technologies 2019, 7, 66. https://doi.org/10.3390/technologies7030066
Zikos D, Zimeras S, Ragina N. A Bayesian Study of the Dynamic Effect of Comorbidities on Hospital Outcomes of Care for Congestive Heart Failure Patients. Technologies. 2019; 7(3):66. https://doi.org/10.3390/technologies7030066
Chicago/Turabian StyleZikos, Dimitrios, Stelios Zimeras, and Neli Ragina. 2019. "A Bayesian Study of the Dynamic Effect of Comorbidities on Hospital Outcomes of Care for Congestive Heart Failure Patients" Technologies 7, no. 3: 66. https://doi.org/10.3390/technologies7030066
APA StyleZikos, D., Zimeras, S., & Ragina, N. (2019). A Bayesian Study of the Dynamic Effect of Comorbidities on Hospital Outcomes of Care for Congestive Heart Failure Patients. Technologies, 7(3), 66. https://doi.org/10.3390/technologies7030066





