Abstract
Laser-assisted hatching (LAH) is used during in vitro fertilization (IVF) to improve the chances of embryo implantation into the uterine wall by creating a small, precise opening in its outer shell (zona pellucida). The primary objective of this study was to evaluate the safety profile of LAH performed using an infrared femtosecond laser system (λ = 1028 nm, E = 155 nJ, and I = 6.5 TW/cm2). We aimed to identify and quantify the potential biological effects of the laser and compare them with results from previous studies that used visible wavelength laser pulses (λ = 514 nm, E = 49 nJ, and I = 2.5 TW/cm2). To achieve this, we designed a controlled experiment using a mouse model. A critical component of our safety assessment involved quantifying the levels of reactive oxygen species (ROS) and analyzing the expression of heat-shock proteins (HSPs). Robust analyses revealed no statistically significant differences in either ROS production or HSP expression—assessed at both the protein and mRNA levels—between embryos in the negative control group and those subjected to the femtosecond LAH procedure. This key finding indicates that neither infrared nor visible femtosecond laser microsurgery of the zona pellucida induced a detectable oxidative or thermal stress response within the tested parameters.
1. Introduction
Laser technologies have been established as powerful and versatile tools across a remarkably broad spectrum of biological and medical disciplines. Their integration has revolutionized standard practices in ophthalmology, dermatology, and various surgical fields primarily due to their unparalleled precision, minimal invasiveness, and capacity for targeted energy delivery [1,2,3,4,5]. The fundamental appeal of laser-based applications lies in their ability to perform non-contact, precise micromanipulations at cellular and subcellular levels, opening new frontiers in research and clinical practice. Technologies such as laser-assisted microdissection enable the selective isolation of specific cells, overcoming critical challenges in areas such as cancer research [6,7]. Parallel advancements in regenerative medicine have been equally impressive. In particular, laser-assisted bioprinting has advanced from the initial concepts of laser-guided direct writing [8] into robust technologies that can create sophisticated tissue constructs, including vascular networks, cardiac patches, and liver organoids [9,10].
The continuous evolution of laser technology has consistently expanded biological applications. Following the initial identification of their significance in the fields of cell and developmental biology [11,12,13], perhaps the most transformative breakthrough emerged with the development of ultrafast femtosecond lasers—an innovation recognized with the 2018 Nobel Prize in Physics. These systems generate ultrashort pulses (typically in the femtosecond range) with exceptionally high peak intensities through chirped-pulse amplification techniques. The physics of femtosecond (fs) laser–matter interaction is fundamentally different from that of longer-pulsed or continuous-wave systems. Short durations of the pulse, coupled with nonlinear optical effects in tightly focused volumes of the laser beam, confine energy deposition to a microscopic focal point. This precise confinement virtually eliminates adverse effects such as out-of-focus light absorption and substantive heat transfer to the surrounding media. This facilitates high-precision microsurgery with minimal collateral damage [14,15]. Beyond ablation, the principles of the nonlinear excitation of matter—a distinctive attribute of these lasers—have also revolutionized imaging, giving rise to modalities such as multiphoton microscopy (see the reviews in [16,17,18] and the references therein). The latter offers exceptional resolutions and deep-tissue penetration, and it often eliminates the need for exogenous fluorescent markers.
These technological advancements hold particular significance for addressing challenges in reproductive medicine, a field where precision and minimal invasiveness are paramount. Infertility, affecting more than 180 million people globally [19], presents a complex array of etiologies requiring equally diverse therapeutic strategies. A significant implantation barrier arises from impaired embryo hatching—the critical process whereby a developing blastocyst escapes its protective glycoprotein shell, the zona pellucida (z.p.), before uterine implantation [20]. The z.p. can exhibit abnormal morphological or biochemical characteristics, becoming excessively thickened, hardened, or otherwise dysfunctional, thus preventing natural hatching. The etiology of these z.p. abnormalities is multifactorial, with strong correlations with advanced maternal age, genetic predispositions, and iatrogenic factors such as cryopreservation-induced hardening [21,22,23].
To overcome this challenge, assisted reproductive technologies have been used to develop several assisted hatching (AH) techniques. Among mechanical, chemical, and laser-based approaches, laser-assisted hatching (LAH) [24,25] has gained prominence in clinical practice due to its precision, speed, and reproducibility. However, the majority of commercial LAH systems utilize millisecond or nanosecond lasers, which operate through photothermal mechanisms. A significant limitation of these systems is the substantial transfer of thermal energy from the focal point of the laser beam, resulting in the undesirable heating of the surrounding culture medium and adjacent embryonic structures [26,27]. This heat propagation poses a non-negligible risk to embryo viability, necessitating stringent protocol optimizations regarding pulse duration, beam distance from blastomeres, and overall energy exposure [26,28]. To minimize the risk of thermal damage, LAH should be performed at an early stage of development (day 2 or 3), when there is sufficient perivitelline space between the z.p. and blastomeres. However, this approach hinders subsequent trophectoderm biopsy due to the inability to control whether the embryo hatches “TE-forward” or “ICM-forward” [29] (here, TE and ICM stand for trophectoderm and inner cell mass, respectively). Furthermore, z.p.’s opening during the late stages of development results in blastocyst collapse for an indefinite period of time, which also complicates TE biopsy.
In this context, femtosecond laser-assisted hatching (fs-LAH) emerges as a profoundly promising alternative. The core advantage lies in the radically different energy profile: the total energy of a femtosecond pulse is several orders of magnitude lower than that of a millisecond pulse required for the same ablation effect [30]. Furthermore, the mechanism of action—predominantly based on nonlinear and plasma-mediated absorption—ensures that thermal and mechanical effects are exquisitely confined to the immediate focal volume (micron or sub-micron scale). This drastic reduction in collateral energy deposition suggests a significantly improved safety profile.
Visible-wavelength fs lasers have been used successfully in the microsurgery of the z.p. at late blastocyst stages, resulting in increased hatching rates and successful implantation onto an adhesive plastic surface used for modelling in vitro processes [31]. Fs-LAH does not cause blastocyst collapse, significant reactive oxygen species (ROS) production, or heat-shock protein (HSP) overexpression [32]. Thus, the aforementioned fs lasers can serve as a convenient alternative, making the work of embryologists easier and simplifying routine processes in clinical practice.
Nevertheless, the biological safety profiles of infrared (IR) femtosecond lasers, which are often more practical for integrated workstation setups, require thorough investigation. It has been shown that the energy requirements for effective IR (λ = 1028 nm) fs-LAH are higher than for visible light systems [33], raising pertinent questions about the biological effects induced by the specific interaction of IR wavelengths with the z.p. and the pericellular medium. Therefore, a comprehensive assessment of ROS and HSP expressions, alongside key developmental metrics such as hatching rates, is imperative to validate the safety and efficacy of IR fs-LAH. This study aims to conduct such an assessment—comparing outcomes between IR fs-LAH-treated embryos and control groups—to provide robust data for the future standardization of this highly promising technique in clinical embryology. In addition, we compared the obtained results to those of visible femtosecond radiation (λ = 514 nm) [32]. Thus, femtosecond lasers offer a versatile and powerful toolset, driving progress in modern biomedicine through enhanced precision and minimal invasiveness.
2. Materials and Methods
2.1. Hormonal Stimulation of Ovulation
First-generation CBA × C57Bl/6J hybrid mice (females about 3 weeks old and weighing 12–13 g) were used in this study. To obtain a large number of zygotes, a well-known protocol for ovulation stimulation using follicle-stimulating hormones and chorionic gonadotropin was applied. Hormonal stimulation was carried out according to a two-step protocol: At 13:00 on the first day, FSH (Follimag, Mosagrogen, Moscow, Russia) was administered intraperitoneally at a dose of 5 IU per animal, followed by an injection of hCG (Chorulon, Merck Animal Health, Madison, NJ, USA) intraperitoneally after 48 h at a dose of 10 IU per animal; after this step, these females were paired with males for mating. The fact of mating was determined the next morning by the presence of a vaginal plug.
2.2. Embryo Collection
Embryos were isolated at the zygote stage (0.5 days post-coitus, E0.5) and subsequently cultured in vitro. To retrieve embryos, female animals were euthanized via cervical dislocation. Following this procedure, their oviducts were excised and placed into a pre-warmed M2 medium heated to 37 °C. Using needles from syringes and forceps, adipose tissue was separated from the oviducts. Then, oviducts were transferred to a fresh drop of 100 μL M2 medium where the ampullary region of the oviduct was opened to extract oocyte–cumulus complexes. These complexes were further treated with hyaluronidase (Lidaza, Microgen, Moscow, Russia) to remove cumulus cells surrounding the zygotes. Subsequently, the obtained zygotes were washed sequentially through four drops of M2 medium before being transferred to the culture medium, and they were incubated under controlled conditions (37 °C, 5% CO2/air).
2.3. Embryo Culture
Embryos were cultured up to the morula stage (approximately 2.5 days post-coitus, E2.5) in 4-well plates (Thermo Scientific Nunc, Waltham, MA, USA) using embryo culture medium EmbryoMax KSOM (Merck, Darmstadt, Germany). From the morula stage until blastocyst formation (E3.5), embryos were cultured in Petri dishes (Cat. # 200350, SPL Lifesciences, Pocheon-si, Republic of Korea) in mineral-oil-coated (Origio, Måløv, Denmark) droplets containing 20 µL of EmbryoMax KSOM medium, with 2–3 embryos in each droplet.
2.4. Transportation of Embryos
The collection and cultivation of embryos up to the morula stage were carried out at IGB RAS, while the subsequent culture to the blastocyst stage and the LAH procedure took place at JIHT RAS. To transport the embryos between IGB RAS and JIHT RAS, they were placed in a preheated M2 medium maintained at 37 °C. Transportation was conducted in 2 mL Eppendorf tubes. To maintain a constant temperature during transportation, the tube containing embryos was kept inside a thermos filled with water previously warmed to 37 °C. Transport durations did not exceed 90 min. Upon arrival, embryos were rinsed three times in a culture medium by transferring them via drops.
2.5. Fluorescent Staining of Embryos for Determining ROS Levels
Groups of embryos were stained with CM-H2DCFDA (Thermo Scientific, Waltham, MA, USA) following the manufacturer’s protocol. Embryos were incubated in EmbryoMax KSOM—containing the indicator’s working concentration (500 nM)—for 30 min at 37 °C in a CO2 incubator. Afterward, the embryos were washed of the indicator with three drops of M2. Then, the embryos were loaded into Petri dishes containing a single 20 μL drop of M2 medium covered with mineral oil. Finally, their fluorescence intensity was assessed using a Leica STELLARIS 5 confocal microscope (Leica Microsystems, Wetzlar, Germany).
2.6. Fluorescent Staining of Embryos for Determining HSPs
In order to assess heat-shock protein (HSP) expression following laser irradiation, we initially labelled the embryos using an anti-mouse HSP70 primary antibody from Cell Signaling Technology (Danvers, MA, USA), followed by an Alexa Fluor 488-conjugated fluorescent secondary anti-rabbit IgG antibody as per the manufacturer’s instructions (Thermo Scientific, Waltham, MA, USA). Specifically, embryos underwent fixation in 4% paraformaldehyde, permeabilization via 0.3% Triton X-100 (Merck, Darmstadt, Germany) dissolved in phosphate-buffered saline (PBS), and subsequent blocking in fetal bovine serum (5% FBS, Cytiva, Pasching, Austria) supplemented with 0.1% Triton X-100. The primary antibody was prepared at a concentration of 1:50 in 1% bovine serum albumin within PBS, while the secondary antibody was similarly diluted, but at a ratio of 1:500. Following blocking, incubation occurred with the primary antibody overnight at 4 °C and subsequently with the secondary antibody for one hour under ambient conditions. Additionally, nuclei were visualized through staining with a DAPI reagent provided by Servicebio (Wuhan, China).
We further evaluated both negative and positive controls. For the negative control, embryos were maintained in a standard CO2 incubator set at 37 °C and 5% CO2 instead of the LAH procedure. Conversely, the positive control consisted of embryos cultured in an EmbryoMax medium inside a CO2 incubator heated to 43 °C for three hours to provoke HSP synthesis before being promptly fixed in paraformaldehyde. Post-staining, all samples received three consecutive five-minute rinses in an M2 medium containing an HEPES buffer to eliminate excess stain.
Upon the completion of the staining protocol, the embryos were relocated onto glass-bottomed Petri dishes containing droplets of M2 medium coated with mineral oil to avoid evaporation prior to microscopic evaluation. Visual data acquisition took place on a Leica STELLARIS 5 confocal microscope (Leica Microsystems, Wetzlar, Germany).
2.7. Corrected Total Cell Fluorescence
The fluorescence of the stained embryos was evaluated using ImageJ software (version 1.52e). Z-stacks were split by fluorescence channels; then, the sum projections were produced for ROS-stained stacks. Huang’s threshold was applied to the projections to generate regions of interest (ROIs). The area, integrated density, and mean intensity values were measured for each embryo ROI and in the arbitrary background area on the projection images. The corrected total cell fluorescence (CTCF) of the embryo was calculated as follows: CTCF = Integrated Density − (Area of ROI × Mean grey value of background readings).
2.8. Real-Time PCR
RNA extraction was performed using a TRIzol reagent according to the manufacturer’s instructions (Thermo Scientific, Waltham, MA, USA). Reverse transcription was carried out using the MMLV RT kit to generate complementary DNA (cDNA), as recommended by the manufacturer (Evrogen, Moscow, Russia). Quantitative real-time PCR for gene expression analysis was performed using a CFX96 amplifier (Bio-Rad, Hercules, CA, USA) and a ready-to-use qPCRmix-HS SYBR mixture (Evrogen, Moscow, Russia). cDNA samples derived from blastocysts were amplified in triplicate using primers specific for the reference gene Hprt and heat-shock protein genes Hsp90aa1 and Hspa5. The nucleotide sequences of the primers used are presented in Table 1. The level of gene expression for Hsp90aa1 and Hspa5 was calculated using the commonly known ΔCt method [34].

Table 1.
Primers (the sequences are given in the 5′→3′ direction).
2.9. Experimental Setup
The femtosecond laser scalpel–optical tweezer setup was designed and assembled at the JIHT RAS (Figure 1) [35]. This system was built around a TETA infrared femtosecond laser (Avesta LLC, Troitsk, Russia), which served as the radiation source. Such a Yb-doped diode-pumped solid-state laser is ideally suitable for industrial and scientific research applications. The laser generated ultrashort pulses with the following parameters: duration: τ = 280 fs; pulse energy: E = 330 μJ; central wavelength: λ = 1028 nm; repetition rate: f = 2.5 kHz. The main difference in the experimental setup used for studies on visible radiation wavelength (λ = 514 nm) [29,32] was the removal of a second harmonic generation (SHG) unit consisting of potassium dihydrogen phosphate (DKDP) crystal that converted radiation into its second harmonic, in addition to a notch filter that blocked out radiation at its fundamental frequency.

Figure 1.
Schematic diagram of a femtosecond laser scalpel: 1—femtosecond laser; 2—attenuation unit; 3—SHG unit (optional); 4—glass plate; 5—photodiode; 6—telescope unit; 7—mirrors for laser radiation wavelength; 8—mechanical shutter; 9—microscope objective; 10—microscope motorized stage; 11—microscope condenser; 12—microscope illuminator; 13—Petri dish with embryos; 14—tube lens; 15—video camera; 16—inverted microscope.
The laser output was coupled into the right-side port of an inverted Olympus IX-71 microscope (Olympus Corporation, Tampa, FL, USA). Focusing was carried out using a 20× UPlanFL objective lens with a numerical aperture (NA) of 0.5. This produced a focal spot with a radius of ω0 = 1.56 μm (measured at the 1/e intensity level). In order to regulate the pulse energy delivered to the embryo precisely, a half-wave plate and a Glan–Thompson prism were integrated into the optical path to form an attenuation unit.
Additionally, a telescopic optical system was incorporated to match the laser beam’s diameter to the entrance aperture of the objective. This alignment was critical for maximizing light throughput and minimizing energy loss—which would otherwise occur due to vignetting at the back aperture of the microscope objective.
A portion of the laser beam was directed onto a DET36A2 photodiode (Thorlabs Inc., Newton, NJ, USA) using a thin glass plate via Fresnel reflection. This photodiode was used to monitor the energy of the individual laser pulses. The signal from the photodiode was digitized using a TDS 5054 oscilloscope (Tektronix, Beaverton, OR, USA). The calibration of the photodiode signal amplitude was performed using a power meter comprising an S120VC photodiode sensor and a PM100D console (Thorlabs Inc., Newton, NJ, USA). For this calibration, the sensor was temporarily placed on the microscope’s motorized stage, SCAN IM 120 × 80 (Märzhäuser Wetzlar, Wetzlar, Germany), to measure the laser radiation directly exiting the microscope objective.
To perform microsurgery, embryos were placed in glass-bottomed Petri dishes; laser radiation was focused through the bottom. The Petri dish was mounted on the motorized microscope stage, which allowed for the precise movement of the embryo relative to the stationary laser beam. Embryo imaging was accomplished using a DFK 72AUC02 CMOS camera (The Imaging Source, Bremen, Germany), with the video displayed on a personal computer monitor.
The microsurgery procedure was automated via custom software developed in LabView 2014 (National Instruments, Austin, TX, USA). This software enabled the operator to control several key laser parameters—including pulse energy, pulse repetition rate, and beam divergence—and define the path of the laser beam by drawing it directly over the live image of the embryo. The dissection of the embryo’s zona pellucida was performed using a sequence of laser pulses fired at a specified pulse repetition rate f while the embryo was translated at a constant velocity υ relative to the beam.
Adjustments of the divergence of the laser radiation, and consequently the height of its focus along the optical axis of the objective, were carried out by controlling the distance between the lenses within the telescope. This distance was carefully selected to ensure that laser radiation was focused precisely on the plane corresponding to the embryo’s maximum diameter—its “equatorial” plane (Figure 2a).

Figure 2.
Laser beam focusing for fs-LAH (a); mouse embryo photo prior to (b) and after (c) fs-LAH. Scalebar is 20 µm.
2.10. Laser-Assisted Hatching
Embryo microsurgery was performed in Petri dishes with a glass bottom measuring 170 µm thick (Cat. # 200350, SPL Lifesciences, Pocheon-si, Republic of Korea), and these dishes contained drops of EmbryoMax® KSOM culture medium (20 μL volume), covered with mineral oil (Figure 2a). To carry out the laser-assisted hatching procedure using femtosecond pulses (fs-LAH), the operator superimposed a trajectory for the laser beam directly onto the live image of the embryo, thereby defining the shape of the incision to be made in the zona pellucida (z.p.). Consistent with previous studies [31,32], a U-shaped incision pattern was used (violet polyline, Figure 2b), typically penetrating 80–90% of the total z.p. thickness.
The software used to perform the microsurgery was set to the automated mode: It guided the embryo along a predetermined path relative to a stationary laser beam and controlled the exact laser radiation timing. The experimental parameters were set as follows: the embryo translation speed was υ = 10 µm/s, the laser pulse repetition rate was f = 2.5 kHz, and the pulse energy was E = 155 nJ, corresponding to a peak intensity of I = 6.5 TW/cm2.
Embryo pictures were taken both immediately before and after the fs-LAH procedure. Following the experiment, the Petri dish was returned to the incubator. The effectiveness of the LAH was typically assessed 24 h later, coinciding with the expected time of embryo hatching.
The entire LAH procedure, including focus adjustments, trajectory drawing, laser cutting of the z.p., and saving pre-operative and post-operative images, typically required 30 to 60 s for completion. The actual laser cutting process itself was highly efficient, lasting approximately 2–3 s.
2.11. Evaluation of Hatching Coefficient After LAH Procedure
The LAH procedure was performed at the blastocyst stage. Embryos were divided into two groups: the experimental group (Experiment) consisted of 46 embryos, and the parallel control group (PC) contained 28 embryos. Three drops of culture media were prepared in Petri dishes with glass bottoms and covered with mineral oil. Each drop contained three embryos. Embryos from one of the drops represented the PC, while others underwent laser treatment. The hatching coefficient, characterizing the effectiveness of LAH, was determined 24 h after the procedure using the following formula:
where Nh is the number of embryos that reached the hatching stage, and Nt is the total number of embryos in the group.
2.12. Embryo Groups
In the experiments dedicated to the evaluation of biological effects, embryos were divided into three groups—a negative control, a positive control, and an experimental group—with 30 embryos in each group:
Negative Control: Embryos cultivated under standard conditions (5% CO2/air, 37 °C) in an incubator.
Positive Heat-Shock Control: Blastocysts subjected to thermal stress at 43 °C for 30 min. Thermal exposure was carried out in a CO2 incubator (5% CO2/air, 43 °C). During the exposure, embryos remained in the same culture medium and plates as intact embryos. Afterward, blastocysts from this group were cultured under standard conditions for 2 h to allow the embryos to develop a response to thermal stress.
Positive Reactive Oxygen Species (ROS) Control: Embryos were incubated in 500 nM of hydrogen peroxide (H2O2) diluted in an EmbryoMax culture medium for 30 min in a CO2 incubator to induce reactive oxygen species production. Post-exposure, H2O2-treated embryos were washed three times (each time for 5 min) in an M2 medium to eliminate residual peroxide prior to further manipulation. Additionally, after staining procedures, embryos were again washed three times (for 5 min each) in an M2 medium to remove any remaining stain.
Experimental Group: This group consisted of embryos for fs-LAH exposure. The microsurgery of the zona pellucida was performed at the blastocyst stage (E3.5). Detailed group descriptions are given in the Laser-Assisted Hatching and Embryo Culture subsections. On the day of the experiment, the Petri dish was removed from the incubator and fixed onto the microscope stage. The total time spent outside the incubator did not exceed 10 min.
2.13. Statistics
Data were analyzed using the software packages Statistica 7.0 (Dell, Round Rock, TX, USA) and Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA). To check whether the data exhibited normal distributions, Kolmogorov–Smirnov and Shapiro–Wilk tests were used. In cases where data distributions differed from normal conditions, nonparametric criteria were employed for the analysis. In such instances, median, quartiles (25–75%), and variations (minimum and maximum values) in the measured variables were used as significant indicators. The Mann–Whitney U test was utilized for comparing two independent groups, and the Kruskal–Wallis test was used for multiple comparisons.
For the analysis of numbered data contingency tables, a 2 × 2 table was used, and Yates’s chi-squared test was applied. A critical significance level of 0.05 was considered significant for all statistical analyses.
3. Results
3.1. Hatching Rate Assessment
To evaluate the efficacy of fs-LAH, we analyzed the embryonic hatching rate. The LAH procedure was deliberately performed on the side of the inner cell mass (ICM). This approach presents the highest potential risk of trauma to the embryo, as the laser interacts with the zona pellucida in close proximity to the cells responsible for fetal development. The results of the hatching assessment are presented in Table 2. There are statistically significant differences (p = 0.009) in hatching rates between LAH-treated and control embryos.

Table 2.
The embryo hatching rate in the experimental group after the fs-LAH procedure using IR laser pulses compared to the parallel control group.
3.2. Heat-Shock Protein Expression Analysis
We investigated the impact of laser microsurgery on the expression of heat-shock protein genes Hsp90aa1 (HSP90 family) and Hspa5 (HSP70 family) in embryos from the negative control, positive control, and experimental (IR fs-LAH) groups (Figure 3a,b). The exact p-values of the Kruskal–Wallis test are given in Table A1 in the Appendix A. The analysis revealed a statistically significant difference in the expression levels of both Hsp90 and Hsp70 genes between the negative and positive control groups.


Figure 3.
Expression of the genes for the heat-shock proteins, Hsp90 (a) and Hsp70 (b), in the negative control group, positive control group, and experimental group of embryos for IR fs-LAH (λ = 1028 nm, this study); (c,d): the same for visible fs-LAH (λ = 514 nm, data from [32]). Observed statistically significant differences between Control+ vs. fs-LAH and Control+ vs. Control− groups for both wavelengths (λ = 1028 nm and 514 nm) and both mRNA products—Hsp70 and Hsp90, * p < 0.05.
Crucially, no significant differences were found between the experimental group and the negative control, nor between the experimental and positive control groups, for either gene. The same outcome was observed when fs-LAH was carried out using visible laser pulses (Figure 3c,d; λ = 514 nm [32]).
We also examined the presence of HSP70 in the embryos from the positive and negative control groups, as well as the experimental (fs-LAH) group. To visualize the nuclei, we used DAPI (4′,6-diamidino-2-phenylindole). Our results showed that all three groups of embryos were positive for HSP70, indicating that this protein is synthesized in embryos at a basal level (Figure 4). However, the fluorescence intensity varied between the groups. The negative control and the LAH-treated group showed little difference in fluorescence intensity, while the fluorescence of the stained HSP70 protein was significantly higher in the positive control group. The same pattern emerged when fs-LAH was performed using visible laser pulses (Figure 4d,h,l; λ = 514 nm).
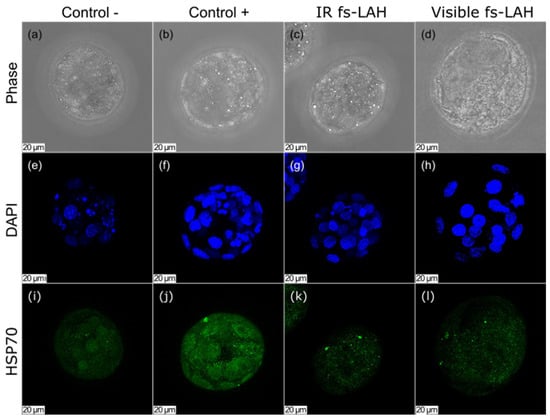
Figure 4.
Staining for HSP70 (i–k) and DAPI (e–g) for the negative control, positive control, and experimental groups (IR fs-LAH): (a–c) phase contrast. Maximum projection images are given. Scale bar is 20 μm. Figures for visible fs-LAH (d,h,l) are presented for comparison (λ = 514 nm, [32]).
3.3. Evaluation of Reactive Oxygen Species Levels
Previous studies have indicated that exposure to femtosecond laser pulses can potentially cause cell damage or even death through the generation of reactive oxygen species (ROS) [36]. While cells tightly regulate ROS levels under normal conditions, stress (oxidative or thermal) can increase ROS production, resulting in DNA, lipid, and protein damage.
Although fs-LAH does not involve the direct irradiation of embryonic cells, the procedure at the blastocyst stage is performed with cells closely apposed to the zona pellucida near the laser’s focus. Therefore, confirming the absence of laser-induced oxidative stress in adjacent cells is essential for establishing the safety profile of femtosecond LAH.
Mouse embryos were divided into three groups: an experimental group undergoing LAH, a negative control, and a positive control. Subsequent measurements of ROS levels demonstrated that the fluorescence intensity in embryos following IR fs-LAH did not exceed the levels observed in the control group embryos (Figure 5). The same result was observed for visible fs-LAH [32].

Figure 5.
ROS staining for 3 groups of embryos. Phase contrast of negative control (a); experimental groups of IR fs-LAH (b) and visible fs-LAH [32] (d); positive control embryos (c); fluorescence signal (e–h). Arrow indicates the site of z.p. dissection via the laser (U-cut). Scale bar is 30 µm.
As anticipated, there were statistically significant differences between the positive and negative control groups (p = 0.00433) and between the positive control and experimental group (p = 0.00952). This finding suggests that the fs-LAH procedure (both IR and visible) does not induce detectable oxidative stress.
The level of reactive oxygen species (ROS) was quantified using the corrected total cell fluorescence method (CTCF) (Figure 6). The CTCF of ROS did not exhibit a statistically significant difference between the negative control and the experimental (fs-LAH) group (p = 0.58095).
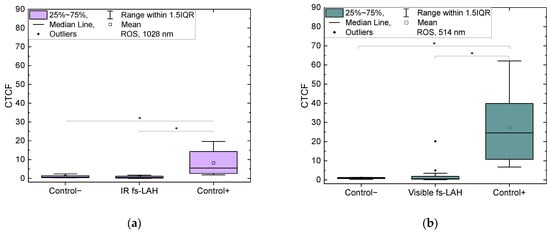
Figure 6.
CTCF of ROS for negative control, positive control, and experimental (fs-LAH) groups (a) for IR (λ = 1028 nm, this study) and (b) visible laser wavelengths (λ = 514 nm [32]). Observed statistically significant differences between Control+ vs. fs-LAH and Control+ vs. Control− groups for both wavelengths (λ = 1028 nm and 514 nm), * p < 0.05.
4. Discussion
4.1. The Choice of Parameters of Infrared Femtosecond Lasers for Zona Pellucida Dissection
The feasibility of using infrared femtosecond laser pulses for embryonic zona pellucida dissection was initially demonstrated in pilot studies [30]. These investigations confirmed that laser-assisted hatching using both infrared and visible radiation does not adversely affect blastocyst development dynamics. Subsequent research [33] established the permissible energy range for effective z.p. dissection.
The authors of [37] revealed that laser pulse intensities—rather than energy—represent the critical parameter for successful microsurgery. Notably, IR picosecond pulses at the same wavelength and similar laser beam parameters required an order of magnitude more energy compared to femtosecond pulses to achieve comparable dissection effects. Consequently, this study utilizes peak intensity I rather than pulse energy E as the primary operational parameter. For Gaussian spatial and temporal profiles, the peak intensity of laser pulses can be calculated as follows:
where τd = 0.95 is the transmission of the Petri dish glass at the laser wavelength; E is the pulse energy; ω0 = 1.56 μm is the beam waist radius at the 1/e level; τ = 280 fs is the pulse duration at FWHM (full width at half maximum).
The authors of [37] demonstrated a direct correlation between required laser intensity I and embryo translation velocity υ. As shown in Figure 6, a predetermined incision width D in z.p. can be achieved through various intensity and velocity combinations. The upper intensity limit is constrained by the optical breakdown threshold in aqueous environments. This is the creation process of a local, short-lived plasma when a high-intensity focused laser pulse exceeds a specific intensity threshold. The rapid heating and expansion of formed plasma generates shockwaves, which can potentially damage adjacent blastocyst cells. The lower intensity limit is determined by a minimum achievable incision width of D ∼ 0.7 μm.
While low velocities (υ < 1 μm/s) require impractical processing times exceeding 20 s, high velocities (υ ∼ 100 μm/s) substantially narrow the usable intensity range. Accordingly, we selected intermediate parameters from the permissible range—υ = 10 μm/s and I = 6.5 TW/cm2 (E = 155 nJ)—as indicated by the red circle in Figure 7. The embryo translation velocity was the same as for the visible fs-LAH. In combination with velocity υ, laser intensity I enabled us to obtain a similar cut width D ~ 1.7 μm to that achieved with an intensity of I = 2.5 TW/cm2 at a wavelength of λ = 514 nm [32]. Selecting laser parameters for both radiation wavelengths, which result in similar ZP microsurgery outcomes, allows the biological effects of IR and visible fs-LAH to be compared.
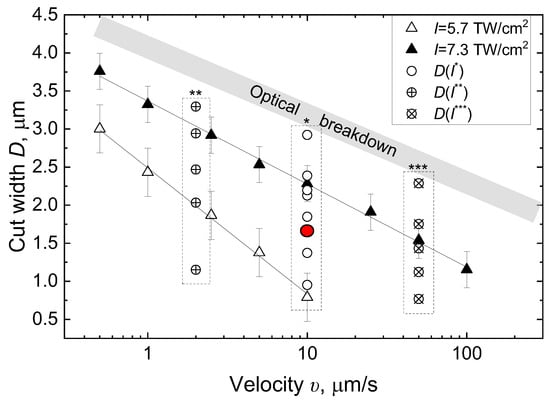
Figure 7.
Incision width of zona pellucida as a function of laser pulse intensity I and embryo translational velocity υ. I* = 5.7–8 TW/cm2; I** = 4.9–7.8 TW/cm2; I*** = 6.4–8 TW/cm2.
4.2. Thermal Considerations
Although z.p. dissection at IR (λ = 1028 nm) wavelengths requires higher intensities compared to visible (λ = 514 nm) radiation—6.5 TW/cm2 versus 2.5 TW/cm2 for forming an incision width of D ∼ 1.7 μm (due to lower photon energy)—the thermal spatial profile at the laser focus remains favorable. While millisecond laser systems (250 mW, 4 μm beam size) can generate aqueous medium temperatures reaching 370 °C—with substantial heat diffusion [27] to the adjacent area (temperatures can reach 50 °C at a distance of 10 µm from the laser beam’s center)—femtosecond pulses exhibit fundamentally different behaviors. Despite the theoretical temperature rise in ΔT ∼ 300 °C at the laser focus for an IR femtosecond laser pulse (I1030 = 6.5 TW/cm2, τ = 250 fs, λ = 1028 nm) [38], the extremely short duration of femtosecond pulses spatially and temporally confines thermal effects. The temperature spike halves within <1 μs, effectively restricting the thermal impact zone to approximately the beam’s diameter (ω0 = 1.56 μm) and allowing complete thermal relaxation between pulses [39].
Since precise thermal modeling remains challenging due to unknown zona pellucida parameters (heat capacity and conductivity), studying the biological effects seems to be the only way to assess the potential negative effects of IR femtosecond pulses.
4.3. Benefits and Biological Safety of Femtosecond Laser-Assisted Hatching
Femtosecond LAH demonstrated a significant positive effect, increasing the hatching rate in the experimental group compared to the control group. This beneficial outcome aligns with our previous observations using visible-wavelength femtosecond pulses [31]. A notable advantage of femtosecond irradiation is the absence of blastocyst collapse during microsurgery—the embryo remains unaffected by the procedure. This stands in favorable contrast to millisecond laser systems (λ = 1480 nm, 5–10 ms pulse duration), where such collapse has been observed [32].
Culturing embryos at non-physiological high temperatures (≥39 °C) is known to upregulate heat-shock protein (HSP) gene expression, a key cellular stress response [40]. To assess the thermal impact of IR fs-LAH, we quantified the expression levels of HSP genes Hsp90aa1 (HSP90 family) and Hspa5 (HSP70 family) across experimental, negative control, and positive control groups. Our analysis revealed no statistically significant differences in HSP expression between the fs-LAH group and the negative controls. This finding was corroborated by confocal microscopy, which exhibited visually comparable fluorescence intensities between these groups (Figure 4). The positive control group, as expected, exhibited significantly elevated expression levels, validating the experimental methodology. These results strongly indicate that IR fs-LAH (λ = 1028 nm), similarly to visible fs-LAH (λ = 514 nm), does not induce a measurable heat-shock response in mouse blastocysts despite the higher infrared pulse intensities compared to those at a visible wavelength.
Although femtosecond laser pulses can potentially generate reactive oxygen species and cause cell damage when exposed [36], the fs-LAH procedure does not involve direct cell irradiation. However, due to the close apposition of blastocyst cells to the targeted zona pellucida, we specifically investigated potential ROS induction. Fluorescence intensity analyses (Figure 5) revealed nearly identical ROS levels in the negative control and fs-LAH groups (both IR and visible). Quantitative assessments using the CTCF method confirmed the absence of statistically significant differences between these groups, while a substantial difference was observed with the positive control. This allows us to conclude that fs-LAH does not increase overall ROS levels in blastocysts, regardless of the wavelength used.
It is important to note that individual cells within a blastocyst may exhibit elevated ROS levels due to intrinsic processes such as apoptosis [41], as occasionally observed even in controls (Figure 5e). Such isolated occurrences in late-stage blastocysts are not known to substantially impair embryonic development, analogous to how trophectoderm biopsy in human blastocysts does not hinder development—unlike cleavage-stage biopsy [42]. The absence of measurable biological effects in our study—as demonstrated through ROS and HSP analyses—provides evidence for the safety profile of both infrared and visible fs-LAH within the established operational parameters.
A limitation of this study is its focus on HSP and ROS responses exclusively. Future investigations should expand the spectrum of biological effect monitoring to include additional markers, particularly apoptosis indicators, in order to further validate the safety profile of femtosecond LAH procedures. Studying the results of LAH-treated embryo transfers in pseudopregnant mice would help us better understand the embryos’ developmental potential and the long-term effects of fs-LAH treatments. This remains the subject of our ongoing research.
4.4. Considerations for Infrared Wavelength Application
The biological safety of IR fs-LAH, consistent with results for visible radiation wavelengths (λ = 514 nm) [32], appears to stem from the extreme spatial localization of laser effects, confined to approximately the beam’s diameter (ω0 = 1.56 μm). As the laser dissects the z.p. for only 80–90% of its thickness without direct cell contact, collateral damage is minimized.
A notable technical consideration for IR wavelengths is the narrower range of suitable peak intensities (I1028 = 5.7–7.3 TW/cm2, Imax/Imin = 1.3 in Figure 7) compared to visible radiation (I514 = 1.3–2.75 TW/cm2, Imax/Imin = 2.1) [29]. This narrower window of intensities, a disadvantage under equivalent conditions (2.5 kHz pulse repetition rate and 10 μm/s translation speed), imposes stricter requirements on the stability of laser pulse parameters. Zona pellucida heterogeneities could potentially trigger optical breakdowns (due to increased absorption) even at intensities below 8 TW/cm2, resulting in large vapor bubble formations that risk damaging adjacent cells. Therefore, operating near the upper intensity limit is not recommended. Furthermore, the exceptional pulse-to-pulse energy stability of femtosecond laser radiation is paramount for safe and effective IR fs-LAH application.
From the perspective of potential clinical applications of the obtained results, the following considerations can be made. The application of fs-LAH to human embryos has its own peculiarities: (i) the human z.p. is wider than that of the mouse model. Therefore, cutting through 80–90% of its thickness is expected to have less impact on adjacent cells than in a mouse embryo. (ii) Analyses of the zona pellucida images of human embryos have shown that its structure is more non-uniform. This means there is an increased risk of absorption at these heterogeneities and of optical breakdown. Therefore, it may be advisable to set the intensity closer to the lower limit of the acceptable range, which would further lower the Imax/Imin value for IR radiation. Therefore, using laser radiation in the visible spectral range seems preferable.
5. Conclusions
Our comparative analysis of results performed for 1028 nm (current study) and 514 nm [32] wavelengths demonstrates that laser-assisted hatching can be effectively performed using both infrared and visible femtosecond laser radiation. It is worth noting that the required peak intensity for infrared wavelengths is significantly higher than that needed for visible light. Despite this difference, our results confirm that infrared femtosecond pulses are not only applicable for precise zona pellucida microsurgery but also induce no detectable biological effects in adjacent trophectoderm cells.
To comprehensively assess the potential negative effects of IR fs-LAH, we conducted detailed analyses of heat-shock protein (HSP) expression and reactive oxygen species (ROS) levels, comparing results with both negative and positive control blastocysts. No significant differences were observed between the experimental and negative control groups. Similar results were observed for visible pulses. Critically, embryos remained unaffected by the laser procedure—maintaining normal morphologies with no instances of blastocyst collapse.
The primary limitation of infrared femtosecond pulses lies in their narrower operational intensity range compared to visible radiation. Furthermore, the incision width demonstrates stronger intensity dependence in IR fs-LAH, requiring more precise parameter control to avoid optical breakdown within the zona pellucida and potential collateral damage to adjacent cells through shockwave formation. These characteristics render IR femtosecond LAH (λ = 1028 nm) technically feasible, though it is somewhat more challenging to implement compared to visible wavelength approaches, despite sharing a lack of induced biological effects. Simultaneously, femtosecond LAH based on visible laser radiation does not suffer from these limitations and offers better spatial resolution and microsurgery accuracy due to diffraction-focusing constraints.
Our findings clearly demonstrate that both infrared and visible radiation wavelengths of femtosecond laser pulses can be applied in laser-assisted hatching even at the blastocyst stage of embryo development with no detectable biological effects in cells, thereby providing evidence of a favorable safety profile.
Author Contributions
Conceptualization, D.S.S. and M.A.F.; methodology, V.S.A. and Y.Y.S.; software, L.A.I.; formal analysis, M.V.K., D.S.S. and L.A.I.; investigation, D.S.S., M.V.K., D.E.M. and M.A.F.; writing—original draft preparation, D.S.S. and M.A.F.; writing—review and editing, D.S.S. and M.A.F.; visualization, A.V.T. and M.V.K.; project administration, D.S.S.; funding acquisition, D.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 23–19-00424 (https://rscf.ru/en/project/23-19-00424/, accessed on 1 April 2023).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Commission of the Institute of Gene Biology of the Russian Academy of Sciences.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The experiments were conducted using the Unique Facility “Terawatt Femtosecond Laser Complex” at the Center for Collective Usage “Femtosecond Laser Complex” of the Joint Institute for High Temperatures of the Russian Academy of Sciences (JIHT RAS).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AH | Assisted hatching; |
| DAPI | 4′,6-diamidino-2-phenylindole; |
| fs | Femtosecond; |
| FBS | Fetal bovine serum; |
| FSH | Follicle-stimulating hormone; |
| hCG | Human chorionic gonadotropin; |
| HSP | Heat-shock proteins; |
| ICM | Inner cell mass; |
| IGB RAS | Institute of Gene biology Russian Academy of Sciences; |
| JIHT RAS | Joint Institute for High Temperatures of the Russian Academy of Sciences; |
| LAH | Laser-assisted hatching; |
| ms | Millisecond; |
| PBS | Phosphate-buffered saline; |
| PFA | Paraformaldehyde; |
| ROS | Reactive oxygen species; |
| z.p. | Zona pellucida. |
Appendix A

Table A1.
Statistical analysis of Hsp70 and Hsp90 mRNA expression levels (Mann–Whitney U test) for IR fs-LAH.
Table A1.
Statistical analysis of Hsp70 and Hsp90 mRNA expression levels (Mann–Whitney U test) for IR fs-LAH.
| HSP90 (p-Value) | HSP70 (p-Value) | |||
|---|---|---|---|---|
| Control- | Experiment | Control- | Experiment | |
| Experiment 1028 | 0.28097 | – | >0.9999 | – |
| Control+ | 0.0004 | 0.00005 | 0.0004 | 0.00004 |
References
- Litvinova, K.S.; Rafailov, I.E.; Dunaev, A.V.; Sokolovski, S.G.; Rafailov, E.U. Non-Invasive Biomedical Research and Diagnostics Enabled by Innovative Compact Lasers. Prog. Quantum Electron. 2017, 56, 1–14. [Google Scholar] [CrossRef][Green Version]
- Prasad, A. Laser Techniques in Ophthalmology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9781003144304. [Google Scholar][Green Version]
- Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Fioranelli, M.; Roccia, M.G.; Gianfaldoni, R.; Lotti, T. An Overview of Laser in Dermatology: The Past, the Present and … the Future (?). Open Access Maced. J. Med. Sci. 2017, 5, 526–530. [Google Scholar] [CrossRef]
- Khalkhal, E.; Rezaei-Tavirani, M.; Zali, M.R.; Akbari, Z. The Evaluation of Laser Application in Surgery: A Review Article. J. Lasers Med. Sci. 2019, 10, S104–S111. [Google Scholar] [CrossRef]
- Chung, S.H.; Mazur, E. Surgical Applications of Femtosecond Lasers. J. Biophotonics 2009, 2, 557–572. [Google Scholar] [CrossRef]
- Lehmann, U.; Kreipe, H. Laser-Assisted Microdissection and Isolation of DNA and RNA BT. In Breast Cancer Research Protocols; Brooks, S.A., Harris, A., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 65–75. ISBN 978-1-59259-969-1. [Google Scholar]
- Han, T.S.; Oshima, M. Laser Microdissection of Cellular Compartments for Expression Analyses in Cancer Models BT. In Inflammation and Cancer: Methods and Protocols; Jenkins, B.J., Ed.; Humana Press: New York, NY, USA, 2018; pp. 143–153. ISBN 978-1-4939-7568-6. [Google Scholar]
- Odde, D.J.; Renn, M.J. Laser-Guided Direct Writing for Applications in Biotechnology. Trends Biotechnol. 1999, 17, 385–389. [Google Scholar] [CrossRef]
- Duan, B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 195–209. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef]
- Thalhammer, S.; Lahr, G.; Clement-Sengewald, A.; Heckl, W.M.; Burgemeister, R.; Schütze, K. Laser Microtools in Cell Biology and Molecular Medicine. Laser Phys. 2003, 13, 681–691. [Google Scholar]
- Kohli, V.; Elezzabi, A.Y. Prospects and Developments in Cell and Embryo Laser Nanosurgery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Berns, M.W.; Aist, J.; Edwards, J.; Strahs, K.; Girton, J.; McNeill, P.; Rattner, J.B.; Kitzes, M.; Hammer-Wilson, M.; Liaw, L.H.; et al. Laser Microsurgery in Cell and Developmental Biology. Science 1981, 213, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Stern, D. Corneal Ablation by Nanosecond, Picosecond, and Femtosecond Lasers at 532 and 625 Nm. Arch. Ophthalmol. 1989, 107, 587. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; Da Silva, L.B.; Rubenchik, A.M.; Feit, M.D.; Glinsky, M.E.; Perry, M.D.; Mammini, B.M.; Small, W.; Stuart, B.C. Plasma Mediated Ablation of Biological Tissues with Nanosecond-to-Femtosecond Laser Pulses: Relative Role of Linear and Nonlinear Absorption. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 801–809. [Google Scholar] [CrossRef]
- Ilina, I.; Sitnikov, D. From Zygote to Blastocyst: Application of Ultrashort Lasers in the Field of Assisted Reproduction and Developmental Biology. Diagnostics 2021, 11, 1897. [Google Scholar] [CrossRef]
- Ilina, I.V.; Sitnikov, D.S. Application of Ultrashort Lasers in Developmental Biology: A Review. Photonics 2022, 9, 914. [Google Scholar] [CrossRef]
- Abu-Siniyeh, A.; Al-Zyoud, W. Highlights on Selected Microscopy Techniques to Study Zebrafish Developmental Biology. Lab. Anim. Res. 2020, 36, 12. [Google Scholar] [CrossRef] [PubMed]
- Inhorn, M.C.; Patrizio, P. Infertility around the Globe: New Thinking on Gender, Reproductive Technologies and Global Movements in the 21st Century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Krivonogova, A.S.; Bruter, A.V.; Makutina, V.A.; Okulova, Y.D.; Ilchuk, L.A.; Kubekina, M.V.; Khamatova, A.Y.; Egorova, T.V.; Mymrin, V.S.; Silaeva, Y.Y.; et al. AAV Infection of Bovine Embryos: Novel, Simple and Effective Tool for Genome Editing. Theriogenology 2022, 193, 77–86. [Google Scholar] [CrossRef]
- Kilani, S.S.; Cooke, S.; Kan, A.K.; Chapman, M.G. Do Age and Extended Culture Affect the Architecture of the Zona Pellucida of Human Oocytes and Embryos? Zygote 2006, 14, 39–44. [Google Scholar] [CrossRef]
- Maddirevula, S.; Coskun, S.; Al-Qahtani, M.; Aboyousef, O.; Alhassan, S.; Aldeery, M.; Alkuraya, F.S. ASTL Is Mutated in Female Infertility. Hum. Genet. 2022, 141, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsen, A.; Agerholm, I.; Toft, B.; Hald, F.; Petersen, K.; Aagaard, J.; Feldinger, B.; Lindenberg, S.; Fedder, J. Assisted Hatching Improves Implantation Rates on Cryopreserved–Thawed Embryos. A Randomized Prospective Study. Hum. Reprod. 2004, 19, 2258–2262. [Google Scholar] [CrossRef] [PubMed]
- Obruca, A.; Strohmer, H.; Sakkas, D.; Menezo, Y.; Kogosowski, A.; Barak, Y.; Feichtinger, W. Fertilization and Early Embryology: Use of Lasers in Assisted Fertilization and Hatching. Hum. Reprod. 1994, 9, 1723–1726. [Google Scholar] [CrossRef]
- Elnahas, A.; Elnahas, T.; Azmy, O.; Elnoury, A.; Abdelhalim, A.; Aboelghar, M.; Alhassani, S.; Noureldin, R. The Use of Laser Assisted Hatching of Frozen/Thawed Embryos versus Laser Assisted Hatching of Fresh Embryos in Human Intracytoplasmic Sperm Injection. J. Obstet. Gynaecol. 2018, 38, 729. [Google Scholar] [CrossRef]
- Douglas-Hamilton, D.H.; Conia, J. Thermal Effects in Laser-Assisted Pre-Embryo Zona Drilling. J. Biomed. Opt. 2001, 6, 205. [Google Scholar] [CrossRef]
- Tadir, Y.; Douglas-Hamilton, D.H. Laser Effects in the Manipulation of Human Eggs and Embryos for In Vitro Fertilization. Methods Cell Biol. 2007, 82, 409–431. [Google Scholar] [CrossRef]
- Chatzimeletiou, K.; Morrison, E.E.; Panagiotidis, Y.; Prapas, N.; Prapas, Y.; Rutherford, A.J.; Grudzinskas, G.; Handyside, A.H. Comparison of Effects of Zona Drilling by Non-Contact Infrared Laser or Acid Tyrode’s on the Development of Human Biopsied Embryos as Revealed by Blastomere Viability, Cytoskeletal Analysis and Molecular Cytogenetics. Reprod. Biomed. Online 2005, 11, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Sitnikov, D.S.; Filatov, M.A.; Ilina, I.V. Optimal Exposure Parameters for Microsurgery of Embryo Zona Pellucida Using Femtosecond Laser Pulses. Appl. Sci. 2023, 13, 11204. [Google Scholar] [CrossRef]
- Ilina, I.V.; Khramova, Y.V.; Filatov, M.A.; Semenova, M.L.; Sitnikov, D.S. Femtosecond Laser Assisted Hatching: Dependence of Zona Pellucida Drilling Efficiency and Embryo Development on Laser Wavelength and Pulse Energy. High Temp. 2016, 54, 46–51, Erratum in High Temp. 2024, 61, 144. https://doi.org/10.1134/S0018151X23010248. [Google Scholar] [CrossRef]
- Ilina, I.V.; Khramova, Y.V.; Ivanova, A.D.; Filatov, M.A.; Silaeva, Y.Y.; Deykin, A.V.; Sitnikov, D.S. Controlled Hatching at the Prescribed Site Using Femtosecond Laser for Zona Pellucida Drilling at the Early Blastocyst Stage. J. Assist. Reprod. Genet. 2021, 38, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Filatov, M.A.; Kubekina, M.V.; Tvorogova, A.V.; Ilchuk, L.A.; Zhuravlev, A.D.; Sazonova, E.A.; Ivanova, A.D.; Silaeva, Y.Y.; Sitnikov, D.S. Biological Effects of Femtosecond and Millisecond Lasers Application for Assisted Hatching in Mouse Embryos. J. Assist. Reprod. Genet. 2025, 42, 2219–2230. [Google Scholar] [CrossRef]
- Sitnikov, D.S.; Mukhdina, D.E.; Filatov, M.A.; Silaeva, Y.Y. Determination of the Optimal Impact Parameters for Microdissection of Zona Pellucida Using Femtosecond IR Laser Pulses. High Temp. 2024, 62, 102–109. [Google Scholar] [CrossRef]
- Kubekina, M.; Kalinina, A.; Korshunova, D.; Bruter, A.; Silaeva, Y. Models of Mitochondrial Dysfunction with Inducible Expression of Polg Pathogenic Mutant Variant. Bull. Russ. State Med. Univ. 2022, 2, 11–17. [Google Scholar] [CrossRef]
- Ilina, I.V.; Ovchinnikov, A.V.; Sitnikov, D.S.; Rakityanskiy, M.M.; Agranat, M.B.; Khramova, Y.V.; Semenova, M.L. Application of Femtosecond Laser Pulses in Biomedical Cell Technologies. High Temp. 2013, 51, 173–178. [Google Scholar] [CrossRef]
- Tirlapur, U.K.; König, K.; Peuckert, C.; Krieg, R.; Halbhuber, K.J. Femtosecond Near-Infrared Laser Pulses Elicit Generation of Reactive Oxygen Species in Mammalian Cells Leading to Apoptosis-like Death. Exp. Cell Res. 2001, 263, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Sitnikov, D.S.; Mukhdina, D.E.; Ovchinnikov, M.A. Study of the Applicability of IR Picosecond Laser Pulses for Microdissection of Zona Pellucida of a Mouse Embryo. High Temp. 2024, 62, 394–399. [Google Scholar] [CrossRef]
- Liang, X.X.; Zhang, Z.; Vogel, A. Multi-Rate-Equation Modeling of the Energy Spectrum of Laser-Induced Conduction Band Electrons in Water. Opt. Express 2019, 27, 4672. [Google Scholar] [CrossRef]
- Sitnikov, D.S.; Ilina, I.V.; Pronkin, A.A. Assessment of the Thermal Effect of Femtosecond and Millisecond Laser Pulses in Microsurgery of Mammalian Embryos. Quantum Electron. 2022, 52, 482–490. [Google Scholar] [CrossRef]
- Choi, I.; Dasari, A.; Kim, N.H.; Campbell, K.H.S. Effects of Prolonged Exposure of Mouse Embryos to Elevated Temperatures on Embryonic Developmental Competence. Reprod. Biomed. Online 2015, 31, 171–179. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Scott, R.T.; Upham, K.M.; Forman, E.J.; Zhao, T.; Treff, N.R. Cleavage-Stage Biopsy Significantly Impairs Human Embryonic Implantation Potential While Blastocyst Biopsy Does Not: A Randomized and Paired Clinical Trial. Fertil. Steril. 2013, 100, 624–630. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).