Application of Sonication and Microwave Irradiation to Boost Continuous Fabrication of the Copper(II) Oxide Sub-Micron Particles
Abstract
:1.Introduction
2. Experimental Section
2.1. Chemicals
2.2. Experimental Set-Up and Procedure
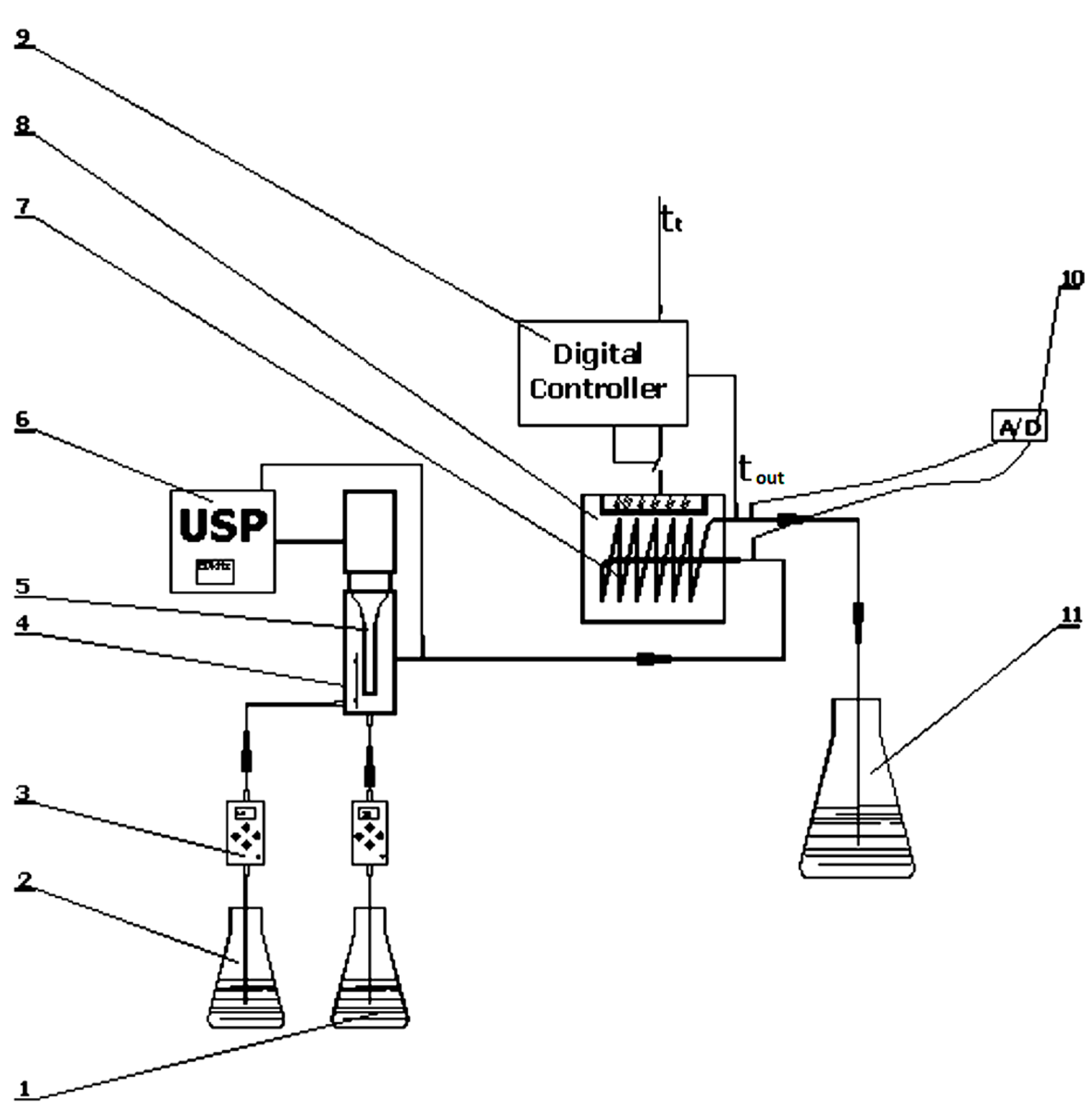

2.3. Analytical Methods
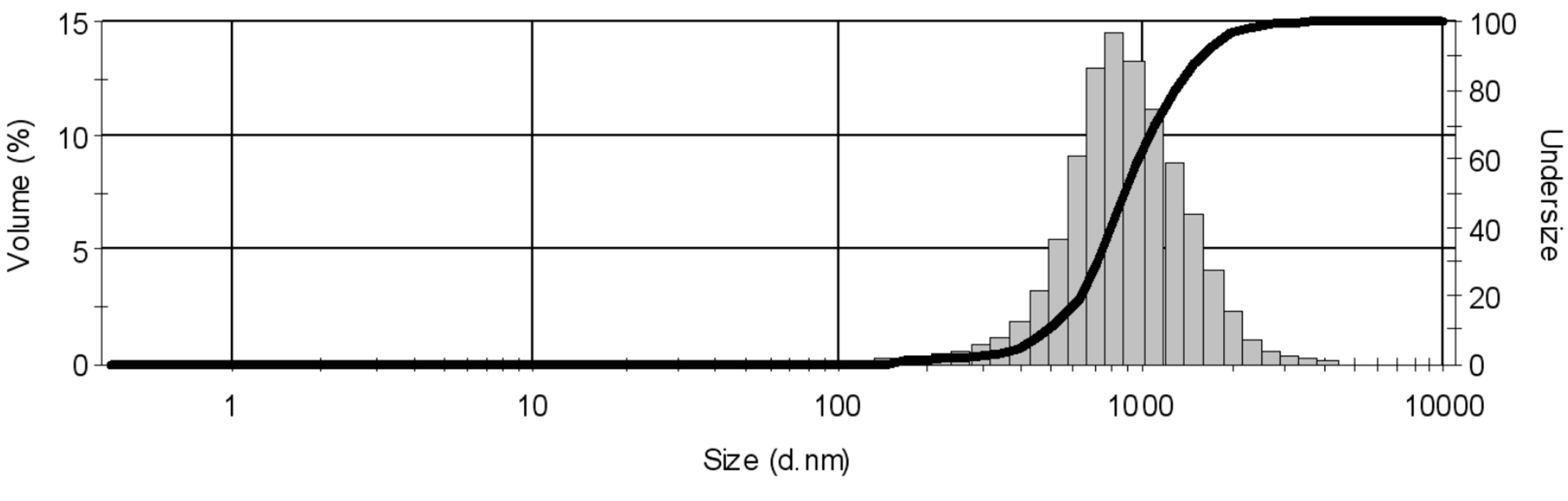
| Sample | CCu(OAc)2 (mol/dm3) | CNaOH (mol/dm3) | tt (°C) | Amplitude a (%) | z-Average (nm) |
|---|---|---|---|---|---|
| 1 | 0.1 | 0.2 | - | 100 | 405.5 |
| 2 | 0.1 | 0.2 | 86 | - | 238.7 |
| 3 | 0.1 | 0.2 | 86 | 80 | 273.5 |
| 4 | 0.1 | 0.2 | 86 | 40 | 379.2 |
| 5 b | 0.1 | 0.2 | 86 | 60 | - |
| 6 | 0.1 c | 0.2 | 86 | 60 | 385.5 |
| 7 | 0.2 | 0.4 d | 89 | 60 | 830.5 |
| 8 | 0.1 | 0.2 | 86 | 60 | 194.5 |
| 9 | 0.05 | 0.1 | 86 | 60 | 206.6 |
| 10 | 0.2 | 0.4 | 89 | 60 | 211.7 |
| 11 e | 0.1 | 0.2 | 86 | - | 496.6 |
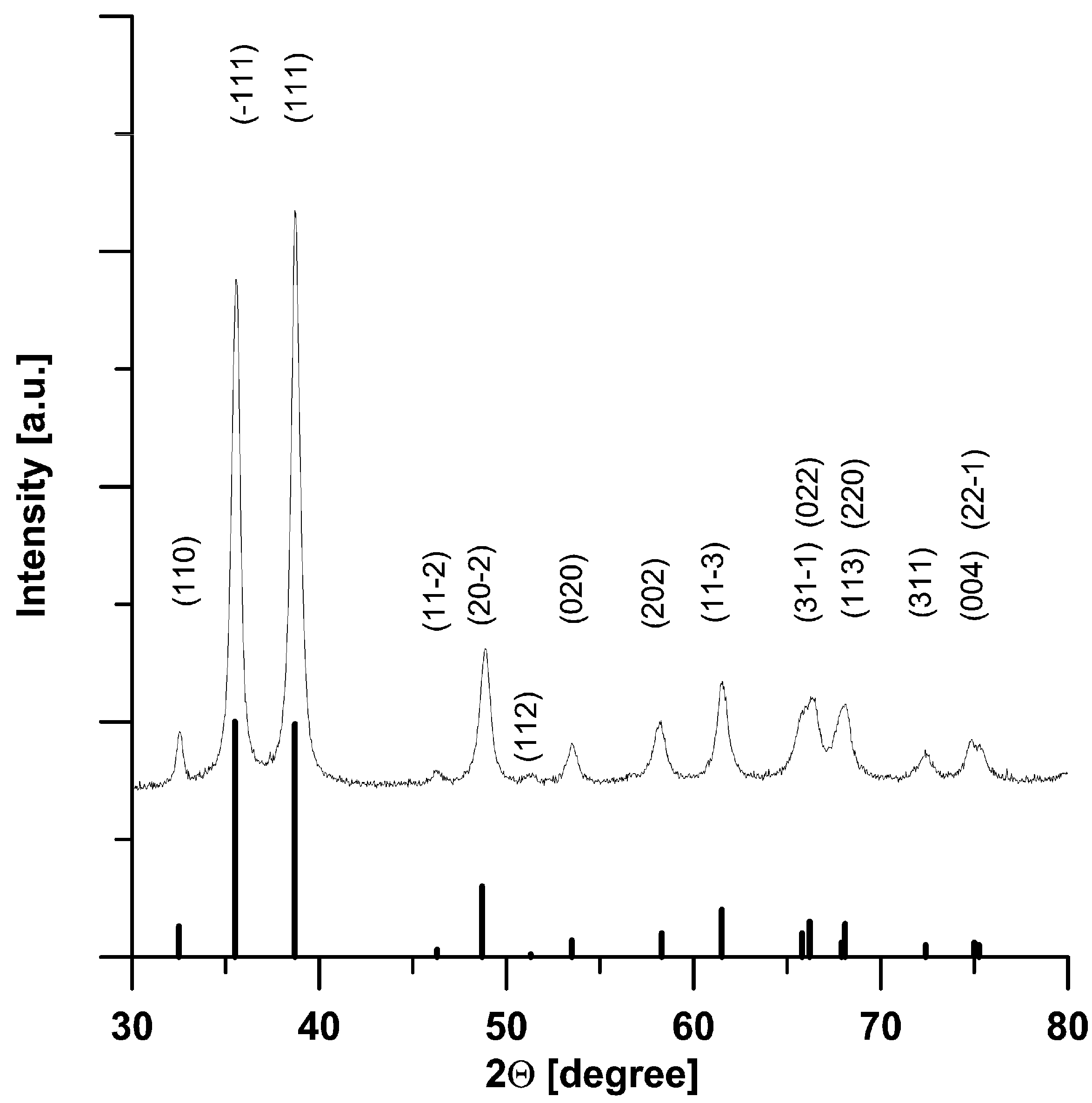
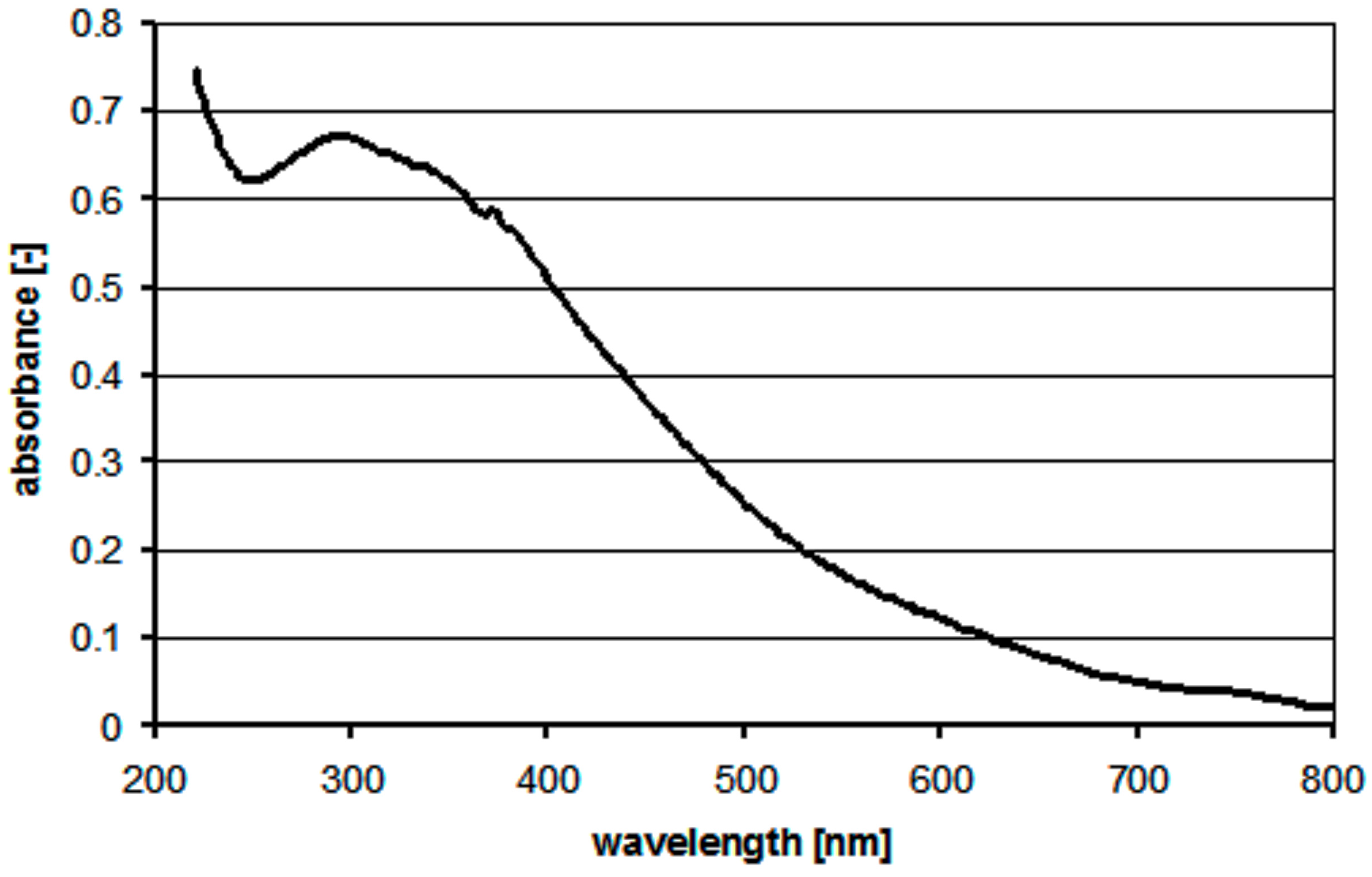
3. Results and Discussion
- -
- working amplitude of about 60% of maximum mixer amplitude; it appears to ensure good homogeneity of the Cu(OH)2 suspension discharged from the mixer while preventing uncontrolled formation of the CuO particles,
- -
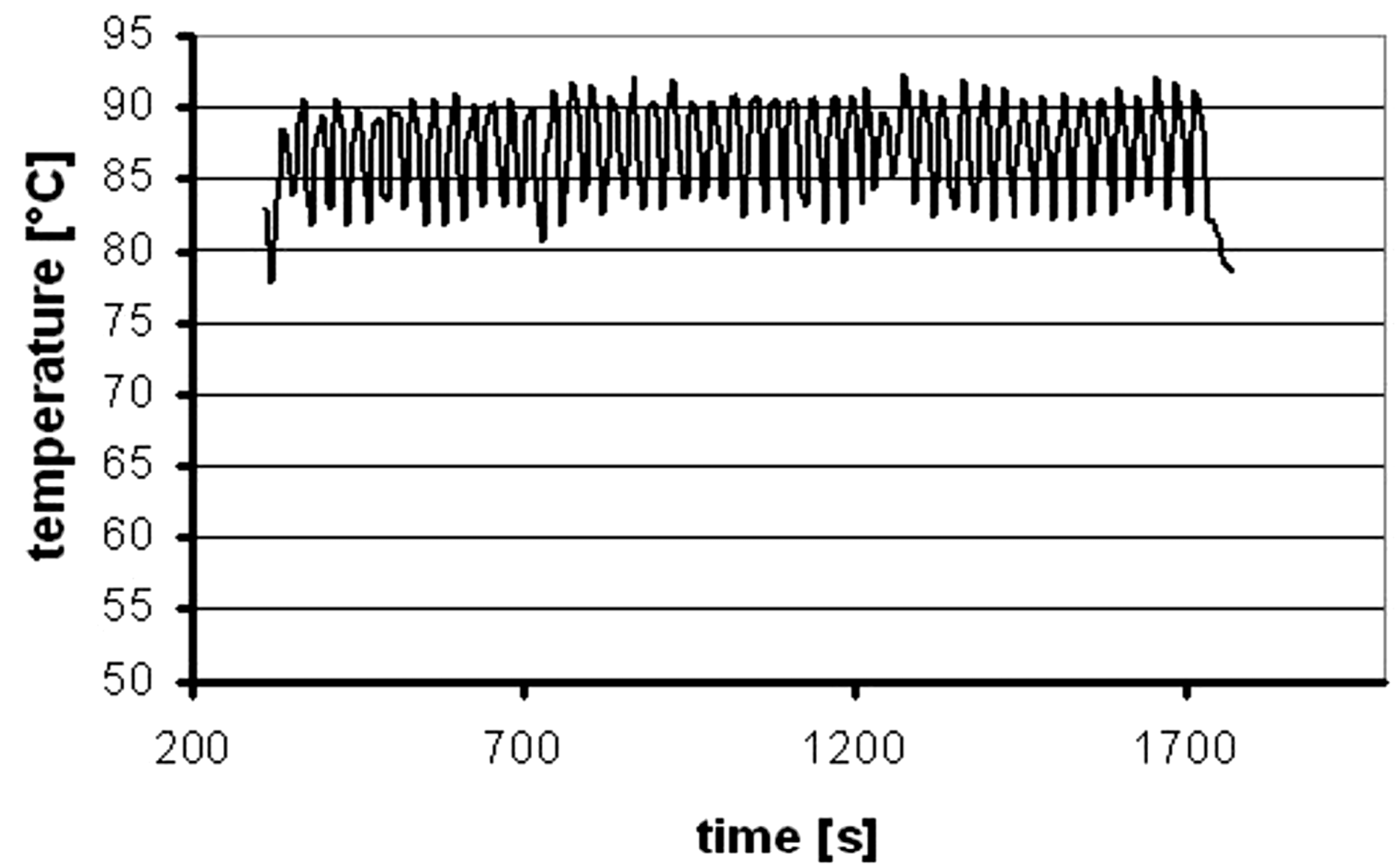
- second stage temperature (tt) of about 86–89 °C, which enables smooth transformation of the uniform suspension Cu(OH)2 and a stable synthesis of sub-micrometric CuO,
- -
- supply of substrates in amounts (streams, concentrations) meeting the stoichiometry of reaction (1).
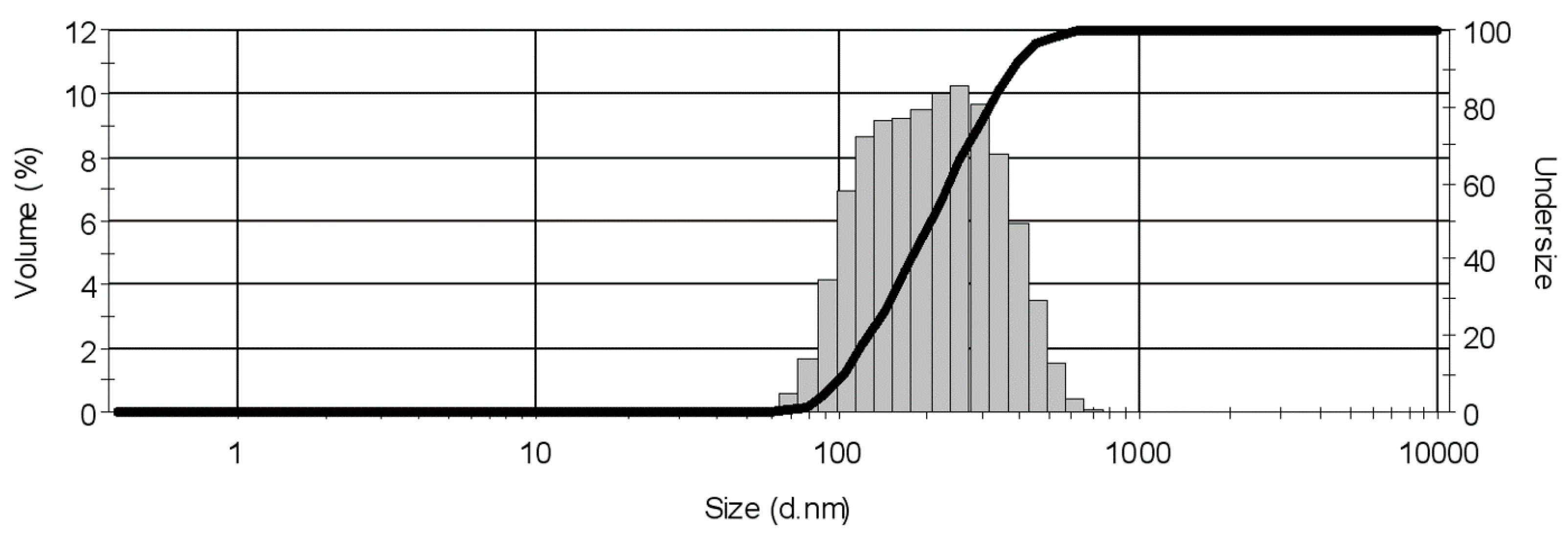
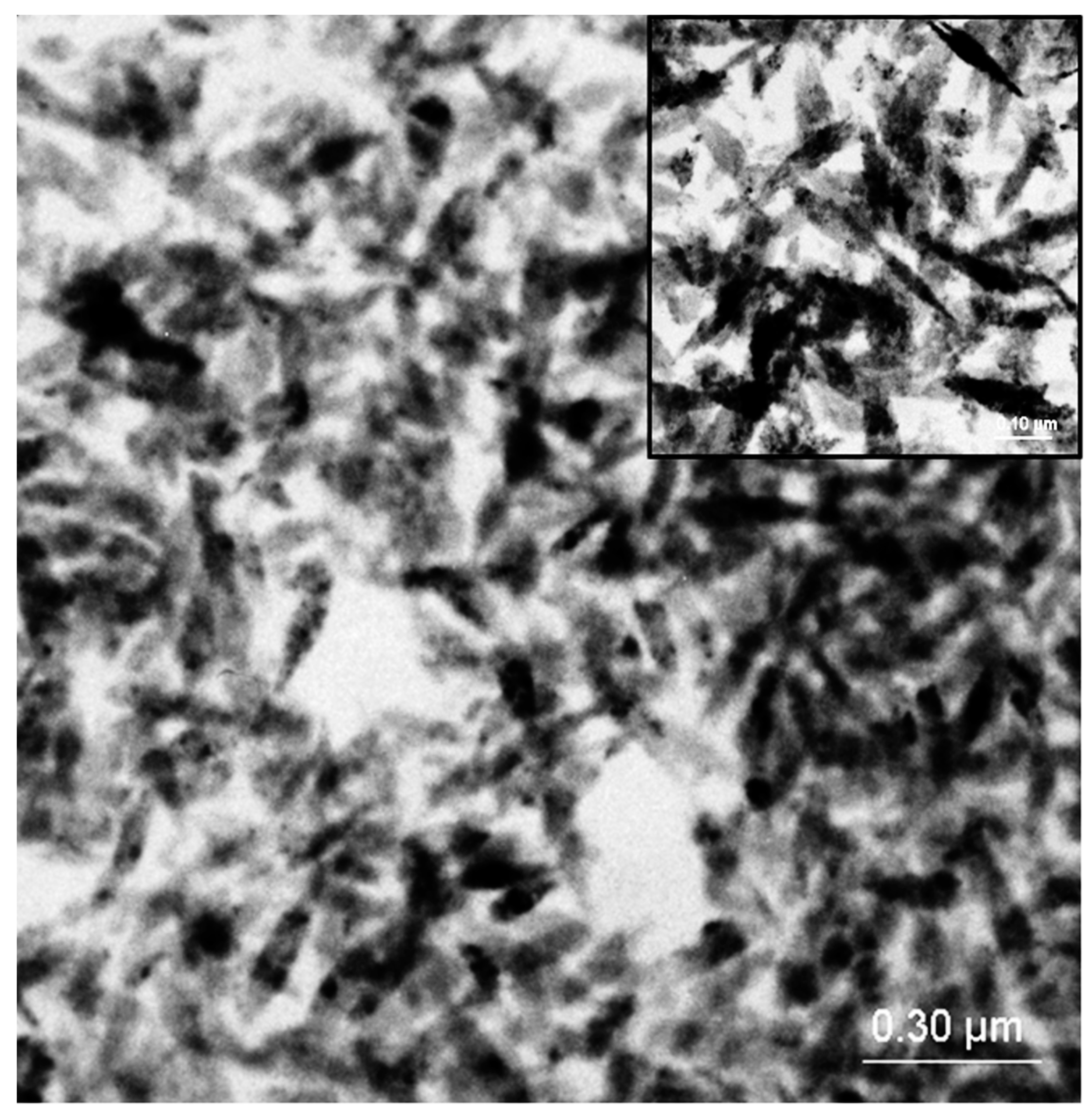
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, M.; He, J.; Hu, X.; Yan, C.; Cheng, Z.; Zhao, Y.; Zuo, G. Copper oxide nanoparticle sensors for hydrogen cyanide detection: Unprecedented selectivity and sensitivity. Sens. Actuators B 2011, 155, 692–698. [Google Scholar]
- Yuan, C.; Xu, Y.; Deng, Y.; Jiang, N.; He, N.; Dai, L. CuO based inorganic-organic hybrid nanowires: A new type of highly sensitive humidity sensor. Nanotechnology 2010, 21, 1–8. [Google Scholar]
- Ko, S.; Lee, J.I.; Yang, H.S.; Park, S.; Jeong, U. Mesoporous CuO particles threaded with CNTs for high-performance lithium-ion battery anodes. Adv. Mater. 2012, 24, 4451–4456. [Google Scholar]
- Zhou, K.; Wang, R.; Xu, B.; Li, Y. Synthesis, characterization and catalytic properties of CuO nanocrystals with various shapes. Nanotechnology 2006, 17, 3939–3943. [Google Scholar]
- Abdelsayed, V.; Aljarash, A.; El-Shall, M.S.; Al Othman, Z.A.; Alghamdi, A.H. Microwave synthesis of bimetallic nanoalloys and CO oxidation on ceria-supported nanoalloys. Chem. Mater. 2009, 21, 2825–2834. [Google Scholar]
- Elazab, H.A.; Moussa, S.; Gupton, B.F.; El-Shall, M.S. Microwave-assisted synthesis of Pd nanoparticles supported on Fe3O4, Co3O4, and Ni(OH)2 nanoplates and catalysis application for CO oxidation. J. Nanopart. Res. 2014, 16, 1–11. [Google Scholar]
- Mahato, T.H.; Singh, B.; Srivastava, A.K.; Prasad, G.K.; Srivastava, A.R.; Ganesan, K.; Vijayaraghavan, R. Effect of calcinations temperature of CuO nanoparticle on the kinetics of decontamination and decontamination products of sulphur mustard. J. Hazard. Mater. 2011, 192, 1890–1895. [Google Scholar]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particulogy 2009, 7, 141–150. [Google Scholar]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper oxide nanoparticles: Synthesis, characterization and their antibacterial activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar]
- Outokesh, M.; Hosseinpour, M.; Ahmadi, S.J.; Mousavand, T.; Sadjadi, S.; Soltanian, W. Hydrothermal synthesis of CuO nanoparticles: Study on effects of operational conditions on yield, purity, and size of the nanoparticles. Ind. Eng. Chem. Res. 2011, 50, 3540–3554. [Google Scholar]
- Kida, T.; Oka, T.; Nagano, M. Synthesis and application of stable copper oxide nanoparticle suspensions for nanoparticulate film fabrication. J. Am. Ceram. Soc. 2001, 90, 107–110. [Google Scholar]
- Fan, H.; Yang, L.; Hua, W.; Wu, X.; Wu, Z.; Xie, S.; Zou, B. Controlled synthesis of monodispersed CuO nanocrystals. Nanotechnology 2004, 15, 37–42. [Google Scholar]
- Ahmad, T.; Chopra, R.; Ramanujachary, K.V.; Lofland, S.E.; Ganguli, A.K. Canted antiferromagnetism in copper oxide nanoparticles synthesized by the reverse-micellar route. Solid State Sci. 2005, 7, 891–895. [Google Scholar]
- Yao, W.T.; Yu, S.H.; Zhou, Y.; Jiang, J.; Wu, Q.S.; Zhang, L.; Jiang, J. Formation of uniform CuO nanorods by spontaneous aggregation: selective synthesis of CuO, Cu2O, and Cu nanoparticles by a solid-liquid phase arc discharge process. J. Phys. Chem. B 2005, 109, 14011–14016. [Google Scholar]
- Radwan, N.R.E.; El-Shall, M.S.; Hassan, H.M.A. Synthesis and characterization of nanoparticle Co3O4, CuO and NiO catalysts prepared by physical and chemical methods to minimize air pollution. Appl. Catal. A Gen. 2007, 331, 8–18. [Google Scholar]
- Zhu, H.; Han, D.; Meng, Z.; Wu, D.; Zhan, C. Preparation and thermal conductivity of CuO nanofluid via a wet chemical method. Nanoscale Res. Lett. 2011, 6, 181–186. [Google Scholar]
- Zhu, H.T.; Zhang, C.Y.; Tang, Y.M.; Wang, J.X. Novel synthesis and thermal conductivity of CuO nanofluid. J. Phys. Chem. C 2011, 111, 1646–1650. [Google Scholar]
- Zhao, Y.; Zhu, J.J.; Hong, J.M.; Bian, N.; Chen, H.Y. Microwave-induced polyol-process synthesis of copper and copper oxide nanocrystals with controllable morphology. Eur. J. Inorg. Chem. 2004, 2004, 4072–4080. [Google Scholar]
- Wang, H.; Xu, J.Z.; Zhu, J.J.; Chen, H.Y. Preparation of CuO nanoparticles by microwave irradiation. J. Cryst. Growth 2002, 244, 88–94. [Google Scholar]
- Li, Z.; Liu, Y.; Gong, P.; Zhai, Y. Preparation of chain copper oxide nanoparticles by microwave. Rare Met. 2007, 26, 476–481. [Google Scholar]
- Pieper, M.; Aman, S.; Hintz, W.; Tomas, J. Optimization of a continuous precipitation process to produce nanoscale BaSO4. J. Chem. Eng. Technol. 2011, 34, 1567–1574. [Google Scholar]
- Eluri, R.; Paul, B. Synthesis of nickel nanoparticles by hydrazine reduction: Mechanistic study and continuous flow synthesis. J. Nanopart. Res. 2012, 14, 1–14. [Google Scholar]
- Chang, M.H.; Liu, H.S.; Tai, C.Y. Preparation of copper oxide nanoparticles and its application in nanofluid. Powder Technol. 2011, 207, 378–386. [Google Scholar]
- Langford, J.I.; Louer, D. High-resolution powder diffraction studies of copper (II) oxide. Appl. Cryst. 1991, 24, 149–155. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzido, G.; Drzazga, M.; Markowski, P.; Jarzębski, A.B. Application of Sonication and Microwave Irradiation to Boost Continuous Fabrication of the Copper(II) Oxide Sub-Micron Particles. Technologies 2015, 3, 37-46. https://doi.org/10.3390/technologies3010037
Dzido G, Drzazga M, Markowski P, Jarzębski AB. Application of Sonication and Microwave Irradiation to Boost Continuous Fabrication of the Copper(II) Oxide Sub-Micron Particles. Technologies. 2015; 3(1):37-46. https://doi.org/10.3390/technologies3010037
Chicago/Turabian StyleDzido, Grzegorz, Michał Drzazga, Piotr Markowski, and Andrzej B. Jarzębski. 2015. "Application of Sonication and Microwave Irradiation to Boost Continuous Fabrication of the Copper(II) Oxide Sub-Micron Particles" Technologies 3, no. 1: 37-46. https://doi.org/10.3390/technologies3010037
APA StyleDzido, G., Drzazga, M., Markowski, P., & Jarzębski, A. B. (2015). Application of Sonication and Microwave Irradiation to Boost Continuous Fabrication of the Copper(II) Oxide Sub-Micron Particles. Technologies, 3(1), 37-46. https://doi.org/10.3390/technologies3010037






