Surface Modification of Polystyrene with O Atoms Produced Downstream from an Ar/O2 Microwave Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, X-ray Photoelectron Spectroscopy (XPS), Contact Angle Goniometry, Atomic Force Microscopy (AFM)
2.2. Production of Oxygen Atoms
3. Results
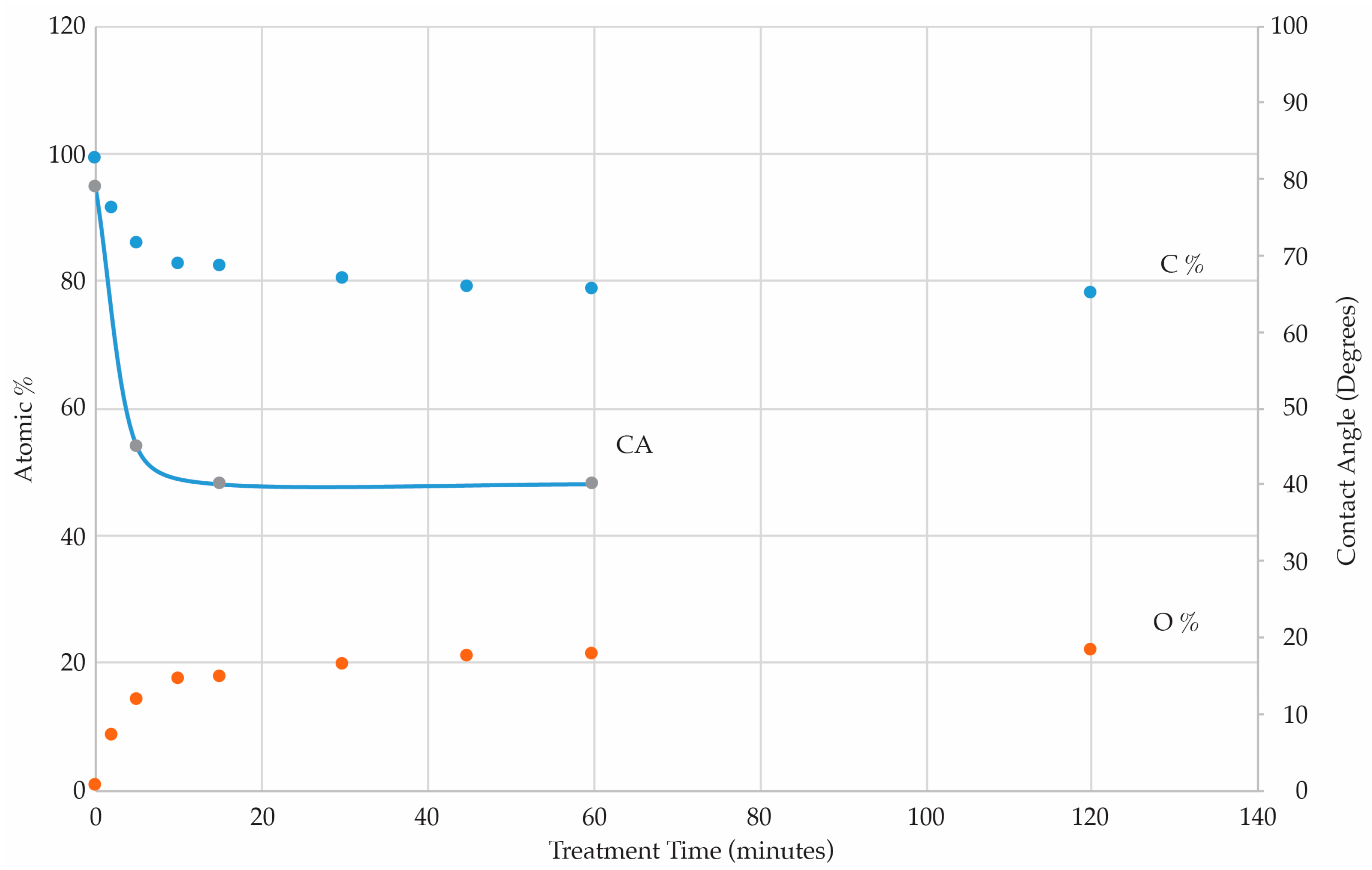
3.1. Treatment with a O Atoms Downstream from an Ar/O2 MW Plasma
3.1.1. PS Quantitative Analyses and Contact Angle Measurements
3.1.2. XPS Chemical State Analyses
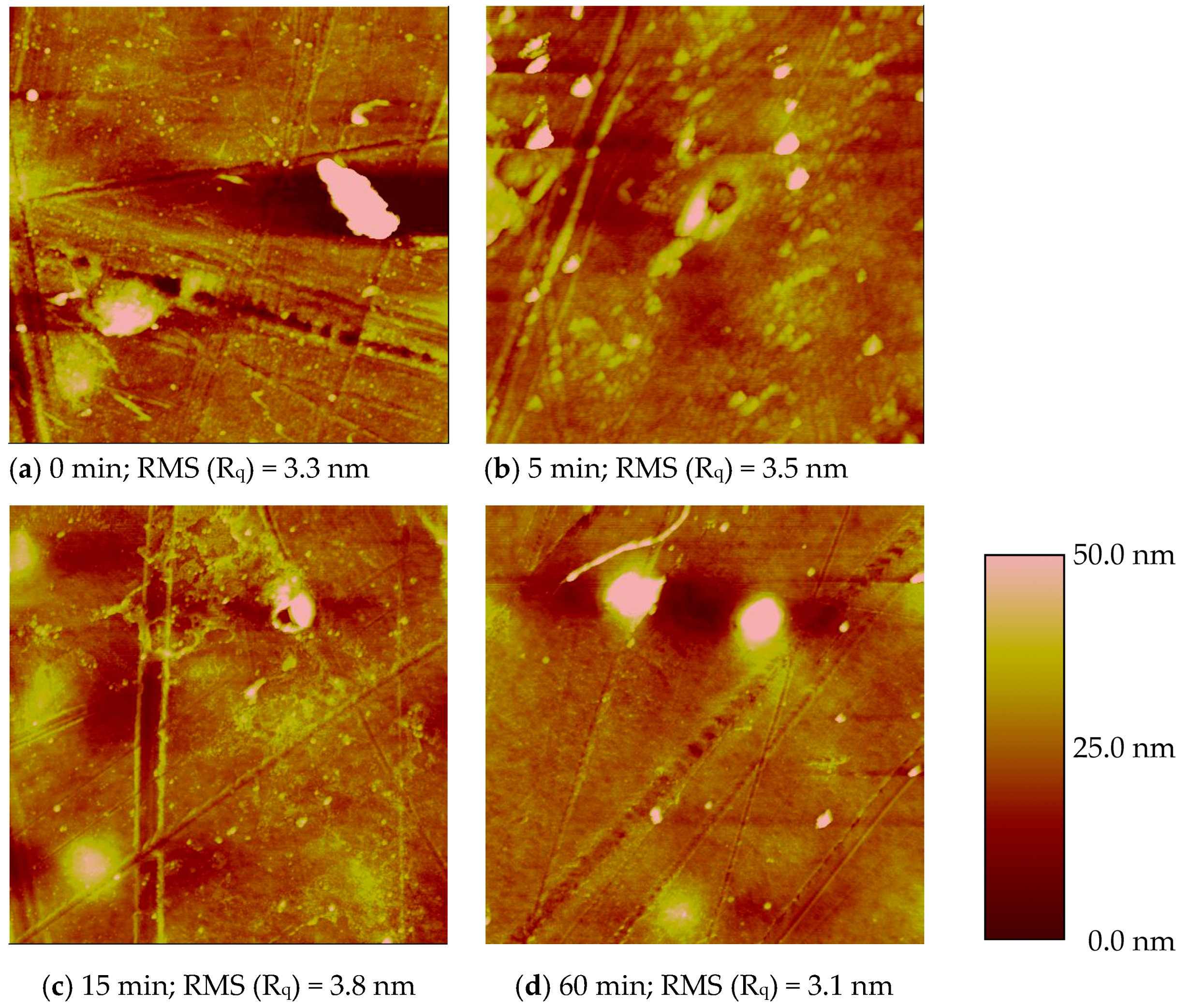
3.1.3 Surface Topography for O Atom Treatment
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bandyopadhyay, A.; Basak, G.C. Studies of photocatalytic degradation of polystyrene. Mater. Sci. Technol. 2007, 23, 305–314. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Optimized synthesis and characterization of polystyrene graft copolymers and preliminary assessment of their biodegradability and application in water pollution alleviation technologies. Polym. Degrad. Stab. 2007, 92, 876–885. [Google Scholar] [CrossRef]
- Ramsey, W.S.; Hertl, W.; Nowlan, E.D.; Binkowski, N.J. Surface treatments and cell attachment. In Vitro 1984, 20, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Toro, M.; Lu, F.; On, J.; Bailey, A.; Debies, T.; Mehan, M.; Gupta, S.K.; Takacs, G.A. Vacuum UV photo-oxidation of polystyrene. J. Adhes. Sci. Technol. 2016, 30, 2212–2223. [Google Scholar] [CrossRef]
- Calvert, J.G.; Pitts, J.N. Photochemistry; Wiley & Sons: New York, NY, USA, 1966; pp. 205–209. [Google Scholar]
- Okabe, H. Photochemistry of Small Molecules; Wiley & Sons: New York, NY, USA, 1978; p. 179. [Google Scholar]
- Morgan, A.; Cocca, M.; Vega, K.; Fleischer, A.; Gupta, S.K.; Mehan, M.; Takacs, G.A. Vacuum UV photo-oxidation of poly(ethylene terephthalate). J. Adhes. Sci. Technol. 2017, 31, 2542–2554. [Google Scholar] [CrossRef]
- Hollander, A.; Klemberg-Sapieha, J.E.; Wertheimer, M.R. The influence of vacuum-ultraviolet radiation on poly(ethylene terephthalate). J. Polym. Sci. A Polym. Chem. 1996, 34, 1511–1516. [Google Scholar] [CrossRef]
- Badey, J.P.; Urbaczewski-Espunche, E.; Jugnet, Y.; Sage, D.; Minh Duc, T.; Chabert, B. Surface modification of poly(tetrafluoroethylene) by microwave plasma downstream treatment. Polymer 1994, 35, 2472–2479. [Google Scholar] [CrossRef]
- Lens, J.P.; Spaay, B.; Terlingen, J.G.A.; Engbers, G.H.M.; Feijen, J. Mechanism of the immobilization of surfactants on polymer surfaces by means of an argon plasma treatment: Influence of polymer of UV radiation. Plasmas Polym. 1999, 4, 159–182. [Google Scholar] [CrossRef]
- Takacs, G.A.; Glass, G.P. Reaction of atomic oxygen with hydrogen bromide. J. Phys. Chem. 1973, 77, 1182–1186. [Google Scholar] [CrossRef]
- Oliveira, L.; Debies, T.; Takacs, G.A. Reaction of multiwalled carbon nanotubes with gaseous oxygen and chlorine atoms. In Recent Advances in Adhesion Science and Technology in Honor of Dr. Kash Mittal; Gutowski, W.V., Dodiuk, H., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 319–329. [Google Scholar]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Dong, X.; Ma, L.Q.; Zhu, Y.; Li, Y.; Gu, B. Mechanistic investigation of mercury sorption by Brazilian pepper biochars of different pyrolytic temperatures based on X-ray photoelectron spectroscopy and flow calorimetry. Environ. Sci. Technol. 2013, 47, 12156–12164. [Google Scholar] [CrossRef] [PubMed]
- Girardeaux, C.; Pireaux, J.-J. Analysis of polystyrene (PS) by XPS. Surf. Sci. Spectra 1996, 4, 130–133. [Google Scholar] [CrossRef]
- Lianos, L.; Parrat, D.; Hoc, T.Q.; Duc, T.M. Secondary ion mass spectrometry time of flight and in situ X-ray photoelectron spectroscopy studies of polymer surface modifications by remote oxygen plasma treatment. J. Vac. Sci. Technol. 1994, 12, 2491–2498. [Google Scholar] [CrossRef]
- Greyson, J.; Ingalls, R.B.; Keen, R.T. ESR studies of the interaction of nitrogen and oxygen atoms with polystyrene. J. Chem. Phys. 1996, 45, 3755–3759. [Google Scholar] [CrossRef]
- Moss, S.J.; Jolly, A.M.; Tight, B.J. Plasma oxidation of polymers. Plasma Chem. Plasma Process. 1986, 6, 401–415. [Google Scholar] [CrossRef]
- Minton, T.K.; Zhang, J.; Garton, D.J.; Seale, J.W. Collision-assisted erosion of hydrocarbon polymers in atomic-oxygen environments. High Perform. Polym. 2000, 12, 27–42. [Google Scholar] [CrossRef]
- Banks, B.A.; Backus, J.A.; Manno, M.V.; Waters, D.L.; Cameron, K.C.; de Groh, K.K. Atomic Oxygen Erosion Yield Prediction for Spacecraft Polymers in Low Earth Orbit; NASA Center for AeroSpace Information (CASI): Hanover, MD, USA, 2009; pp. 1–15.
- De Groh, K.K.; Banks, B.A.; McCarthy, C.E.; Rucker, R.N.; Roberts, L.M.; Berger, L.A. MISSE 2 PEACE Polymers Atomic Oxygen Erosion Experiment on the International Space Station. High Perform. Polym. 2008, 20, 388–409. [Google Scholar] [CrossRef]
- Stambler, A.H.; Inoshita, K.E.; Roberts, L.M.; Barbagallo, C.E.; de Groh, K.K.; Banks, B.A. Ground-laboratory and in-space atomic oxygen correlation for the PEACE polymers. AIP Proc. Prot. Mater. Struct. Space Environ. 2009, 1087, 51–66. [Google Scholar]
- Golub, M.A.; Wydeven, T. Reaction of atomic oxygen (O(3P)) with various polymer films. Polym. Degrad. Stab. 1988, 22, 325–338. [Google Scholar] [CrossRef]
- Hansen, R.H.; Pascale, J.V.; de Benedictis, T.; Rentzepis, P.M. Effect of atomic oxygen on polymers. J. Polym. Sci. 1965, 3, 2205–2214. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N. Atmospheric Chemistry; Wiley & Sons: New York, NY, USA, 1986; pp. 459–469. [Google Scholar]
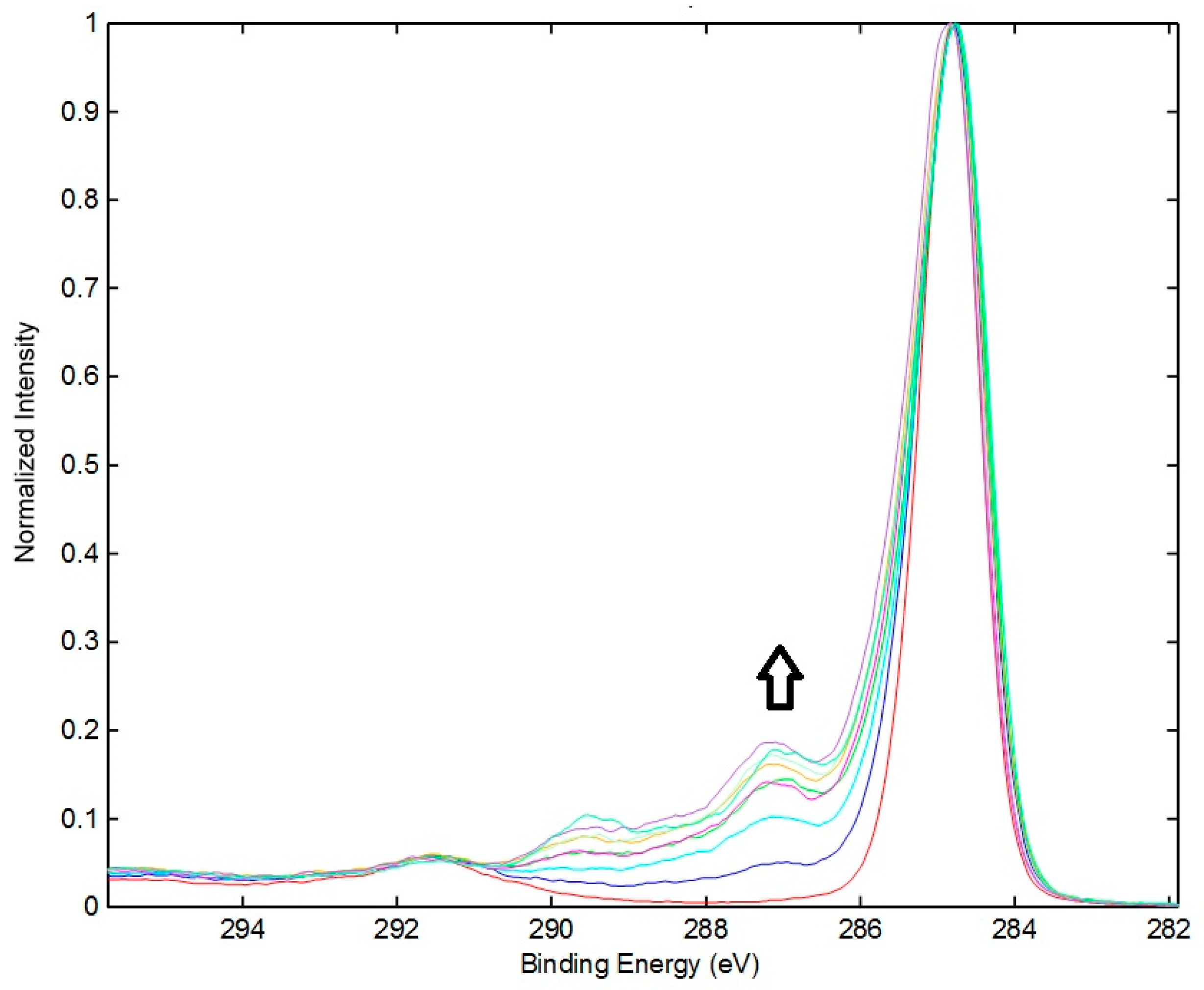
| Binding Energy (eV) | Species | Control | 2 min | 5 min | 10 min | 15 min | 30 min | 45 min | 60 min | 120 min |
|---|---|---|---|---|---|---|---|---|---|---|
| 284.6 | C–C sp2 | 78.0 | 72.3 | 64.5 | 60.4 | 53.5 | 56.0 | 53.1 | 46.1 | 51.7 |
| 285.1 | C–C sp3 | 17.7 | 13.8 | 10.6 | 8.1 | 11.3 | 7.1 | 7.3 | 10.3 | 6.6 |
| 286.0 | 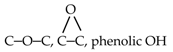 | 0 | 8.5 | 11.1 | 14.1 | 16.0 | 17.0 | 18.2 | 20.1 | 19.3 |
| 287.0 | C=O, aldehyde | 0 | 1.9 | 8.1 | 11.0 | 11.8 | 12.3 | 13.4 | 14.1 | 13.7 |
| 288.6 | O–C=O, carboxylic acid | 0 | 0 | 1.7 | 2.5 | 2.7 | 3.4 | 3.7 | 4.6 | 4.5 |
| 289.8 | O=C–O–C=O, O–(C=O)–O | 0 | 0 | 1.1 | 1.9 | 2.4 | 2.6 | 2.7 | 3.3 | 3.3 |
| 292.0 | Energy Loss | 4.3 | 3.4 | 3.0 | 2.1 | 2.3 | 1.8 | 1.6 | 1.6 | 1.0 |
| Binding Energy (eV) | Species | Control | 60 min | 60 min Washed | 60 min Washed |
|---|---|---|---|---|---|
| 284.6 | C–C sp2 | 78.0 | 46.1 | 67.7 | 61.3 |
| 285.1 | C–C sp3 | 17.7 | 10.3 | 12.5 | 16.2 |
| 286.0 |  | 0 | 20.1 | 8.4 | 7.2 |
| 287.0 | C=O, aldehyde | 0 | 14.1 | 6.2 | 7.1 |
| 288.6 | O–C=O, carboxylic acid | 0 | 4.6 | 3.1 | 5.0 |
| 289.8 | O=C–O–C=O, O–(C=O)–O | 0 | 3.3 | 0.8 | 1.4 |
| 292.0 | Energy Loss | 4.3 | 1.6 | 1.3 | 1.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lu, F.; Cocca, M.; Vega, K.; Fleischer, A.; Bailey, A.; Toro, M.; Sachdev, S.; Debies, T.; Mehan, M.; et al. Surface Modification of Polystyrene with O Atoms Produced Downstream from an Ar/O2 Microwave Plasma. Technologies 2018, 6, 21. https://doi.org/10.3390/technologies6010021
Li X, Lu F, Cocca M, Vega K, Fleischer A, Bailey A, Toro M, Sachdev S, Debies T, Mehan M, et al. Surface Modification of Polystyrene with O Atoms Produced Downstream from an Ar/O2 Microwave Plasma. Technologies. 2018; 6(1):21. https://doi.org/10.3390/technologies6010021
Chicago/Turabian StyleLi, Xinyun, Fei Lu, Matthew Cocca, Katerine Vega, Andrew Fleischer, Alla Bailey, Marc Toro, Shreen Sachdev, Thomas Debies, Michael Mehan, and et al. 2018. "Surface Modification of Polystyrene with O Atoms Produced Downstream from an Ar/O2 Microwave Plasma" Technologies 6, no. 1: 21. https://doi.org/10.3390/technologies6010021
APA StyleLi, X., Lu, F., Cocca, M., Vega, K., Fleischer, A., Bailey, A., Toro, M., Sachdev, S., Debies, T., Mehan, M., Gupta, S. K., & Takacs, G. A. (2018). Surface Modification of Polystyrene with O Atoms Produced Downstream from an Ar/O2 Microwave Plasma. Technologies, 6(1), 21. https://doi.org/10.3390/technologies6010021






